Abstract
Luminal B breast cancer (LBBC) represents an aggressive, high-grade ER+ disease, associated with a high proliferation rate, higher mutation burden, and higher probability of eliciting the immune response. Clinical and pathological data from 89 patients of stage II-III, triple-negative (TN), and luminal B-like BC (LB-like BC) were included in the analysis. All patients were submitted to neoadjuvant chemotherapy (NACT). Quantitative and qualitative evaluations of TILs (Tumor-Infiltrating Lymphocytes) were performed on tissue microarrays constructed from pretreatment core-needle biopsy tumor specimens. The proportion of stromal TILs, CD8, CD4, and PD-L1 positive (+) immune cells (IC), as well as the number of FOXP3, CTLA4, and HSP-70+ IC, was observed concerning tumor immunophenotype, traditional clinicopathological prognostic factors, and tumor response to NACT. There was no statistically significant difference in the proportion of stromal TILs between the LB-like and TNBC (p = 0.344) cohorts. However, a higher CD4/CD8 ratio was associated with the TNBC biology (p = 0.018) and within the LB-like BC cohort with a high proliferation index and metastatic nodal involvement (p = 0.045, p = 0.015). Within the LB-like BC cohort, a higher expression of PD-L1 and HSP70+ IC was associated with a high proliferation index of tumor cells (p = 0.018, p = 0.040), massive metastatic nodal involvement (p = 0.002, p = 0.026), and higher stages of disease (p = 0.004, p = 0.042). Better response to NACT was associated with higher numbers of HSP70+ IC and higher proportions of CD8+ cells within the LB-like BC cohort (p = 0.045, p = 0.012). Routine evaluation of immune markers and HSP70 may help identify high-risk patients of LB-like breast cancer who would have a better response to NACT.
1. Introduction
Breast cancer represents a heterogeneous group of tumors originating from the breast epithelium, with different developmental pathways [1], histological and molecular patterns, clinical characteristics, treatment responses, and patient outcomes [2,3]. Molecular profiling has revealed four primary subtypes of breast cancer: luminal A, luminal B, HER2 enriched, and basal, with different expression profiles of luminal, basal, proliferation, and ERBB2 genes. Immunohistochemical (IHC) evaluations of estrogen receptors (ER), progesterone receptors (PR), and HER2 are a mandatory part of routine breast cancer histopathology reports, as it may approximate the breast cancer molecular subtype [4]. Besides their role as prognostic markers, they have predictive significance, representing molecular targets for specific breast cancer therapy. Therefore, their routine determination in clinical practice has a significant impact on treatment recommendations and has improved the outcomes of ER+ and HER2+ breast cancer patients. However, 15–20% of breast cancers are of the ER-PR-HER2- (triple-negative, TNBC) phenotype, lacking in molecular targets for specific anti-cancer treatment, as well as in prognostic and predictive biomarkers [5]. In addition, a significant proportion of ER+PR+/-HER2- luminal B breast cancers (LBBC) are resistant to endocrine treatment (ET), due to the activation of aberrant signaling pathways for proliferation, independent of the ER mitogen signal [6]. Nevertheless, in routine clinical practice, ET is still a primary systemic treatment modality for all ER+ breast cancers. Both TNBC and LBBC are aggressive, high-grade breast cancer subtypes associated with a high proliferation rate of tumor cells and poor patient outcomes [7]. Identifying new tumor-specific molecular markers that may improve the prognostic classification of LBBC and TNBC patients and enable the prediction of tumor response to systemic treatment is essential for adjusting the treatment protocols according to tumor characteristics and optimizing patients’ outcomes.
The stromal compartment of the tumor is determined by the tumor’s biology and therefore may be a valuable source of disease-specific biomarkers [8]. The proportion of stromal TILs is a surrogate marker for tumor immunogenicity and is associated with a tumor mutation burden. Qualitative and functional variations in TILs reflect the nature of the immune response in the tumor microenvironment (TME). The composition of TILs may vary within equal proportions of stromal TILs due to differences in tumor biology or within the equal subtype of breast cancer due to differences in the stages of the disease [9]. In early TNBC, the proportion of stromal TILs is associated with better patient outcomes and better tumor response to neoadjuvant chemotherapy (NACT). Therefore, it is already accepted as a prognostic and predictive biomarker [10,11,12,13]. However, this may not apply to the later stages of the disease, due to the activation of several regulatory mechanisms in TILs as suggested by the concept of immunoediting [14,15,16,17]. Following the aforementioned, in the early stage of TNBC, the efficient immune response against tumor cells is pro-inflammatory and is characterized by a high proportion of CD8+ T cells. However, by the stage of disease progression, several regulatory mechanisms may be activated, enabling the escape, progression, and metastatic spread of tumor cells. The immunosuppressive mechanisms of CTLA4 [18,19] and HSP70 [20,21,22,23] are in general mediated primarily through the functional inactivation of dendritic cells in tumors with the consequent ineffective priming and apoptosis of conventional T lymphocytes upon antigen recognition, or regulatory T lymphocyte (Treg) differentiation. In addition, the stress-induced expression of HSP70 in IC suppresses pro-inflammatory gene expression [24,25]. The PD-L1 regulatory mechanism is mediated through the intracellular inhibition of the T cell receptor (TCR) signal; therefore, both the activation and effector function of T cells may be impaired [26]. Of these three mechanisms, PD-L1 could be the most important inducer of Treg differentiation in tumors [27]. This study aimed to evaluate the proportion and composition of stromal TILs in stage II-III TNBC and luminal B-like breast cancer (LB-like BC) cohorts, to identify subtype-related and stage-related specificities of the immune response and to identify immune markers that may predict tumor response to NACT.
2. Materials and Methods
Clinical and traditional histopathological data from 89 patients were retrospectively included in the present analysis. Table 1 displays the clinicopathological characteristics of the study cohort.

Table 1.
Clinical and traditional histopathological data of the study cohort (n = 89).
All patients were diagnosed consecutively with anatomical stage II-III, triple-negative (n = 36), and luminal B-like (n = 53) invasive breast cancer in the period from 2016 to 2022 in Clinical Hospital Center Rijeka, Croatia, and submitted to NACT before surgical treatment. All patients have received 4 cycles of doxorubicin and cyclophosphamide, followed by 12 cycles of paclitaxel. Patients for whom pretreatment core-needle biopsy (CNB) tissue samples were not available (diagnosed in another institution), those diagnosed with stage IV disease, HER2+ breast cancer, or in whom NACT was not completed per protocol were excluded from the study. The control group for IHC stainings was represented by 36 vacuum-assisted breast biopsy (VABB) tissue specimens, classified as benign breast change (B2) in the histopathology report, primarily fibrocystic change or microcalcifications within the acini. Quantitative evaluation of stromal TILs was performed on whole HE-stained tumor tissue slides, according to recommendations of the TILs working group from 2014 [28]. Qualitative IHC analysis of immune infiltrate was performed on tissue microarrays (TMA), constructed for this research from pretreatment CNB tissue specimens for the study cohort and from VABB tissue specimens for the control group. To overcome the issue of intratumoral heterogeneity, each tumor biopsy was represented with two cylinders of primary tumor tissue of 1.5 mm in diameter. The benign breast change was represented with one cylinder of 2 mm in diameter per biopsy. The “EnVision” IHC method was performed using the Real EnVision Detection System K8000 on an automated immunostainer (DakoCytomation, Autostainer Plus, Glostrup, Denmark), following the manufacturer’s instructions. Detailed specifications for the IHC staining procedures are provided in Table 2.

Table 2.
Immunohistochemical staining and procedure.
The proportions of CD4+ T lymphocytes, CD8+ T lymphocytes, and PD-L1+ immune cells (IC) were estimated relative to the tumor tissue micro-area under a light microscope magnification of 200×. The expression of FOXP3+ lymphocytes, CTLA4+ lymphocytes, and HSP70+ IC were estimated as the total number of positive cells within the tumor tissue micro-area under a light microscope magnification of 400×. All IHC stainings of IC were cytoplasmic and membranous except for the nuclear anti-FOXP3 staining (Figure 1). All IHC readings were performed by two researchers, blinded for the clinical characteristics of the patients and the histological characteristics of the tumors.
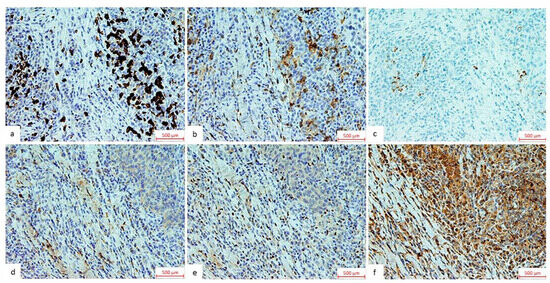
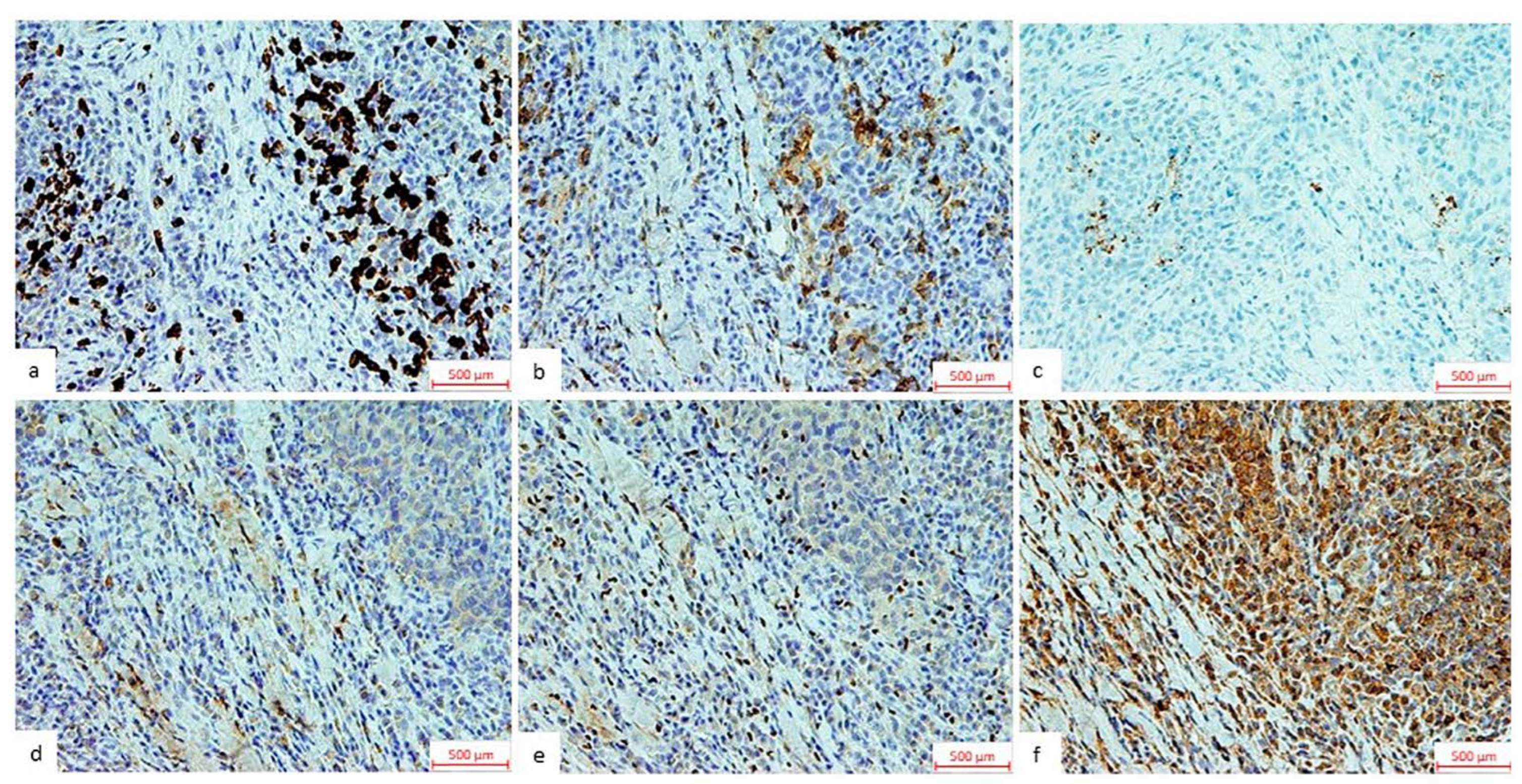
Figure 1.
Representative immunohistochemical stainings of TIL components and HSP70. The images represent immunohistochemical staining of (a) CD8, (b) CD4, and (c) PD-L1, shown as the proportion of positive immune cells relative to the tumor tissue micro-area, observed under a light microscope at 200× magnification and (d) CTLA4, (e) FOXP3, and (f) HSP70 shown as the total number of positive immune cells within the tumor tissue micro-area under a light microscope at 200× magnification.
The proportion of stromal TILs, CD8+ T lymphocytes, CD4+ T lymphocytes, and PD-L1+ IC, as well as the number of FOXP3+ lymphocytes, CTLA4+ lymphocytes, and HSP-70+ IC, were analyzed in relation to the IHC profile of the tumor, pretreatment clinical tumor and nodal status (cT and cN), anatomical and clinical prognostic stages of the disease and tumor nuclear grade (NG), patient oncological outcomes, and tumor response to NACT. The IHC phenotype of breast cancer was determined according to the St. Gallen recommendations [4] and the cT, cN, anatomical, and clinical prognostic stages of the disease according to the last version of the TNM classification [29]. Disease-free survival (DFS) was defined as the time from the beginning of primary neoadjuvant treatment until the first occurrence of locoregional or distant recurrence, or until censoring at the date of the last clinical follow-up in the absence of recurrence. The pathological residual cancer burden score (pRCB score) was calculated for every case with an online calculator available on the MD Anderson Center web page [30].
MedCalc Version 20.013 (Medcalc © 2024 MedCalc Software Ltd., Ostend Belgium) and X-tile analysis version 3.6.1. (X-Tile © Yale University 2003-05) were used for statistical analysis. The normality of the data distribution was calculated by the Kolmogorov–Smirnov test (K-S test). Since all variables were distributed irregularly, the results were presented as median values. Variations in immunological variables in relation to clinical and pathological prognostic factors for two or more groups were evaluated by the Mann–Whitney U test and by the Kruskal–Wallis ANOVA followed by a post hoc Conover test. Intercorrelations between immunological variables were determined by Spearman’s rank correlation. The Spearman’s rank of correlation coefficient was expressed as negligible (less than 0.30); low positive (0.30–0.50); moderately positive (0.50–0.70); high positive (0.70–0.90); and very highly positive (more than 0.90). For the survival analysis, the cut-off values of the results were determined by X-tile analysis, and the survival curves were calculated by the Kaplan–Meier method with appropriate censoring of patients who had not experienced a recurrence by the time of their last clinical evaluation. Differences in survival curves were tested with the log-rank test. For variables in a statistically significant association with the tumor response to NACT, the predictive value of the clinical test was determined by Receiver Operating Characteristic (ROC) analysis and logistic regression. All statistical values were considered significant at a p-level of <0.05.
3. Results
3.1. The Relationship of TILs and TIL Components to Clinicopathological Data of TNBC and LB-like BC
The proportion of stromal TILs was determined for all cases of the entire study cohort. The median value for the TNBC cohort (n = 36) was 12% (range 3–70%) and for the LB-like BC cohort (n = 53) 13% (range 0.5–65%). No statistically significant difference was detected in the proportion of stromal TILs between stage II-III TNBC and LB-like BC in our study cohort (Mann–Whitney U test, p = 0.345), displayed in Table 3. In the LB-like BC cohort, higher values of stromal TILs were associated with a higher nuclear grade (NG) of tumor cells (Mann–Whitney U test, p = 0.049) and with a higher clinical prognostic stage of the disease (Kruskal–Wallis ANOVA by ranks, p = 0.033) (Figure 2a). Quantitative variations in TILs were not associated with the clinical status of the primary tumor (cT), the clinical status of the regional lymph nodes (cN), or the anatomical stage of the disease in the LB-like BC cohort, nor at the level of the entire cohort. Qualitative analysis of stromal TILs, according to the selected IHC markers, was performed for each case of the study cohort. The results were compared between the TNBC and LB-like BC cases, as well as between the study cohort and the control group. The results are displayed in Table 3.

Table 3.
The results of quantitative and qualitative analyses of stromal TILs in the control group and study cohort.
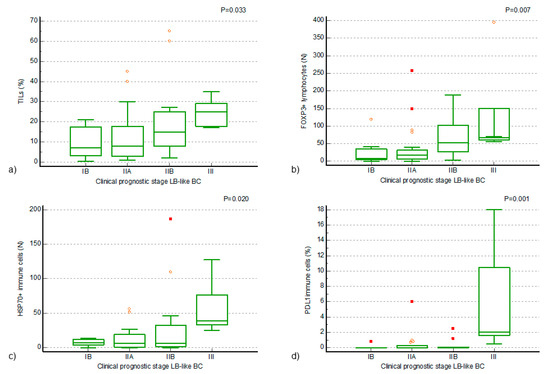
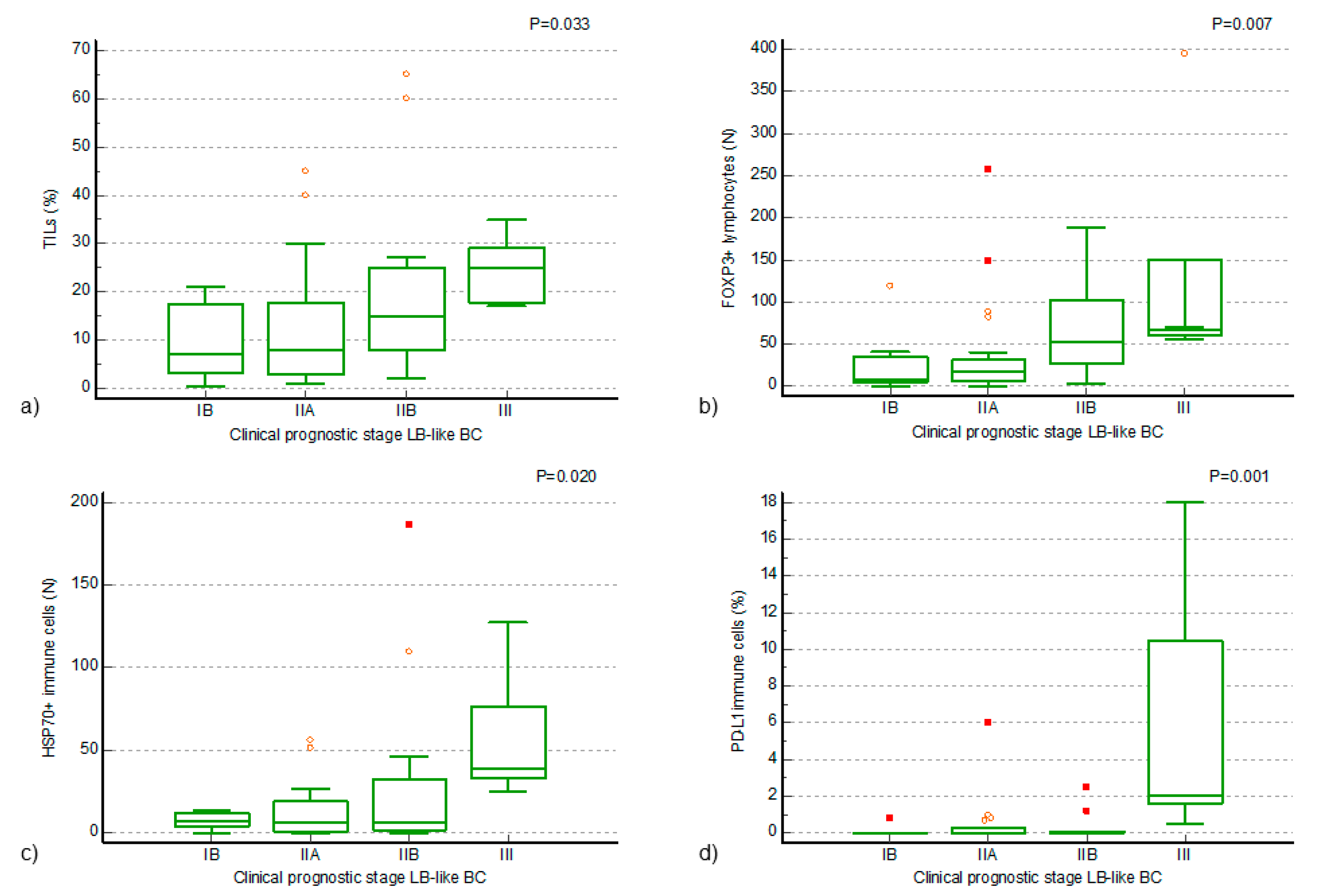
Figure 2.
Positive associations of TILs and TIL components of luminal B-like breast cancer (LB-like BC) to the clinical prognostic stage of the disease. (a) A statistically significant increase in the proportion of TILs in clinical prognostic stage III disease, compared to earlier stages (Kruskal–Wallis ANOVA by ranks, p = 0.033; (b) a statistically significant increase in the number of FOXP3+ lymphocytes in the TILs of clinical prognostic stage IIB and III LB-like BC, compared to earlier stages of disease (Kruskal–Wallis ANOVA by ranks, p = 0.007); (c,d) a statistically significant increase in the number of HSP70+ immune cells and a statistically significant increase in the proportion of PD-L1+ immune cells in the TILs of clinical prognostic stage III LB-like BC, compared to earlier stages of disease (Kruskal–Wallis ANOVA by ranks, p = 0.020, p = 0.001). The small symbols—orange circles and red squares—represent outliers in the data distribution, with circles indicating mild outliers and squares indicating extreme outliers.
All values of IHC stains were significantly higher in the stromal compartment of the breast cancer as compared to the benign breast tissue of the control group (Mann–Whitney U test, p < 0.001). There were no statistically significant differences detected in the proportion of CD8+ and CD4+ T lymphocytes, PD-L1+ IC, nor in the number of FOXP3+ and CTLA4+ lymphocytes, as well as the HSP70+ IC between the TNBC and LB-like BC cohorts (Mann–Whitney U test, p values are displayed in Table 3). However, a higher CD4/CD8 ratio was associated with the TNBC cohort (Mann–Whitney U test, p = 0.018) although with a modest effect size. Within the LB-like BC cohort, a higher CD4/CD8 ratio, a higher proportion of PD-L1+ IC, and a higher number of HSP70+ IC were associated with a high proliferation index of tumor cells (Ki67 > 30%) (Mann–Whitney U test, p = 0.045, p = 0.018, p = 0.040) and with metastatic nodal involvement, described below. Metastatic tumor spread in the regional lymph nodes (cN (+) status) was associated with a higher CD4/CD8 ratio (Mann–Whitney U test, p = 0.015, and in addition, massive nodal involvement (cN2 status) was associated with higher values of PD-L1+ and HSP70+ IC in TILs (Mann–Whitney U test, p = 0.002, p = 0.026).
Positive associations of higher values of FOXP3+ lymphocytes, PD-L1+ IC, and HSP70+ IC with a higher clinical prognostic stage of the disease were found (Kruskal–Wallis ANOVA by ranks, p = 0.007, p = 0.001, p = 0.020) in the LB-like BC cohort. A statistically significant increase in the number of FOXP3+ lymphocytes in the TILs of LB-like BC of clinical prognostic stage IIB and III, compared to earlier stages of the disease, was confirmed by Conover’s post hoc analysis (Figure 2b). A statistically significant increase in the number of HSP70+ IC and in the proportion of PD-L1+ IC in the TILs of the LB-like BC cohort of clinical prognostic stage III, compared to earlier stages of disease, was confirmed by Conover’s post hoc analysis (Figure 2c,d). In addition to the described associations with the clinical prognostic stage of disease, an association of a higher number of FOXP3+ and CTLA4+ lymphocytes with a higher NG was also found (Mann–Whitney U test, p = 0.023, p = 0.024).
Statistically significant differences in DFS, associated with quantitative or qualitative variations in TILs, were not noticed in the present analysis.
3.2. Intercorellations of TIL Components in TNBC and LB-like BC
Several positive correlations, calculated with Sperman’s rank of correlations, were noticed between TIL components within the TNBC and LB-like BC cohorts. In both the TNBC and LB-like BC cohorts, high positive correlations were found between the proportion of stromal TILs and the proportions of CD8+ (rho = 0.744, p = 0.01; rho = 0.842, p = 0.0001) and CD4+ T lymphocytes (rho = 0.653, p = 0.006; rho = 0.842, p = 0.0001). However, different correlations with regulatory markers were noticed concerning breast cancer immunophenotype. Moderate positive correlations were observed between the proportions of CD8+ and CD4+ T lymphocytes and the proportion of PD-L1+ IC (rho = 0.56, p = 0.023; rho = 0.57, p = 0.020), and also between the proportion of CD4+ T lymphocytes and the number of FOXP3+ lymphocytes (rho = 0.53, p = 0.035) in the TNBC cohort. In the LB-like BC cohort, moderate positive correlations were observed between the proportions of stromal TILs and CD4+ and CD8+ T lymphocytes and the number of CTLA4+ lymphocytes (rho = 0.67, p = 0.0001; rho = 0.69, p = 0.0001; rho = 0.57, p = 0.0001) and also between the proportion of stromal TILs and HSP70+ tumor-associated IC (rho = 0.53, p = 0.0001). Several positive correlations also appeared between the number of FOXP3+ lymphocytes and the proportion of PD-L1+ IC (rho = 0.72, p = 0.0001), the number of HSP70+ IC (rho = 0.59, p = 0.0001), and the number of CTLA4+ lymphocytes (rho = 0.45, p = 0.003).
3.3. Predictive Value of TILs and TIL Components in Neoadjuvant Treatment of LB-like BC
For the purposes of statistical analysis, two clinically and prognostically significant categories related to the response to neoadjuvant treatment were formed: one representing a good response to NACT, and the other representing a poor response or no response. The first category of the TNBC cohort was represented by the complete pathological response to NACT (pCR, pRCB0, n = 13), and the second by any residual tumor (pRCB1-3, n = 23) following completion of NACT. In the TNBC cohort, a statistical trend was observed between a higher proportion of CD8+ T lymphocytes and pCR (Mann–Whitney U test, p = 0.0733). In the analysis of the LB-like BC cohort, the first category was created by the fusion of the pRCB 0 and pRCB1 groups (n = 11) and the second by the fusion of the pRCB2 and pRCB3 groups (n = 41). Concerning the two clinically significant prognostic categories in the LB-like BC cohort (pCR + pRCB1 vs. pRCB2 + pRCB3), statistically significant associations were found between a higher proportion of CD8+ T lymphocytes as well as a higher number of HSP70+ IC and a good response to NACT (Mann–Whitney U test, p = 0.012, p = 0.045). A higher proportion of stromal TILs was observed in patients with a good response to NACT, although this difference did not reach statistical significance (p = 0.060, Mann–Whitney U test). Along with the above-mentioned associations related to the TIL components, higher Ki67 values were also connected with good response to NACT in the LB-like BC cohort (Mann–Whitney U test, p = 0.029).
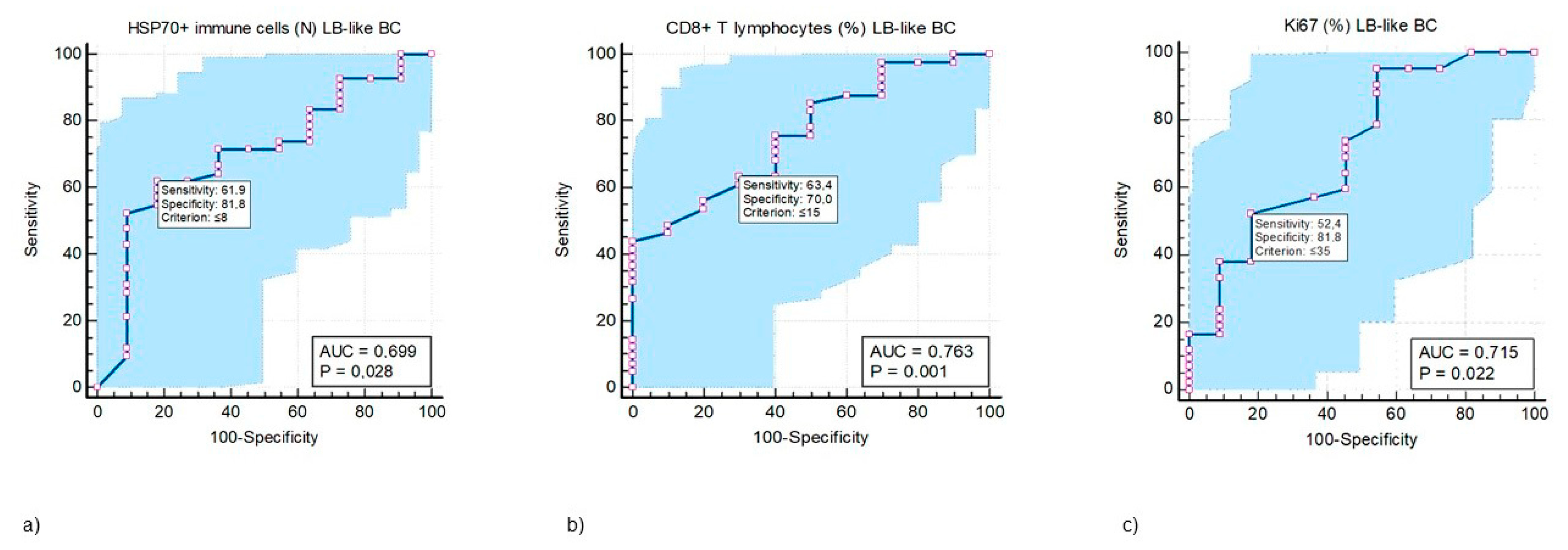
ROC analysis was performed to test the predictive value of CD8+ T lymphocytes and HSP70+ IC in the LB-like BC cohort. Cut-off values, at which the test’s specificity and sensitivity would be highest, were determined for both variables (Figure 3a,b), and the Ki67 proliferation index (Figure 3c).
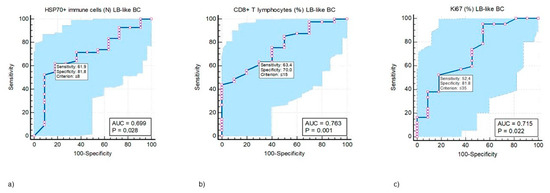
Figure 3.
ROC curve analysis of the predictive values of HSP70+ immune cells (IC) (a), CD8+ T lymphocytes (T ly) (b), and Ki67 (c) in response to neoadjuvant treatment in luminal B-like breast cancer (LB-like BC) categorized as pRCB class 0 + 1 vs. pRCB class 2 + 3. (a) HSP70+ IC in the TILs of LB-like BC can be used as a predictive biomarker (AUC 0.669, p = 0.028) that may differentiate between good and poor responses to NACT associated with a test sensitivity of 61.9% (61.9% of randomly selected LB-like BC patients with a pretreatment value of HSP70+ IC ˃ 8 will have a good response to NACT) and a test specificity of 81.8% (81.8% of randomly selected LB-like BC patients with a pretreatment value of HSP70+ IC ≤ 8 will have a poor response to NACT) at the cut-off value ≤ 8. (b) Proportion of CD8+ T ly in the TILs of LB-like BC can be used as a predictive biomarker (AUC 0.763, p = 0.001) that may differentiate between good and poor responses to NACT associated with a test sensitivity of 63.4% (63.4% of randomly selected LB-like BC patients with a pretreatment value of CD8+ T ly ˃15% will have a good response to NACT) and a test specificity of 70% (70% of randomly selected LB-like BC patients with a pretreatment value of CD8+ T ly ≤ 15% will have a poor response to NACT) at the cut-off value ≤ 15. (c) The predictive value for Ki67 proliferation index with a sensitivity of 52.4% and specificity 81.8% (AUC 0.715, p = 0.022) at the cut-off value 35%. For these three values, the test will have the highest accuracy in predicting good vs. poor tumor response to NACT, i.e., there will be the lowest number of false positive and false negative predictions. The blue-shaded area represents the area under the ROC curve (AUC), indicating the test’s overall ability to discriminate between positive and negative cases. In MedCalc, the blue area extends above and below the curve line to visually emphasize this region.
A comparison of the ROC curves for CD8+ T lymphocytes, HSP70+ IC, and Ki67 revealed that all three variables were equally reliable in predicting the response to NACT in LB-like BC. Areas under the curves between the three mentioned variables did not differ statistically significantly (p ˃ 0.05).
In multivariate analysis, however, neither of the two markers reached statistical significance. Only a statistical trend was found for CD8+ T lymphocytes, along with Ki67 as a confirmed independent predictor of response to NACT in LB-like BC (p = 0.065, p = 0.042) (Table 4).

Table 4.
Odds ratio of biomarkers as predictors of a response to neoadjuvant treatment.
4. Discussion
Luminal breast cancer is generally considered a low immunogenicity tumor, but this might not apply to the entire group of ER-related pathway tumors. It is a highly heterogeneous group with divergent molecular development pathways [1], different gene expressions, and dissimilar clinical features [6,7]. On the molecular level, LBBC has a lower expression of ER-related genes, a higher expression of basal and proliferation genes, a higher mutation burden, and, therefore, a higher probability of eliciting the immune response. The results of several previous studies also imply that this aggressive subtype of ER+ breast cancer may have a higher expression of immune-related genes with a higher proportion of stromal TILs, which may have prognostic and predictive significance in this subtype of breast cancer [31,32,33,34]. However, the results in the literature are inconclusive as the LBBC was rarely investigated isolated from the whole group of luminal tumors. In the present analysis, we did not detect a statistically significant difference in either the proportion of stromal TILs or TIL components between the LB-like BC and TNBC cohorts, matched according to the stage of the disease. Our results endorse that LBBC might be an immunogenic subtype of ER+ breast cancer and we have further evaluated the potential implications of this observation for the current clinical practice.
In healthcare systems where genetic risk scores are still unavailable, clinical risk scores, generated upon validated prognostic factors, are utilized for the selection of LB-like BC patients who may benefit from additional treatments; and every biomarker that may suggest high-risk biology within this subtype of breast cancer is a valuable clinical tool. Herein, we have tested if qualitative and quantitative evaluations of the stromal TILs of the LBBC immunophenotype may optimize the prognostic classification and treatment recommendations accordingly. Although we failed to detect any statistically significant association between quantitative and qualitative (functional) variations in TILs and disease-free survival, probably due to a short follow-up period, several associations were noticed regarding adverse tumor biology (high grade and high proliferation) and disease progression (high stage and regional nodal involvement). A higher proportion of stromal TILs and a higher number of FOXP3+ lymphocytes in the LB-like BC immunophenotype imply a more aggressive, higher-risk disease (higher stage and higher NG). This is concordant with the previous literature data, in which the ER signal was associated with Treg differentiation in the TME and in which higher TILs were associated with adverse patient outcomes within the whole ER+ breast cancer group [34,35,36]. Several regulatory mechanisms are mediated by Tregs [27], all of which contribute to the creation of an immunosuppressive microenvironment that allows cancer to escape the immune system and enable disease progression. The inhibition of priming and the activation of conventional T cells are mediated through the CTLA4 molecule, constitutively expressed on Tregs. Although the expression of PD-L1, CTLA4, and HSP70 molecules in tumor-associated immune cells were all in positive correlation with the number of FOXP3+ lymphocytes within our LB-like BC cohort, the strongest correlation was observed for the expression of PD-L1+ IC. The aggressive biology, higher stages of the disease, and massive metastatic nodal involvement within the LB-like BC cohort were associated with a higher expression of PD-L1 and HSP70 in immune cells. In addition to the ER-related signal, FOXP3+ lymphocytic differentiation may also be mediated through a higher expression of PD-L1 and HSP70 regulatory molecules in TILs that occurs during the tumorogenesis of a higher-risk LBBC, as suggested by the herein presented data, with a consequent higher expression of FOXP3 and CTLA4 proteins in TILs. The previously described regulatory roles of PD-L1 and HSP70 in the immune response support this conclusion [18,20,21,22,23,24,25,26,27]. Interestingly, we did not observe such correlations or associations in the TNBC cohort of the present analysis, possibly due to a small sample size. However, in our previous TIL analysis on a larger TNBC cohort of 68 cases (unpublished data, ref. [37]), similar correlations with regulatory molecules were observed, with the percentage of PD-L1-positive immune cells and CD4-positive lymphocytes shown to be independent prognostic factors for overall survival.
Cytotoxicity failure, which precedes the tumor’s escape from the immune system, is represented by a higher CD4/CD8 ratio in TILs [15,38] and is predominantly caused by the effect of immunosuppressive cytokines and HSP70 chaperokine in the TME, with a consequently higher Treg differentiation rate from the naive CD4+ T lymphocyte population. A higher CD4/CD8 ratio was associated with a triple-negative breast cancer immunophenotype in this analysis, but also with the more aggressive LB-like BC cases with a high proliferation rate and in a more advanced stage of disease with metastatic spread to the regional lymph nodes.
A high proportion of stromal TILs with a high number of FOXP3+ lymphocytes, a high proportion of PD-L1+ and HSP70+ immune cells, and a high CD4/CD8 ratio in LB-like BC suggest aggressive biology and a higher stage of the disease, i.e., a requirement for treatment escalation strategies. The association of an aggressive ER+ phenotype with a high expression of PD-L1+ immune cells is supported by former literature data that have related a higher expression of PD-L1 to a higher expression of immune-related genes and a lower expression of ER-related genes in breast cancer [39]. The resistance to tamoxifen previously associated with the presence of CD4+ FOXP3+ lymphocytes [40] also implies the necessity for different treatment strategies in TILs-high/FOXP3-high LB-like BC. In selected cases of high-risk LB-like BC, unresponsive to conventional treatments, immunotherapy may be a therapeutic option as indicated by the recently published clinical data of KEYNOTE-756 study [41].
Heterogeneous biology within the LB-like BC cohort with different expressions of regulatory molecules in stromal TILs implies that different strategies may be required for the activation of the immune response against the tumor within this cancer subtype. The addition of cytotoxic chemotherapy may be beneficial for the reduction in the Treg fraction, the reactivation of the Th1 response in TILs-high LBBC with a high CD4/CD8 ratio, and the activation of the new immune response in higher stages of LBBC [42]. Further investigations are needed to confirm these observations.
As all patients in the present analysis have received preoperative chemotherapy, we have further analyzed whether tumor response to NACT may be predicted upon quantitative and qualitative variations in TILs in pretreatment CNB specimens. In the TNBC cohort, only a statistical trend was observed between a higher proportion of CD8+ T lymphocytes and a higher pCR rate, probably due to a small sample size, as the proportion of TILs and CD8+ T cells has already been associated with a higher pCR rate in several TNBC trials. The results on the chemosensitivity of ER+ HER2- TILs-high tumors vary in the literature from no associations to better tumor response to cytotoxic treatment [35,36]. In our LB-like BC cohort, a higher proportion of stromal TILs was associated with a better response to neoadjuvant chemotherapy, but this association did not reach statistical significance. However, an increased number of HSP70-positive immune cells and a greater proportion of CD8-positive T lymphocytes in the stromal compartment of pretreatment LB-like BC tumor tissue were significantly associated with a favorable response to neoadjuvant chemotherapy. The genetic risk score is not assessed routinely in our institution because it still is not reimbursed by the healthcare insurance systems in Croatia, but also because its predictive value of tumor response to NACT is limited. All patients of our LB-like BC cohort were classified as high-clinical-risk patients at institutional breast multidisciplinary team meetings and therefore selected for NACT. However, only 20.7% had a pRCB 0 or 1 score following NACT. We, therefore, support the routine IHC determination of CD8+ and HSP70+ IC, along with Ki67 determination, in LB-like BC, as it may optimize the patient selection for NACT.
5. Conclusions
The main advantage of this study is our LB-like BC cohort composed of high-risk ER + HER2- breast cancer patients comparable to the TNBC group, in which the proportion of stromal TILs suggests that it could be an immunogenic subtype of ER+ HER2- breast cancer. In addition to the quantitative evaluation of TILs, a comprehensive IHC analysis of the immune landscape was performed and several implications to the current clinical practice were identified. The assessment of TILs may help with the identification of high-risk LB-like BC patients, for whom additional treatments might be needed, as well as the patients who may benefit from neoadjuvant chemotherapy. Immunotherapy may be considered a therapeutic option for selected high-risk LB-like BC cases, resistant to conventional treatments. Nevertheless, the specific distribution of regulatory molecules in stromal TILs noticed in our LB-like BC cohort suggests different strategies may be required for the activation of the immune response against the tumor within this cancer subtype, which would need to be investigated on a larger sample of LB breast cancers.
The main limitations of the study are its retrospective design, short follow-up period, and relatively small sample size. Also, the clinically low-risk population of LB-like BC patients, diagnosed with cT1 status of the primary tumor, have not been included in this study.
Author Contributions
A.C.P.: conceptualization, data curation, investigation, writing—original draft; K.R.M.: validation, visualization; A.S.V.: validation, visualization; T.G.: investigation; methodology; P.V.Z.: investigation, validation; E.C.T.: investigation, validation; D.J.: investigation, validation; A.P.M.: investigation, supervision; F.L.: investigation, supervision; G.Đ.: investigation, supervision; M.A.: conceptualization, data curation, formal analysis, writing—review and editing, resources. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Rijeka project: “Predictive and prognostic role of immune system cells, PD-1, PD L-1 and heat shock proteins in patients with triple-negative, HER-2 positive and neoadjuvant-treated breast cancer” registered under the code: uniri-biomed-18-259-1428.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Clinical Hospital Center Rijeka, Croatia (protocol code 2170-29-02/1-19-2 and approval date: 24 September 2019).
Informed Consent Statement
Patient consent was waived due to the retrospective design of the research.
Data Availability Statement
The data presented in this study were published in the Repository of the Faculty of Medicine University of Rijeka (MEDRI repository) and are available upon request with the consent of the author and/or editor of the repository. https://repository.medri.uniri.hr/islandora/object/medri:9266 (accessed on 27 May 2025).
Acknowledgments
The authors thank the laboratory engineers Helga Frketić and Ozren Štanfel for their excellent technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bombonati, A.; Sgroi, D.C. The molecular pathology of breast cancer progression. J. Pathol. 2011, 223, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An update on the pathological classification of breast cancer. Histopathology 2023, 82, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Panel Members. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Creighton, C.J. The molecular profile of luminal B breast cancer. Biologics 2012, 6, 289–297. [Google Scholar] [CrossRef]
- Du, X.W.; Li, G.; Liu, J.; Zhang, C.Y.; Liu, Q.; Wang, H.; Chen, T.S. Comprehensive analysis of the cancer driver genes in breast cancer demonstrates their roles in cancer prognosis and tumor microenvironment. World J. Surg. Oncol. 2021, 19, 273. [Google Scholar] [CrossRef]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef]
- de Jong, V.M.T.; Wang, Y.; Ter Hoeve, N.D.; Opdam, M.; Stathonikos, N.; Jóźwiak, K.; Hauptmann, M.; Cornelissen, S.; Vreuls, W.; Rosenberg, E.H.; et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy. J. Clin. Oncol. 2022, 40, 2361–2374. [Google Scholar] [CrossRef]
- Vihervuori, H.; Autere, T.A.; Repo, H.; Kurki, S.; Kallio, L.; Lintunen, M.M.; Talvinen, K.; Kronqvist, P. Tumor-infiltrating lymphocytes and CD8+ T cells predict survival of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 3105–3114. [Google Scholar] [CrossRef] [PubMed]
- Morigi, C. Highlights of the 16th St Gallen International Breast Cancer Conference, Vienna, Austria, 20–23 March 2019: Personalised treatments for patients with early breast cancer. Ecancermedicalscience 2019, 13, 924. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Glajcar, A.; Szpor, J.; Hodorowicz-Zaniewska, D.; Tyrak, K.E.; Okoń, K. The composition of T cell infiltrates varies in primary invasive breast cancer of different molecular subtypes as well as according to tumor size and nodal status. Virchows Arch. 2019, 475, 13–23. [Google Scholar] [CrossRef]
- Ogiya, R.; Niikura, N.; Kumaki, N.; Bianchini, G.; Kitano, S.; Iwamoto, T.; Hayashi, N.; Yokoyama, K.; Oshitanai, R.; Terao, M.; et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016, 107, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Althobiti, M.; Aleskandarany, M.A.; Joseph, C.; Toss, M.; Mongan, N.; Diez-Rodriguez, M.; Nolan, C.C.; Ashankyty, I.; Ellis, I.O.; Green, A.R.; et al. Heterogeneity of tumour-infiltrating lymphocytes in breast cancer and its prognostic significance. Histopathology 2018, 73, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J. Autoimmun. 2013, 45, 49–57. [Google Scholar] [CrossRef]
- Walker, L.S.; Sansom, D.M. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol. 2015, 36, 63–70. [Google Scholar] [CrossRef]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef]
- Linder, M.; Pogge von Strandmann, E. The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells. Cancers 2021, 13, 4721. [Google Scholar] [CrossRef]
- Albakova, Z.; Mangasarova, Y. The HSP Immune Network in Cancer. Front. Immunol. 2021, 12, 796493. [Google Scholar] [CrossRef]
- Ogbodo, E.; Michelangeli, F.; Williams, J.H.H. Exogenous heat shock proteins HSPA1A and HSPB1 regulate TNF-α, IL-1β and IL-10 secretion from monocytic cells. FEBS Open Bio. 2023, 13, 1922–1940. [Google Scholar] [CrossRef]
- Borges, T.J.; Wieten, L.; van Herwijnen, M.J.; Broere, F.; van der Zee, R.; Bonorino, C.; van Eden, W. The anti-inflammatory mechanisms of Hsp70. Front. Immunol. 2012, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.K.; Harel-Bellan, A.; Morimoto, R.I.; Welch, W.J.; Farrar, W.L. Mitogen and lymphokine stimulation of heat shock proteins in T lymphocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 3850–3854. [Google Scholar] [CrossRef] [PubMed]
- Arasanz, H.; Gato-Cañas, M.; Zuazo, M.; Ibañez-Vea, M.; Breckpot, K.; Kochan, G.; Escors, D. PD1 signal transduction pathways in T cells. Oncotarget 2017, 8, 51936–51945. [Google Scholar] [CrossRef]
- Cai, J.; Wang, D.; Zhang, G.; Guo, X. The Role Of PD-1/PD-L1 Axis In Treg Development And Function: Implications For Cancer Immunotherapy. Onco Targets Ther. 2019, 12, 8437–8445. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.Y.; Connolly, J.L.; D’Orsi, C.J.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Giuliano, A. Breast Cancer Staging System: AJCC Cancer Staging Manual, 8th ed.; The American College of Surgeons (ACS): Chicago, IL, USA, 2018; Available online: http://www.breastsurgeonsweb.com/wp-content/uploads/downloads/2020/10/AJCC-Breast-Cancer-Staging-System.pdf (accessed on 15 August 2024).
- Residual Cancer Burden Calculator. Available online: https://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3 (accessed on 15 August 2024).
- Oner, G.; Altintas, S.; Canturk, Z.; Tjalma, W.; Van Berckelaer, C.; Broeckx, G.; Zwaenepoel, K.; Tholhuijsen, M.; Verhoeven, Y.; Berneman, Z.; et al. The immunologic aspects in hormone receptor positive breast cancer. Cancer Treat. Res. Commun. 2020, 25, 100207. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Valenza, C.; Taurelli Salimbeni, B.; Santoro, C.; Trapani, D.; Antonarelli, G.; Curigliano, G. Tumor Infiltrating Lymphocytes across Breast Cancer Subtypes: Current Issues for Biomarker Assessment. Cancers 2023, 15, 767. [Google Scholar] [CrossRef]
- Criscitiello, C.; Vingiani, A.; Maisonneuve, P.; Viale, G.; Viale, G.; Curigliano, G. Tumor-infiltrating lymphocytes (TILs) in ER+/HER2- breast cancer. Breast Cancer Res. Treat. 2020, 183, 347–354. [Google Scholar] [CrossRef]
- He, L.; Wang, Y.; Wu, Q.; Song, Y.; Ma, X.; Zhang, B.; Wang, H.; Huang, Y. Association between levels of tumor-infiltrating lymphocytes in different subtypes of primary breast tumors and prognostic outcomes: A meta-analysis. BMC Women’s Health 2020, 20, 194. [Google Scholar] [CrossRef]
- Gao, Z.H.; Li, C.X.; Liu, M.; Jiang, J.Y. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: A meta-analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef] [PubMed]
- Peterko, A.C.; Rajković-Molek, K.; Gulić, T.; Vujaklija, D.V.; Lovasić, I.B.; Lovasić, F.; Mustać, E.; Avirović, M. HSP70 In triple-negative breast cancer: Prognostic value and clinical significance. Pathol. Res. Pract. 2022, 238, 154127. [Google Scholar] [CrossRef]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.N.; Duvillier, H.; et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology 2016, 6, e1257452. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Ali, H.R.; Viens, P.; Caldas, C.; Birnbaum, D.; Bertucci, F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449–5464. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef]
- Cardoso, F.; McArthur, H.L.; Schmid, P.; Cortés, J.; Harbeck, N.; Telli, M.L.; Cescon, D.W.; O’Shaughnessy, J.; Fasching, P.; Shao, Z.; et al. Phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2– breast cancer. Ann. Oncol. 2023, 34, S1261. [Google Scholar] [CrossRef]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).