Treatment with Upadacitinib in Difficult-to-Treat (D2T) Psoriatic Arthritis (PsA): A National Multicenter Study of the First 134 Patients in Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
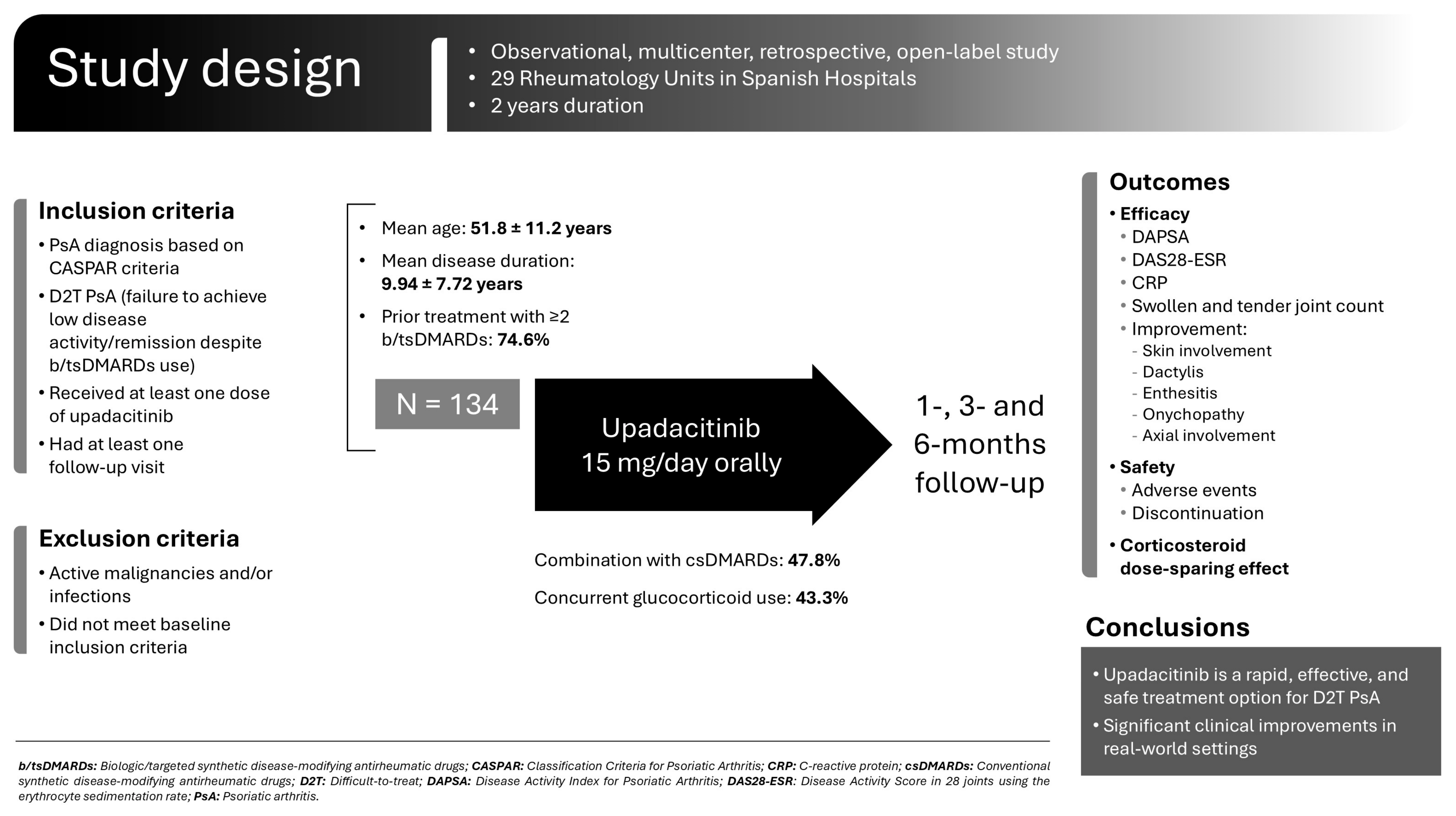
2.1. Patients and Enrollment Criteria
2.2. Study Outcomes
2.3. Data Collection and Statistical Analysis
3. Results
3.1. Baseline Main Clinical Features at UPA Onset
3.2. UPA Treatment and Efficacy
3.3. Adverse Effects
3.4. Subgroup Analysis of Patients with at Least Two b/tsDMARDs with Different Mechanisms of Action
3.5. Comparative Study of a Clinical Practice Cohort and SELECT-PsA 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. List of Study Collaborators. Upadacitinib for PsA Clinical Practice COLLABORATIVE STUDY GROUP
References
- Ogdie, A.; Weiss, P. The Epidemiology of Psoriatic Arthritis. Rheum. Dis. Clin. North Am. 2015, 41, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; van Onna, M.; Boonen, A.; van Tubergen, A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res. 2016, 68, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Helliwell, P.S. Psoriatic Arthritis: State of the Art Review. Clin. Med. Lond. Engl. 2017, 17, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Soriano, E.R.; Corp, N.; Bertheussen, H.; Callis Duffin, K.; Campanholo, C.B.; Chau, J.; Eder, L.; Fernández-Ávila, D.G.; FitzGerald, O.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated Treatment Recommendations for Psoriatic Arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef]
- Gossec, L.; McGonagle, D.; Korotaeva, T.; Lubrano, E.; de Miguel, E.; Østergaard, M.; Behrens, F. Minimal Disease Activity as a Treatment Target in Psoriatic Arthritis: A Review of the Literature. J. Rheumatol. 2018, 45, 6–13. [Google Scholar] [CrossRef]
- Smolen, J.S.; Siebert, S.; Korotaeva, T.V.; Selmi, C.; Bergmans, P.; Gremese, E.; Joven-Ibáñez, B.; Katsifis, G.; Noël, W.; Nurmohamed, M.T.; et al. Effectiveness of IL-12/23 Inhibition (Ustekinumab) versus Tumour Necrosis Factor Inhibition in Psoriatic Arthritis: Observational PsABio Study Results. Ann. Rheum. Dis. 2021, 80, 1419–1428. [Google Scholar] [CrossRef]
- Brahe, C.H.; Ørnbjerg, L.M.; Jacobsson, L.; Nissen, M.J.; Kristianslund, E.K.; Mann, H.; Santos, M.J.; Reino, J.G.; Nordström, D.; Rotar, Z.; et al. Retention and Response Rates in 14 261 PsA Patients Starting TNF Inhibitor Treatment-Results from 12 Countries in EuroSpA. Rheumatol. Oxf. Engl. 2020, 59, 1640–1650. [Google Scholar] [CrossRef]
- Glintborg, B.; Ostergaard, M.; Krogh, N.S.; Andersen, M.D.; Tarp, U.; Loft, A.G.; Lindegaard, H.M.; Holland-Fischer, M.; Nordin, H.; Jensen, D.V.; et al. Clinical Response, Drug Survival, and Predictors Thereof among 548 Patients with Psoriatic Arthritis Who Switched Tumor Necrosis Factor α Inhibitor Therapy: Results from the Danish Nationwide DANBIO Registry. Arthritis Rheum. 2013, 65, 1213–1223. [Google Scholar] [CrossRef]
- Mease, P.J.; Karki, C.; Liu, M.; Li, Y.; Gershenson, B.; Feng, H.; Hur, P.; Greenberg, J.D. Discontinuation and Switching Patterns of Tumour Necrosis Factor Inhibitors (TNFis) in TNFi-Naive and TNFi-Experienced Patients with Psoriatic Arthritis: An Observational Study from the US-Based Corrona Registry. RMD Open 2019, 5, e000880. [Google Scholar] [CrossRef]
- Nagy, G.; Roodenrijs, N.M.; Welsing, P.M.; Kedves, M.; Hamar, A.; van der Goes, M.C.; Kent, A.; Bakkers, M.; Blaas, E.; Senolt, L.; et al. EULAR Definition of Difficult-to-Treat Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80, 31–35. [Google Scholar] [CrossRef]
- Lubrano, E.; Scriffignano, S.; Perrotta, F.M. Difficult to Treat and Refractory to Treatment in Psoriatic Arthritis. Rheumatol. Ther. 2023, 10, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Ribeiro, A.; Torgutalp, M.; Mease, P.J.; Proft, F. Difficult-to-Treat Psoriatic Arthritis (D2T PsA): A Scoping Literature Review Informing a GRAPPA Research Project. RMD Open 2024, 10, e003809. [Google Scholar] [CrossRef] [PubMed]
- Sanmartí, R.; Corominas, H. Upadacitinib for Patients with Rheumatoid Arthritis: A Comprehensive Review. J. Clin. Med. 2023, 12, 1734. [Google Scholar] [CrossRef] [PubMed]
- RINVOQ. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf (accessed on 4 March 2025).
- McInnes, I.B.; Anderson, J.K.; Magrey, M.; Merola, J.F.; Liu, Y.; Kishimoto, M.; Jeka, S.; Pacheco-Tena, C.; Wang, X.; Chen, L.; et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N. Engl. J. Med. 2021, 384, 1227–1239. [Google Scholar] [CrossRef]
- Mease, P.J.; Lertratanakul, A.; Anderson, J.K.; Papp, K.; Van den Bosch, F.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.; Wang, X.; Zhong, S.; et al. Upadacitinib for Psoriatic Arthritis Refractory to Biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021, 80, 312–320. [Google Scholar] [CrossRef]
- Booth, C.M.; Tannock, I.F. Randomised Controlled Trials and Population-Based Observational Research: Partners in the Evolution of Medical Evidence. Br. J. Cancer 2014, 110, 551–555. [Google Scholar] [CrossRef]
- Sanson-Fisher, R.W.; Bonevski, B.; Green, L.W.; D’Este, C. Limitations of the Randomized Controlled Trial in Evaluating Population-Based Health Interventions. Am. J. Prev. Med. 2007, 33, 155–161. [Google Scholar] [CrossRef]
- Annemans, L.; Aristides, M.; Kubin, M. Real-Life Data: A Growing Need. ISPOR Connect 2007, 13, 8–12. [Google Scholar]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification Criteria for Psoriatic Arthritis: Development of New Criteria from a Large International Study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Gómez Reino, J.; Loza, E.; Andreu, J.L.; Balsa, A.; Batlle, E.; Cañete, J.D.; Collantes Estévez, E.; Fernández Carballido, C.; Fernández Sueiro, J.L.; García de Vicuña, R.; et al. Consensus statement of the Spanish Society of Rheumatology on risk management of biologic therapy in rheumatic patients. Reumatol. Clin. 2011, 7, 284–298. [Google Scholar] [CrossRef]
- Fries, J.F.; Spitz, P.W.; Young, D.Y. The Dimensions of Health Outcomes: The Health Assessment Questionnaire, Disability and Pain Scales. J. Rheumatol. 1982, 9, 789–793. [Google Scholar] [PubMed]
- Fredriksson, T.; Pettersson, U. Oral Treatment of Pustulosis Palmo-Plantaris with a New Retinoid, Ro 10-9359. Dermatologica 1979, 158, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified Disease Activity Scores That Include Twenty-Eight-Joint Counts. Development and Validation in a Prospective Longitudinal Study of Patients with Rheumatoid Arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Schoels, M. Psoriatic Arthritis Indices. Clin. Exp. Rheumatol. 2014, 32, S109–S112. [Google Scholar]
- Mease, P.J. Measures of Psoriatic Arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res. 2011, 63 (Suppl. 11), S64–S85. [Google Scholar] [CrossRef]
- Fredriksson, T.; Pettersson, U. Severe Psoriasis--Oral Therapy with a New Retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D. Assessment of SpondyloArthritis international Society Development of an ASAS-Endorsed Disease Activity Score (ASDAS) in Patients with Ankylosing Spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef]
- Ariza-Ariza, R.; Hernández-Cruz, B.; Navarro-Sarabia, F. La versión española del BASDAI es fiable y se correlaciona con la actividad de la enfermedad. Rev. Esp. Reumatol. 2004, 31, 372–378. [Google Scholar]
- Werner, S.G.; Baraliakos, X.; Reckert, S.; Bohl-Bühler, M.; Laliberté, M.-C.; Girard, T.; Jeromin, K.; Baschuk, N.; Fritz, B.; Bessette, L.; et al. Treatment with Upadacitinib in Active Psoriatic Arthritis: Efficacy and Safety Data of the First 192 Patients from the UPJOINT Study, a Multicentre, Observational Study in Clinical Practice. Rheumatol. Ther. 2023, 10, 1503–1518. [Google Scholar] [CrossRef]
- Fagni, F.; Motta, F.; Schett, G.; Selmi, C. Difficult-to-Treat Psoriatic Arthritis: A Conceptual Approach. Arthritis Rheumatol. Hoboken NJ 2024, 76, 670–674. [Google Scholar] [CrossRef]
- Schett, G.; Rahman, P.; Ritchlin, C.; McInnes, I.B.; Elewaut, D.; Scher, J.U. Psoriatic Arthritis from a Mechanistic Perspective. Nat. Rev. Rheumatol. 2022, 18, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Rendas-Baum, R.; Wallenstein, G.V.; Koncz, T.; Kosinski, M.; Yang, M.; Bradley, J.; Zwillich, S.H. Evaluating the Efficacy of Sequential Biologic Therapies for Rheumatoid Arthritis Patients with an Inadequate Response to Tumor Necrosis Factor-α Inhibitors. Arthritis Res. Ther. 2011, 13, R25. [Google Scholar] [CrossRef] [PubMed]
- Schmalzing, M.; Behrens, F.; Schwaneck, E.C.; Koehm, M.; Greger, G.; Gnann, H.; Burkhardt, H.; Tony, H.-P. Does Concomitant Methotrexate Confer Clinical Benefits in Patients Treated with Prior Biologic Therapy? Analysis of Data from a Noninterventional Study of Rheumatoid Arthritis Patients Initiating Treatment with Adalimumab. Medicine 2020, 99, e20201. [Google Scholar] [CrossRef]
- Haddad, A.; Gazitt, T.; Feldhamer, I.; Feld, J.; Cohen, A.D.; Lavi, I.; Tatour, F.; Bergman, I.; Zisman, D. Treatment Persistence of Biologics among Patients with Psoriatic Arthritis. Arthritis Res. Ther. 2021, 23, 44. [Google Scholar] [CrossRef]
- Pina Vegas, L.; Hoisnard, L.; Bastard, L.; Sbidian, E.; Claudepierre, P. Long-Term Persistence of Second-Line Biologics in Psoriatic Arthritis Patients with Prior TNF Inhibitor Exposure: A Nationwide Cohort Study from the French Health Insurance Database (SNDS). RMD Open 2022, 8, e002681. [Google Scholar] [CrossRef]
- Harkins, P.; Burke, E.; Swales, C.; Silman, A.; Conway, R. Are Janus Kinase Inhibitors Safe and Effective in Treating the Key Clinical Domains of Psoriatic Arthritis? A Systematic Review and Meta-Analysis. Int. J. Rheum. Dis. 2023, 26, 31–42. [Google Scholar] [CrossRef]
| Clinical Practice n = 134 | SELECT-PsA 2 n = 211 | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 51.82 ± 11.22 | 53.0 ± 12.0 | 0.362 |
| Sex (female), n (%) | 97 (72.4) | 113 (53.6) | <0.001 |
| Clinical characteristics | |||
| Duration since PsA diagnosis (years), mean ± SD | 9.94 ± 7.72 | 9.5 ± 8.4 | 0.625 |
| Swollen joint count, mean ± SD | 4.33 ± 5.01 | 11.3 ± 8.2 | <0.001 |
| Tender joint count, media ± SD | 6.10 ± 5.6 | 24.9 ± 17.3 | <0.001 |
| Enthesitis, n (%) | 29 (21.6) (MASES) | 172 (81.5) (SPARCC) | <0.001 |
| Dactylitis, n (%) | 14 (10.5) | 55 (26.1) | <0.001 |
| CRP (mg/L), mean ± SD | 8.36 ± 14.47 | 11.2 ± 18.5 | 0.133 |
| HAQ-DI, mean ± SD | 1.00 ± 0.63 | 1.10 ± 0.6 | 0.140 |
| PASI score, mean ± SD | 0.95 ± 1.65 | 10.1 ± 9.2 | <0.001 |
| Glucocorticoid use, n (%) | 58 (43.28) | 22 (10.4) | <0.001 |
| Prior biologic DMARDs | |||
| Prior biologic DMARD use, n (%) | 123 (91.8) | 195 (92.4) | 0.833 |
| Number of prior biologic DMARD, n (%) | |||
| 0 | 11 (8.2) | 18 (8.5) * | 0.916 |
| 1 | 22 (16.4) | 135 (63.7) | <0.001 |
| 2 | 21 (15.7) | 35 (16.5) | 0.822 |
| ≥3 | 80 (59.7) | 24 (11.3) | <0.001 |
| Upadacitinib at baseline | |||
| Monotherapy, n (%) | 70 (52.24) | 113 (53.6) | 0.811 |
| In combination with csDMARDs, n (%) | 64 (47.76) | 98 (46.4) | 0.811 |
| Baseline n = 134 | Month 1 n = 89 | Month 3 n = 84 | Month 6 n = 55 | |
|---|---|---|---|---|
| Swollen joint count | ||||
| Median [IQR] | 3 [1.00;6.00] | 1 [0.00; 4.00] | 0 [0.00; 2.00] | 0 [0.00; 2.00] |
| p-value vs. baseline | p < 0.001 | p < 0.001 | p < 0.001 | |
| Tender joint count | ||||
| Median [IQR] | 5 [2.00; 8.00] | 2 [0.00; 4.00] | 2 [0.00; 6.00] | 1 [0.00; 2.00] |
| p-value vs. baseline | p < 0.001 | p < 0.001 | p < 0.001 | |
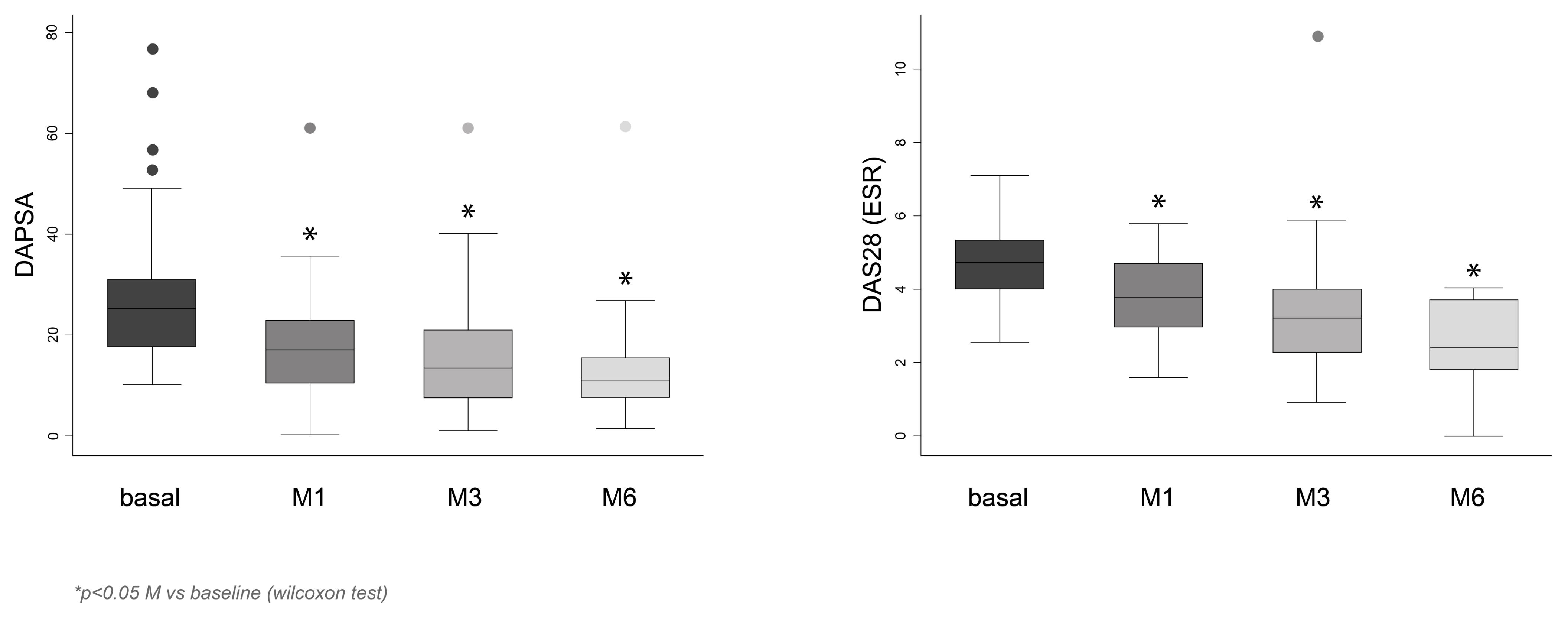
| DAS28-ESR | ||||
| Median [IQR] | 4.7 [3.97; 5.38] | 3.77 [2.87; 4.76] | 3.17 [2.16; 4.06] | 2.38 [1.73; 3.68] |
| p-value vs. baseline | p < 0.001 | p < 0.001 | p < 0.001 | |
| DAPSA | ||||
| Median [IQR] | 25 [18.06; 30.60] | 17 [10.10; 22.60] | 13.29 [7.32; 21.27] | 12 [7.27; 16.00] |
| p-value vs. baseline | p < 0.001 | p < 0.001 | p < 0.001 | |
| Axial involvement (ASDAS-CRP and BASDAI) | ||||
| Improvement *, n (%) | - | 9 (52.94) | 10 (45.45) | 5 (35.71) |
| Skin involvement (PASI) | ||||
| Improvement *, n (%) | - | 10 (52.63) | 14 (87.50) | 9 (69.23) |
| Nail involvement (number of affected nails) | ||||
| Improvement *, n (%) | - | 2 (20) | 4 (57.14) | 0 (0.0) |
| Enthesitis (MASES) | ||||
| Improvement *, n (%) | - | 9 (64.29) | 10 (50) | 7 (53.85) |
| Dactylitis (presence/absence) | ||||
| Improvement *, n (%) | - | 7 (77.78) | 4 (66.67) | 4 (80) |
| CRP (mg/dL) | ||||
| Median [IQR] | 2.90 [1.00; 8.95] | 1.50 [0.43; 4.90] | 2.02 [0.59; 5.12] | 1.00 [0.3; 5.60] |
| p-value vs. baseline | p = 0.001 | p = 0.235 | p < 0.001 | |
| Prednisone dose (mg/day) | ||||
| Mean ± SD | 8.26 ± 5.58 | 7.73 ± 4.18 p = 0.049 | 5.60 ± 3.41 p = 0.003 | 6.16 ± 3.52 p = 0.031 |
| Baseline n = 134 | Month 1 n = 89 | Month 6 n = 55 | |
|---|---|---|---|
| Hemoglobin, g/dL | 13.82 ± 1.53 | 13.65 ± 1.47 | 13.44 ± 1.35 |
| Neutrophils, count/µL | 4516.8 ± 1960.8 | 4138.9 ± 1816.3 | 4759.5 ± 1871.1 |
| Lymphocytes, count/µL | 2388.1 ± 885.5 | 2359.9 ± 893.9 | 2438.6 ± 934.9 |
| Platelets, count/µL | 273,593.8 ± 77,868.4 | 259,372.7 ± 80,370.8 | 266,084.4 ± 64,475.4 |
| Creatinine, mg/dL | 0.77± 0.18 | 0.80 ± 0.19 | 0.78 ± 0.22 |
| AST, U/L | 22.7 ± 9.64 | 24.9 ± 10.6 | 25.7 ± 9.3 |
| ALT, U/L | 23.7 ± 13.2 | 25.2 ± 12.8 | 25.8 ± 11.8 |
| Cholesterol, mg/dL | 193.7 ± 37.4 | 207.2 ± 45.2 | 207.3 ± 45.5 |
| HDL, mg/dL | 64.8 ± 25.1 | 63.1 ± 22.0 | 67.5 ± 17.0 |
| LDL, mg/dL | 116.0 ± 37.4 | 127.3 ± 39.3 | 132.2 ± 42.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galíndez-Agirregoikoa, E.; Prieto-Peña, D.; García-Vivar, M.L.; Belzunegui Otano, J.M.; Joven-Ibáñez, B.; Vergara-Dangond, C.; Pavía-Pascual, M.; Urionaguena-Onaindia, I.; Vega Alvarez, L.; Puche Larrubia, M.Á.; et al. Treatment with Upadacitinib in Difficult-to-Treat (D2T) Psoriatic Arthritis (PsA): A National Multicenter Study of the First 134 Patients in Clinical Practice. Sci 2025, 7, 67. https://doi.org/10.3390/sci7020067
Galíndez-Agirregoikoa E, Prieto-Peña D, García-Vivar ML, Belzunegui Otano JM, Joven-Ibáñez B, Vergara-Dangond C, Pavía-Pascual M, Urionaguena-Onaindia I, Vega Alvarez L, Puche Larrubia MÁ, et al. Treatment with Upadacitinib in Difficult-to-Treat (D2T) Psoriatic Arthritis (PsA): A National Multicenter Study of the First 134 Patients in Clinical Practice. Sci. 2025; 7(2):67. https://doi.org/10.3390/sci7020067
Chicago/Turabian StyleGalíndez-Agirregoikoa, Eva, Diana Prieto-Peña, Maria Luz García-Vivar, Joaquin Maria Belzunegui Otano, Beatriz Joven-Ibáñez, Cristina Vergara-Dangond, Marina Pavía-Pascual, Irati Urionaguena-Onaindia, Lucia Vega Alvarez, M. Ángeles Puche Larrubia, and et al. 2025. "Treatment with Upadacitinib in Difficult-to-Treat (D2T) Psoriatic Arthritis (PsA): A National Multicenter Study of the First 134 Patients in Clinical Practice" Sci 7, no. 2: 67. https://doi.org/10.3390/sci7020067
APA StyleGalíndez-Agirregoikoa, E., Prieto-Peña, D., García-Vivar, M. L., Belzunegui Otano, J. M., Joven-Ibáñez, B., Vergara-Dangond, C., Pavía-Pascual, M., Urionaguena-Onaindia, I., Vega Alvarez, L., Puche Larrubia, M. Á., Ramos Giráldez, C., Garcia-Vicuña, R., Jovani, V., Martínez-Ferrer, A., Moreno Martínez-Losa, M., González Hernández, T., Almodóvar González, R., Urruticoechea-Arana, A., Macía-Villa, C., ... Upadacitinib PsA Clinical Practice COLLABORATIVE STUDY GROUP. (2025). Treatment with Upadacitinib in Difficult-to-Treat (D2T) Psoriatic Arthritis (PsA): A National Multicenter Study of the First 134 Patients in Clinical Practice. Sci, 7(2), 67. https://doi.org/10.3390/sci7020067






