Effects of Physicochemical Characteristics of Two Soils on Agro-Morphological Traits of Two Chickpea Varieties (Cicer arietinum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Analysis
2.1.1. Soil Texture
2.1.2. pH and Organic Matter
2.1.3. Phosphorus
2.1.4. Potassium
2.1.5. Nitrogen
2.2. Plant Material
2.3. Growth Conditions and Experimental Treatment
2.4. Data Collection
2.4.1. Nutritional Quality Assessment and Physicochemical Characterization of the Seeds
2.4.2. Mineral Analysis of the Leaves After Harvest
2.5. Statistical Analysis
3. Results
3.1. Soil Analysis
3.1.1. Soil Texture
3.1.2. pH and Organic Matter
3.1.3. Phosphorus and Potassium
3.1.4. Nitrogen
3.2. Morphological Traits
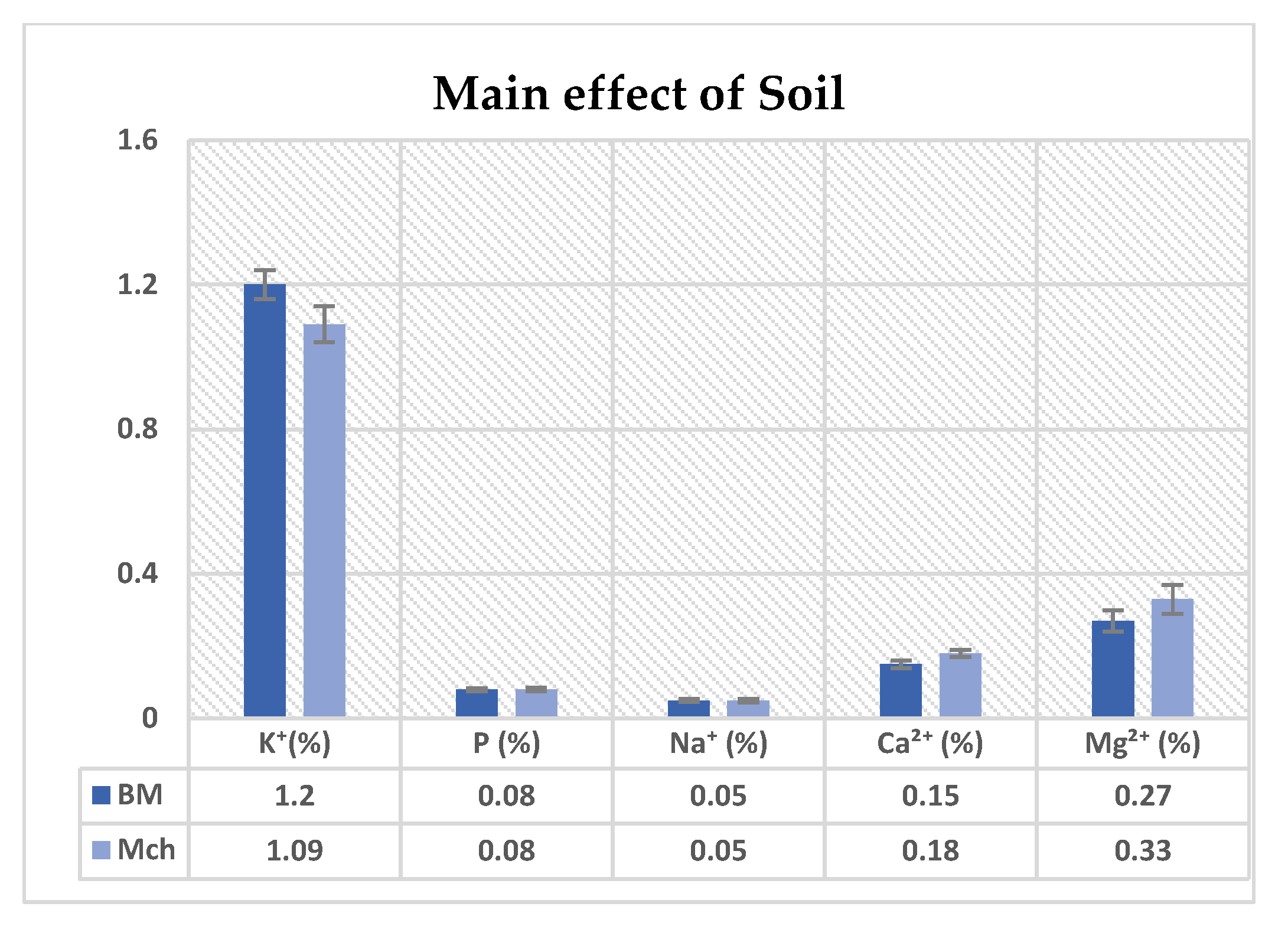
3.3. Mineral Analysis of Leaflets
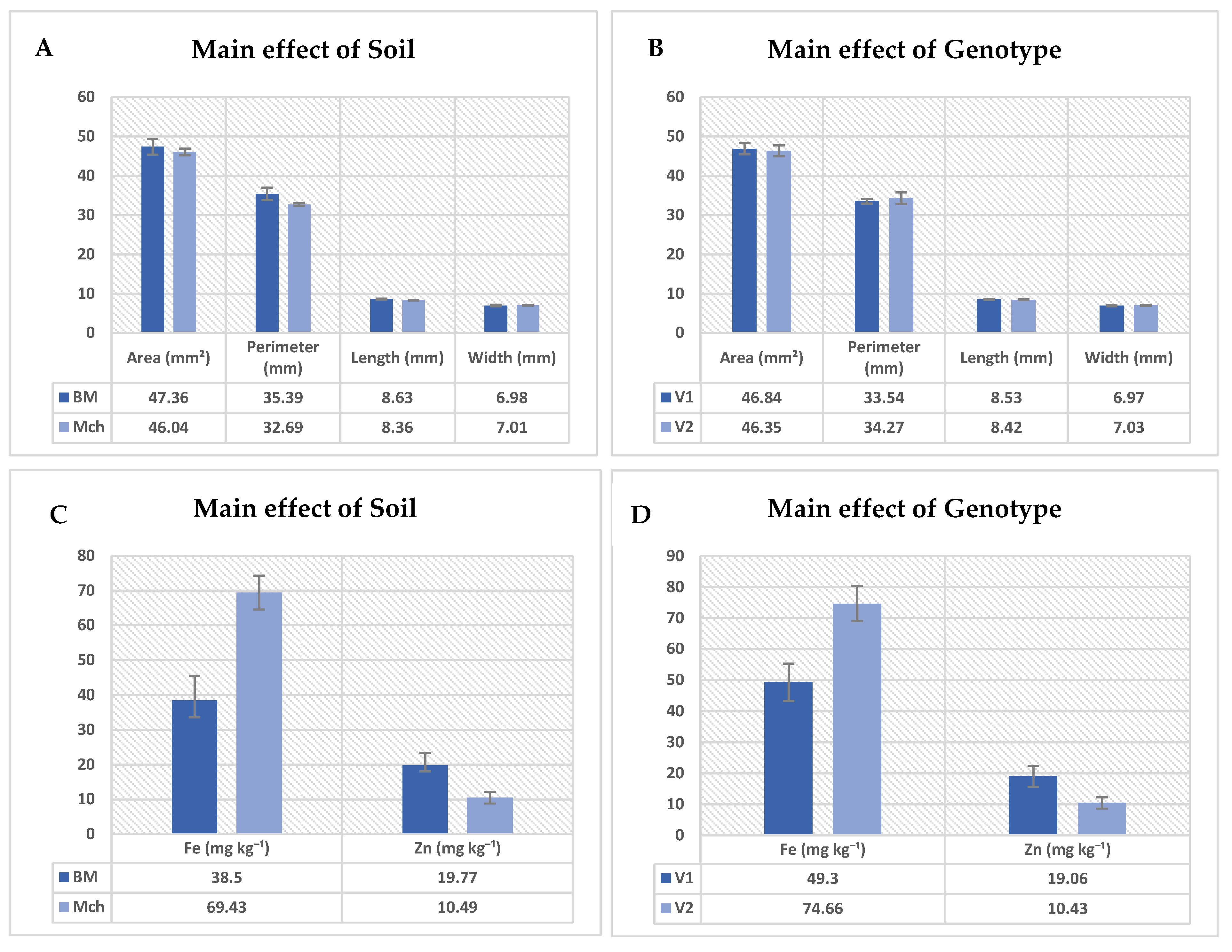
3.4. Analysis of Harvested Seeds
4. Discussion
4.1. Soil Analysis
4.1.1. pH
4.1.2. Organic Matter
4.1.3. Phosphorus and Potassium Analysis
4.1.4. Nitrogen
4.2. Morphologic Traits
4.3. Mineral Analysis
4.4. Analysis of Harvested Seeds
5. Conclusions
- Genotypic effects were minor and had no significant impact on overall production, though slight differences in Fe and Zn accumulation were observed.
- Selecting appropriate soil types and implementing effective nutrient management strategies are more critical than genotype selection for maximizing crop yields.
- The complex interactions between soil characteristics, chickpea genotypes, and productivity highlight the need for further research to enhance agricultural outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nations, U. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. 2017. Available online: https://www.un.org/tr/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 3 June 2024).
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Redden, R.J.; Berger, J.D. History and origin of chickpea. In Chickpea Breeding and Management; Yadav, S.S., Redden, R.J., Chen, W., Sharma, B., Eds.; CABI: Oxfordshire, UK, 2007; pp. 1–13. ISBN 978-1-84593-213-8. [Google Scholar]
- Venkidasamy, B.; Selvaraj, D.; Nile, A.S.; Ramalingam, S.; Kai, G.; Nile, S.H. Indian pulses: A review on nutritional, functional and biochemical properties with future perspectives. Trends Food Sci. Technol. 2019, 88, 228–242. [Google Scholar] [CrossRef]
- Rastgoo, M.; Nezami, A.; Hasanfard, A.; Nabati, J.; Ahmadi-Lahijani, M.J. Freezing stress induces changes in the morphophysiological of chickpea and wild mustard seedlings. Legume Sci. 2023, 5, e173. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Madurapperumage, A.; Tang, L.; Thavarajah, P.; Bridges, W.; Shipe, E.; Vandemark, G.; Thavarajah, D. Chickpea (Cicer arietinum L.) as a Source of Essential Fatty Acids—A Biofortification Approach. Front. Plant Sci. 2021, 12, 734980. [Google Scholar] [CrossRef]
- Elhazzat, N.; Adnani, M.; Berber, F.; Boughribil, S.; Mouden, N.; Errifi, A.; Chliyeh, M.; Selmaoui, K.; Benkirane, R.; Ouazzani Touhami, A.; et al. Comparative pathogenesis of Fusarium spp. obtained from diseased chickpea plants in Morocco. Not. Sci. Biol. 2023, 15, 11361. [Google Scholar] [CrossRef]
- Johnson, N.; Johnson, C.R.; Thavarajah, P.; Kumar, S.; Thavarajah, D. The roles and potential of lentil prebiotic carbohydrates in human and plant health. Plants People Planet 2020, 2, 310–319. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Narnoliya, L.; Das, S.; Kumar, V.; Gowda, C.L.L.; Sharma, S.; Tyagi, A.K.; Parida, S.K. Genome-Wide Scans for Delineation of Candidate Genes Regulating Seed-Protein Content in Chickpea. Front. Plant Sci. 2016, 7, 302. [Google Scholar] [CrossRef]
- Lahmar, M.; El Khodrani, N.; Omrania, S.; Dakak, H.; Douaik, A.; Laaich, H.; Yachou, H. GIS and statistical evaluation of soil quality of Sidi Yahya, Gharbplain (Morocco). J. Exp. Biol. Agric. Sci. 2021, 9, 881–893. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil Quality: A Concept, Definition, and Framework for Evaluation (A Guest Editorial). Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Azeem, M.; Hayat, R.; Hussain, Q.; Ahmed, M.; Pan, G.; Ibrahim Tahir, M.; Imran, M.; Irfan, M.; Mehmood-ul-Hassan. Biochar improves soil quality and N2-fixation and reduces net ecosystem CO2 exchange in a dryland legume-cereal cropping system. Soil Tillage Res. 2019, 186, 172–182. [Google Scholar] [CrossRef]
- Fikre, A.; Desmae, H.; Ahmed, S. Tapping the Economic Potential of Chickpea in Sub-Saharan Africa. Agronomy 2020, 10, 1707. [Google Scholar] [CrossRef]
- Parwada, C.; Parwada, T.F.; Chipomho, J.; Mapope, N.; Chikwari, E.; Mvumi, C. Evaluation of Cicer arietinum (chickpea) growth performance and yield in different soil types in Zimbabwe. J. Curr. Opin. Crop Sci. 2022, 3, 16–27. [Google Scholar] [CrossRef]
- Davies, S.L.; Turner, N.C.; Siddique, K.H.M.; Leport, L.; Plummer, J.A. Seed growth of desi and kabuli chickpea (Cicer arietinum L.) in a short-season Mediterranean-type environment. Aust. J. Exp. Agric. 1999, 39, 181. [Google Scholar] [CrossRef]
- Sijilmassi, B.; Filali-Maltouf, A.; Fahde, S.; Ennahli, Y.; Boughribil, S.; Kumar, S.; Amri, A. In-Vitro Plant Growth Promotion of Rhizobium Strains Isolated from Lentil Root Nodules under Abiotic Stresses. Agronomy 2020, 10, 1006. [Google Scholar] [CrossRef]
- Weather and Climate. Afourer, Béni Mellal-Khénifra, MA Climate. Zone, Monthly Averages, Historical Weather Data. Available online: https://weatherandclimate.com/morocco/beni-mellal-khenifra/afourer (accessed on 21 May 2024).
- Lahmar, M.; El Khodrani, N.; Omrania, S.; Dakak, H.; Moussadek, R.; Douaik, A.; Iaaich, H.; El Azzouzi, M.; Mekkaoui, M.; Zouahri, A. Assessment of the Quality of Soil and Groundwater of the Agricultural Area of Sidi Yahya Region, Morocco. E3S Web Conf. 2020, 150, 01001. [Google Scholar] [CrossRef]
- Petard, J. Les Méthodes D’analyse; ORSTON: Nottinghamshire, UK, 1993; p. 196. [Google Scholar]
- Mweetwa, A.M.; Lubungo, A.C.; Chishala, B.H.; Phiri, M. Selected Chemical Properties, Microbial Activity and Biomass of Soils Amended with Aqueous Neem Leaf Extract. SAR 2016, 5, 103. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Tévez, H.R.; Afonso, M.D. pH dependence of Glyphosate adsorption on soil horizons. Boletín Soc. Geológica Mex. 2015, 67, 509–516. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, pp. 595–624. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- Ekpong, B. Effects of seed maturity, seed storage and pre-germination treatments on seed germination of cleome (Cleome gynandra L.). Sci. Hortic. 2009, 119, 236–240. [Google Scholar] [CrossRef]
- Aloui, K.; Choukri, H.; El Haddad, N.; Gupta, P.; El Bouhmadi, K.; Emmrich, P.M.F.; Singh, A.; Edwards, A.; Maalouf, F.; Bouhlal, O.; et al. Impact of Heat and Drought Stress on Grasspea and Its Wild Relatives. Plants 2023, 12, 3501. [Google Scholar] [CrossRef]
- Kehel, Z.; Sanchez-Garcia, M.; El Baouchi, A.; Aberkane, H.; Tsivelikas, A.; Charles, C.; Amri, A. Predictive Characterization for Seed Morphometric Traits for Genebank Accessions Using Genomic Selection. Front. Ecol. Evol. 2020, 8, 32. [Google Scholar] [CrossRef]
- Farida Traoré, F.; El-Baouchi, A.; En-nahli, Y.; Hejjaoui, K.; Metougui, M.L.; Hamwieh, A.; Sohail, Q.; Istanbuli, T.; Boughribil, S.; Amri, M. Exploring the Genetic Variability and Potential Correlations Between Nutritional Quality and Agro-Physiological Traits in Kabuli Chickpea Germplasm Collection (Cicer arietinum L.). Front. Plant Sci. 2022, 13, 905320. [Google Scholar] [CrossRef]
- Horneck, D.A.; Sullivan, D.M.; Owen, J.S.; Hart, J.M. Soil Test Interpretation Guide; Oregon State University: Corvallis, OR, USA, 2011. [Google Scholar]
- Center for Agriculture, Food, and the Environment Interpreting Your Soil Test Results. Available online: https://ag.umass.edu/soil-plant-nutrient-testing-laboratory/fact-sheets/interpreting-your-soil-test-results (accessed on 21 May 2024).
- Omrania, S.; Khodrani, N.E.; Zouahri, A.; Douaik, A.; Iaaich, H.; Lahmar, M.; Hajjaji, E. Physico-Chemical Characterization of Water and Soil of the M’nasra region in the Gharb plain (Northwest Morocco). Moroc. J. Chem. 2019, 7, 482–492. [Google Scholar]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Slimane, S. Evaluation the Impact of Intensive Agriculture on Physic-Chemical Properties of Soil and Groundwater in the Rural Commune Sfafaa. J. Mater. Environ. Sci. 2017, 8, 2339–2346. [Google Scholar]
- Edahbi, M.; Khaddor, M.; Salmoun, F. Caractérisation des sols du Nord du Maroc (Bassin Loukkos) (Characterization of the soils in North Morocco (Loukkos area)). J. Mater. Environ. Sci. 2014, 5, 2133–2138. [Google Scholar]
- Laghrour, M.; Moussadek, R.; Mrabet, R.; Dahan, R.; El-Mourid, M.; Zouahri, A.; Mekkaoui, M. Long and Midterm Effect of Conservation Agriculture on Soil Properties in Dry Areas of Morocco. Appl. Environ. Soil Sci. 2016, 2016, 6345765. [Google Scholar] [CrossRef]
- Moussadek, R. Impacts de l’agriculture de conservation sur les propriétés et la productivité des vertisols du Maroc Central1. Afr. Focus 2012, 25, 2. [Google Scholar] [CrossRef]
- Coale, F.J.; Sims, J.T.; Leytem, A.B. Accelerated Deployment of an Agricultural Nutrient Management Tool: The Maryland Phosphorus Site Index. J. Environ. Qual. 2002, 31, 1471–1476. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Toor, G.S. Stormwater runoff driven phosphorus transport in an urban residential catchment: Implications for protecting water quality in urban watersheds. Sci. Rep. 2018, 8, 11681. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Quinn, P.; Barber, N.; Burke, S. Identifying Flow Pathways for Phosphorus Transport Using Observed Event Forensics and the CRAFT (Catchment Runoff Attenuation Flux Tool). Water 2020, 12, 1081. [Google Scholar] [CrossRef]
- Delfim, J.; Moreira, A.; Moraes, L.A.C.; Silva, J.F.; Moreira, P.A.M.; Lima Filho, O.F. Soil Phosphorus Availability Impacts Chickpea Production and Nutritional Status in Tropical Soils. J. Soil Sci. Plant Nutr. 2024, 24, 3115–3130. [Google Scholar] [CrossRef]
- Olivera, M.; Tejera, N.; Iribarne, C.; Ocaña, A.; Lluch, C. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): Effect of phosphorus. Physiol. Plant 2004, 121, 498–505. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of Soil Phosphorus on Legume Production. In Legumes for Soil Health and Sustainable Management; Meena, R.S., Das, A., Yadav, G.S., Lal, R., Eds.; Springer: Singapore, 2018; pp. 487–510. ISBN 9789811302527. [Google Scholar]
- Jakobsen, I. The role of phosphorus in nitrogen fixation by young pea plants (Pisum sativum). Physiol. Plant 1985, 64, 190–196. [Google Scholar] [CrossRef]
- Ahmed, K.; Mohammed, B.; Cherk, G. Effect of Soil Available Phosphorus Levels on Chickpea (Cicer arietinum L.)—Rhizobia Symbiotic Association. Legume Res. Int. J. 2020, 43, 878–883. [Google Scholar] [CrossRef]
- Shukla, U.C.; Yadav, O.P. Effect of phosphorus and zinc on nodulation and nitrogen fixation in chickpea (Cicer arietinum L.). Plant Soil 1982, 65, 239–248. [Google Scholar] [CrossRef]
- Mohammed, S.B.; Dzidzienyo, D.K.; Yahaya, A.L.; Umar, M.; Ishiyaku, M.F.; Tongoona, P.B.; Gracen, V. High Soil Phosphorus Application Significantly Increased Grain Yield, Phosphorus Content but Not Zinc Content of Cowpea Grains. Agronomy 2021, 11, 802. [Google Scholar] [CrossRef]
- Adusei, G.; Gaiser, T.; Boukar, O.; Fatokun, C. Growth and yield responses of cowpea genotypes to soluble and rock P fertilizers on acid, highly weathered soil from humid tropical West Africa. Int. J. Biol. Chem. Sci. 2017, 10, 1493. [Google Scholar] [CrossRef]
- Krasilnikoff, G.; Gahoonia, T.; Nielsen, N.E. Variation in phosphorus uptake efficiency by genotypes of cowpea (Vigna unguiculata) due to differences in root and root hair length and induced rhizosphere processes. Plant Soil 2003, 251, 83–91. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the World’s Phosphorus for a Food Secure Future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, A.S.; Rajani, A.V.; Kaneriya, S.C.; Hirpara, D.V. Nutrient Content, Uptake, Quality of Chickpea (Cicer arietinum L.) and Fertility Status of Soil as Influenced by Fertilization of Potassium and Sulphur. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2351–2355. [Google Scholar] [CrossRef]
- Agriculture Victoria Understanding Soil Tests for Pastures—Agriculture. Available online: https://agriculture.vic.gov.au/farm-management/soil/understanding-soil-tests-for-pastures (accessed on 19 May 2024).
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Gurav, P.P.; Ray, S.K.; Choudhari, P.L.; Biswas, A.K.; Shirale, A.O. A review on soil potassium scenario in vertisols of India. Open Access J. Sci. 2018, 2, 89–90. [Google Scholar] [CrossRef][Green Version]
- Daliparthy, J.; Barker, A.V.; Mondal, S.S. Potassium fractions with other nutrients in crops: A review focusing on the tropics. J. Plant Nutr. 1994, 17, 1859–1886. [Google Scholar] [CrossRef]
- Li, M.; Han, X.; Li, L.-J. Total Nitrogen Stock in Soil Profile Affected by Land Use and Soil Type in Three Counties of Mollisols. Front. Environ. Sci. 2022, 10, 945305. [Google Scholar] [CrossRef]
- Horiba Instruments Soil Nitrate Measurement for Determination of Plant-Available Nitrogen. Available online: https://www.horiba.com/deu/water-quality/applications/agriculture-crop-science/soil-nitrate-measurement-for-determination-of-plant-available-nitrogen/ (accessed on 21 May 2024).
- Chiroma, H.D.; Ayinla, G.T.; Orish, O.E.; Emmanuel, O.O.; Jigam, A.A.; Makun, H.A.; Sani, G.M.; Johnson, A.O.; Humphrey, P. Seasonal Nitrate Content of Stream Water, Soil and Some Foodstuffs Samples in Abuja Municipal Area of Federal Capital Territory, Nigeria. J. Health Sci. 2007, 53, 359–364. [Google Scholar] [CrossRef][Green Version]
- Zhu, L.; Liang, A.; Wang, R.; Shi, Y.; Li, J.; Wang, R.; Wang, M.; Guo, S. Harnessing nitrate over ammonium to sustain soil health during monocropping. Front. Plant Sci. 2023, 14, 1190929. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Factors Affecting Nitrification in Soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef]
- Boer, W.D.; Kowalchuk, G.A. Nitrification in acid soils: Micro-organisms and mechanisms. Soil Biol. Biochem. 2001, 33, 853–866. [Google Scholar] [CrossRef]
- Hakimzadeh Ardakani, M.A.; Haghjoo, M.; Moradi, G.; Esfandiari, M. Investigating the effect of different soil textures on morphological characteristics and the amount of essential oil of Lippia citriodora medicinal Plant. Water Soil Manag. Model. 2022, 3, 14–25. [Google Scholar] [CrossRef]
- Bolland, M.D.A.; Siddique, K.H.M.; Loss, S.P.; Baker, M.J. Comparing responses of grain legumes, wheat and canola to applications of superphosphate. Nutr. Cycl. Agroecosyst. 1999, 53, 157–175. [Google Scholar] [CrossRef]
- Wouterlood, M.; Cawthray, G.R.; Turner, S.; Lambers, H.; Veneklaas, E.J. Rhizosphere carboxylate concentrations of chickpea are affected by genotype and soil type. Plant Soil 2004, 261, 1–10. [Google Scholar] [CrossRef]
- Edelman, M.; Colt, M. Nutrient Value of Leaf vs. Seed. Front. Chem. 2016, 4, 32. [Google Scholar] [CrossRef]
- Yadav, S.S.; Longnecker, N.; Dusunceli, F.; Bejiga, G.; Yadav, M.; Rizvi, A.H.; Manohar, M.; Reddy, A.A.; Xaxiao, Z.; Chen, W. Uses, Consumption and Utilization. In Chickpea Breeding and Management; CABI: Wallingford, UK, 2007; pp. 72–100. [Google Scholar] [CrossRef]
- Ibrikci, H.; Knewtson, S.J.; Grusak, M.A. Chickpea leaves as a vegetable green for humans: Evaluation of mineral composition. J. Sci. Food Agric. 2003, 83, 945–950. [Google Scholar] [CrossRef]
- Ma, S.; Khetarpaul, N.; Chand, G. Minerals Profile and Antioxidants Properties of Chickpea Leave of Desi and Kabuli Varieties at Different Stages of Maturity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3171–3177. [Google Scholar] [CrossRef][Green Version]
- Yu, P.; Christensen, D.A.; McKinnon, J.J. In situ rumen degradation kinetics of timothy and alfalfa as affected by cultivar and stage of maturity. Can. J. Anim. Sci. 2004, 84, 255–263. [Google Scholar] [CrossRef]
- Khattak, A.M.; Ullah, S.; Anjum, F.; Shah, H.U.; Alam, S. Proximate Composition and Mineral Content of Selected Chickpea Cultivars. Sarhad J. Agric. 2021, 37, 683–689. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Kumar, S.; Gowda, C.L.L.; Singh, S. Two major genes for seed size in chickpea (Cicer arietinum L.). Euphytica 2006, 147, 311–315. [Google Scholar] [CrossRef][Green Version]
- Biçer, B.T. The effect of seed size on yield and yield components of chickpea and lentil. Afr. J. Biotechnol. 2009, 8, 1482–1487. [Google Scholar]
- Sastry, D.; Upadhyaya, H.; Gowda, C. Determination of Physical Properties of Chickpea Seeds and their Relevance in Germplasm Collections. Indian J. Plant Genet. Resour. 2014, 27, 1–9. [Google Scholar]
- Avelar, R.I.S.; Costa, C.A.D.; Brandão, D.D.S., Jr.; Paraíso, H.A.; Nascimento, W.M. Production and quality of chickpea seeds in different sowing and harvest periods. J. Seed Sci. 2018, 40, 146–155. [Google Scholar] [CrossRef]
- Ozgun, O.S.; Bicer, B.T.; Sakar, D. Agronomic and Morphological Characters of Chickpea Under Irrigated Conditions in Turkey. Int. J. Agric. Biol. 2004, 6, 606–610. [Google Scholar]
- Kahraman, A.; Pandey, A.; Khan, M.K. Nutritional Diversity Assessment in Chickpea-A Prospect for Nutrient Deprived World. Harran Tarım Gıda Bilim. Derg. 2017, 21, 357–363. [Google Scholar] [CrossRef]
- Vandemark, G.J.; Grusak, M.A.; McGee, R.J. Mineral concentrations of chickpea and lentil cultivars and breeding lines grown in the U.S. Pacific Northwest. Crop J. 2018, 6, 253–262. [Google Scholar] [CrossRef]
- Gaur, P.M.; Singh, M.K.; Samineni, S.; Sajja, S.B.; Jukanti, A.K.; Kamatam, S.; Varshney, R.K. Inheritance of protein content and its relationships with seed size, grain yield and other traits in chickpea. Euphytica 2016, 209, 253–260. [Google Scholar] [CrossRef]


| Abbreviation | Genotype | Type | Weight of 100 Seeds (g) | Protein Content (%) | Area (mm2) | Perimeter (mm) | Length (mm) | Width (mm) |
|---|---|---|---|---|---|---|---|---|
| V1 | S170126 | Kabuli | 27.98 | 29.073 | 41.76 | 31.01 | 7.93 | 6.76 |
| V2 | S170310 | Kabuli | 38.36 | 31.323 | 50.45 | 33.96 | 8.73 | 7.42 |
| V3 | S170279 | Kabuli | 33.76 | 31.057 | 52.73 | 36.23 | 9.84 | 7.14 |
| V4 | S170160 | Kabuli | 40.8 | 30.996 | 43.83 | 31.41 | 8.07 | 6.95 |
| Texture (%) | pH (KCl) | Nitrogen (mg kg−1) | P2O5 (mg kg−1) | K2O (mg kg−1) | Organic Matter (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil | Clay | Silt | Sand | Total Nitrogen | Ammonium (NH4+) | Nitrate (NO3−) | ||||
| Marchouch | 63.7 | 15.9 | 22.1 | 6.9 | 1300 | 50 | 42.9 | 62.9 | 539.2 | 3.1 |
| Beni Mellal | 15.0 | 10.0 | 75.8 | 7.7 | 2300 | 64.3 | 28.6 | 20.3 | 162.7 | 4.3 |
| SH (cm) | Leaflet | Chlo (%) | SN | SW (g) | RDW (g) | SDW (g) | |
|---|---|---|---|---|---|---|---|
| Source of Variation (p Value) | |||||||
| Soil | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.948 ns |
| Genotype | 0.018 * | 0.028 * | 0.001 *** | 0.975 ns | 0.480 ns | 0.437 ns | 0.482 ns |
| Soil ∗ Genotype | 0.109 ns | 0.245 ns | 0.236 ns | 0.392 ns | 0.668 ns | 0.728 ns | 0.789 ns |
| K+ (%) | P (%) | Na+ (%) | Ca2+ (%) | Mg2+ (%) | |
|---|---|---|---|---|---|
| Source of Variation (p Value) | |||||
| Soil | 0.100 ns | 0.653 ns | 0.953 ns | 0.172 ns | 0.578 ns |
| Genotype | 0.403 ns | 0.748 ns | 0.770 ns | 0.537 ns | 0.778 ns |
| Soil × Genotype | 0.771 ns | 0.182 ns | 0.770 ns | 0.849 ns | 0.539 ns |
| Area (mm2) | Perimeter (mm) | Length (mm) | Width (mm) | Protein (g/100g) | Fe (mg kg−1) | Zn (mg kg−1) | |
|---|---|---|---|---|---|---|---|
| Source of Variation (p Value) | |||||||
| Soil | 0.454 ns | 0.050 ns | 0.070 ns | 0.973 ns | 0.226 ns | 0.006 ** | 0.040 * |
| Genotype | 0.990 ns | 0.352 ns | 0.808 ns | 0.681 ns | 0.476 ns | 0.007 ** | 0.032 * |
| Soil×Genotype | 0.238 ns | 0.190 ns | 0.114 ns | 0.377 ns | 0.790 ns | 0.384 ns | 0.014 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahde, S.; Boughribil, S.; Ed-daoudy, L.; Dadi, Y.; El Mekkaoui, A.; Sijilmassi, B.; Kehel, Z.; Amri, A. Effects of Physicochemical Characteristics of Two Soils on Agro-Morphological Traits of Two Chickpea Varieties (Cicer arietinum L.). Sci 2025, 7, 45. https://doi.org/10.3390/sci7020045
Fahde S, Boughribil S, Ed-daoudy L, Dadi Y, El Mekkaoui A, Sijilmassi B, Kehel Z, Amri A. Effects of Physicochemical Characteristics of Two Soils on Agro-Morphological Traits of Two Chickpea Varieties (Cicer arietinum L.). Sci. 2025; 7(2):45. https://doi.org/10.3390/sci7020045
Chicago/Turabian StyleFahde, Sara, Said Boughribil, Lamyae Ed-daoudy, Youssef Dadi, Abdelali El Mekkaoui, Badreddine Sijilmassi, Zakaria Kehel, and Ahmed Amri. 2025. "Effects of Physicochemical Characteristics of Two Soils on Agro-Morphological Traits of Two Chickpea Varieties (Cicer arietinum L.)" Sci 7, no. 2: 45. https://doi.org/10.3390/sci7020045
APA StyleFahde, S., Boughribil, S., Ed-daoudy, L., Dadi, Y., El Mekkaoui, A., Sijilmassi, B., Kehel, Z., & Amri, A. (2025). Effects of Physicochemical Characteristics of Two Soils on Agro-Morphological Traits of Two Chickpea Varieties (Cicer arietinum L.). Sci, 7(2), 45. https://doi.org/10.3390/sci7020045






