Evaluating Various Lactose Types as Solid Carriers for Improving Curcumin Solubility in Solid Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDSs) for Oral Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Test
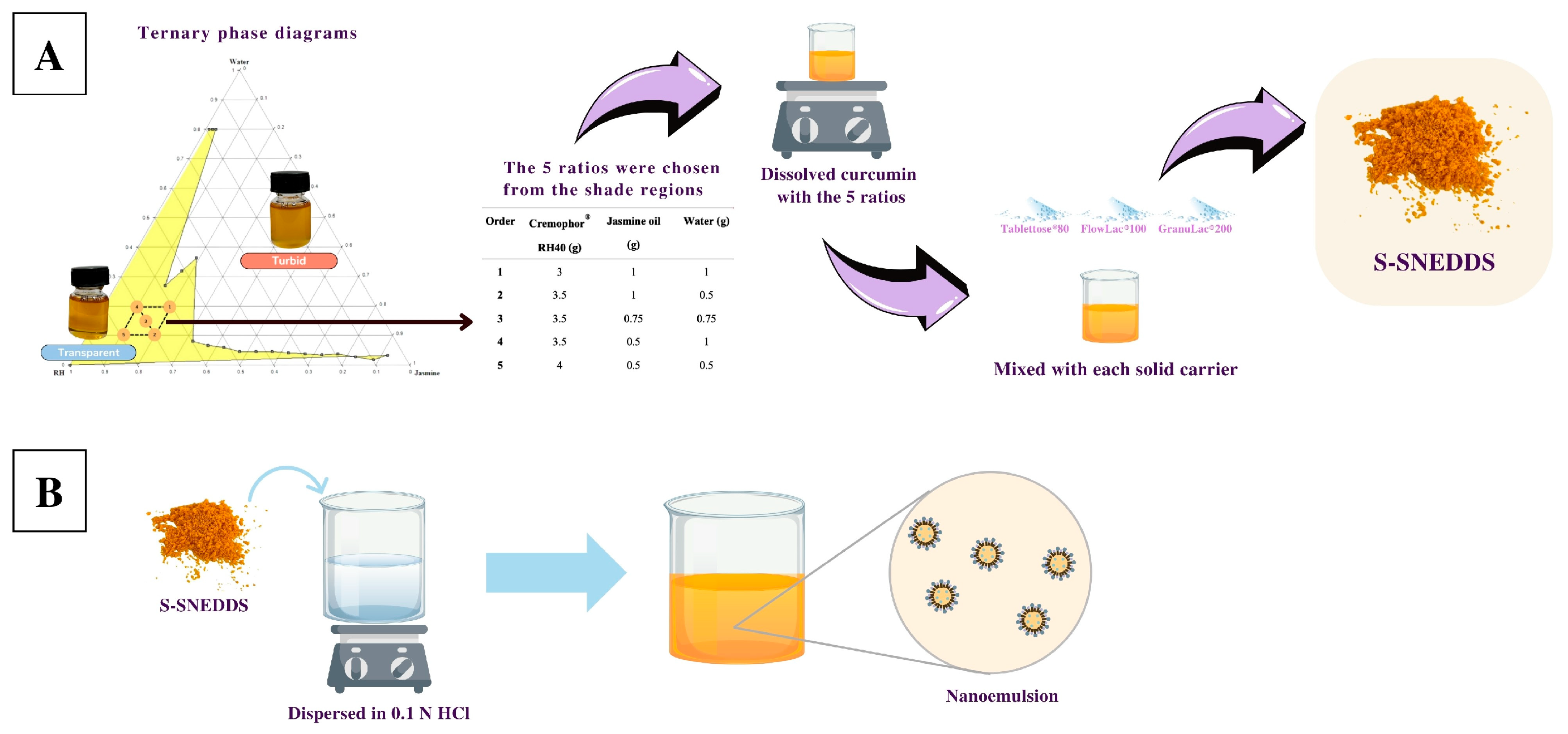
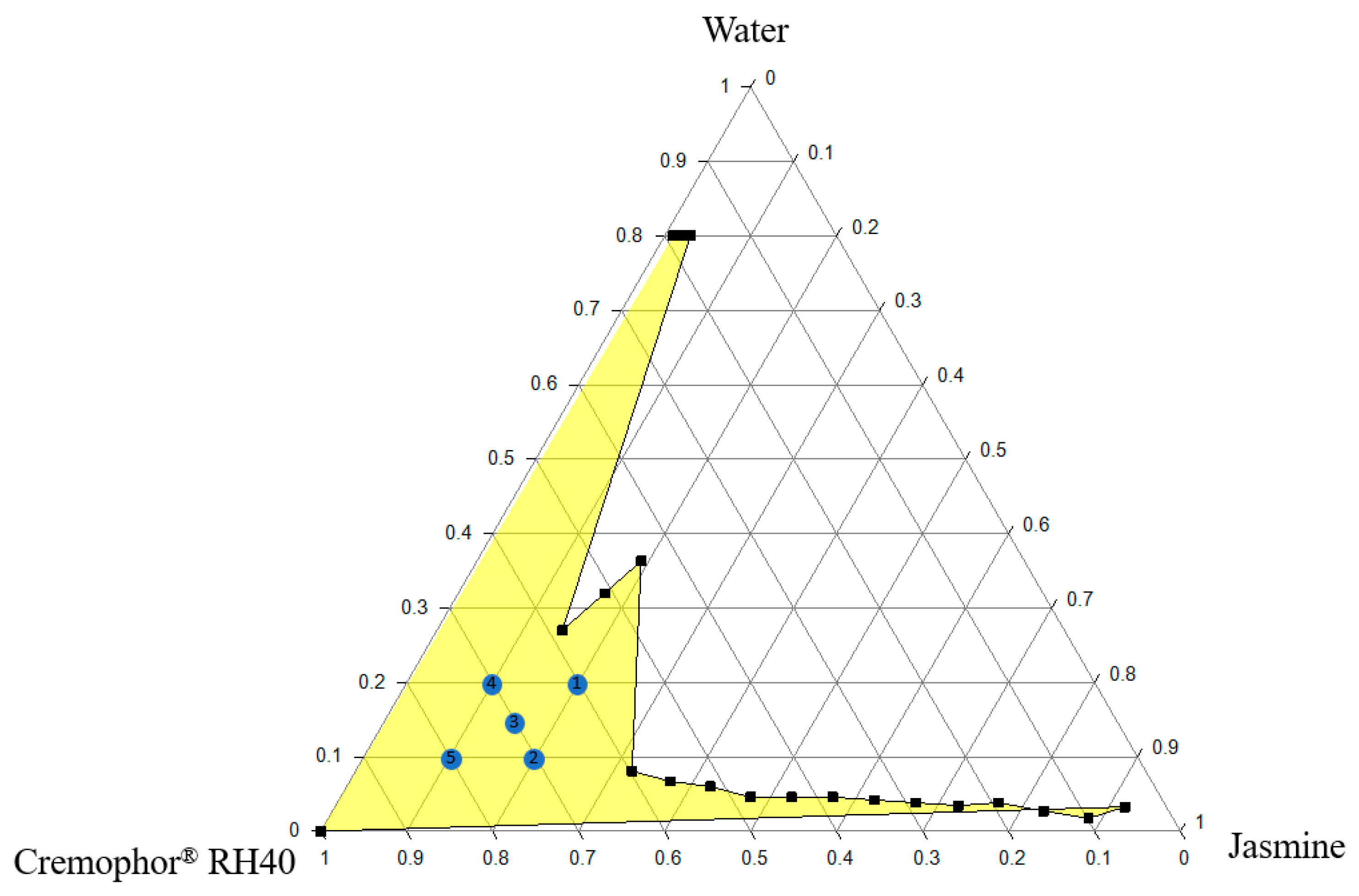
2.3. Construction of Ternary Phase Diagram of Microemulsion
2.4. Preparation of S-SNEDDSs
2.5. Flow Property Study
2.6. Particle Size, PDI, and Zeta Potential Analysis
2.7. Curcumin Content
2.8. High-Performance Liquid Chromatography (HPLC)
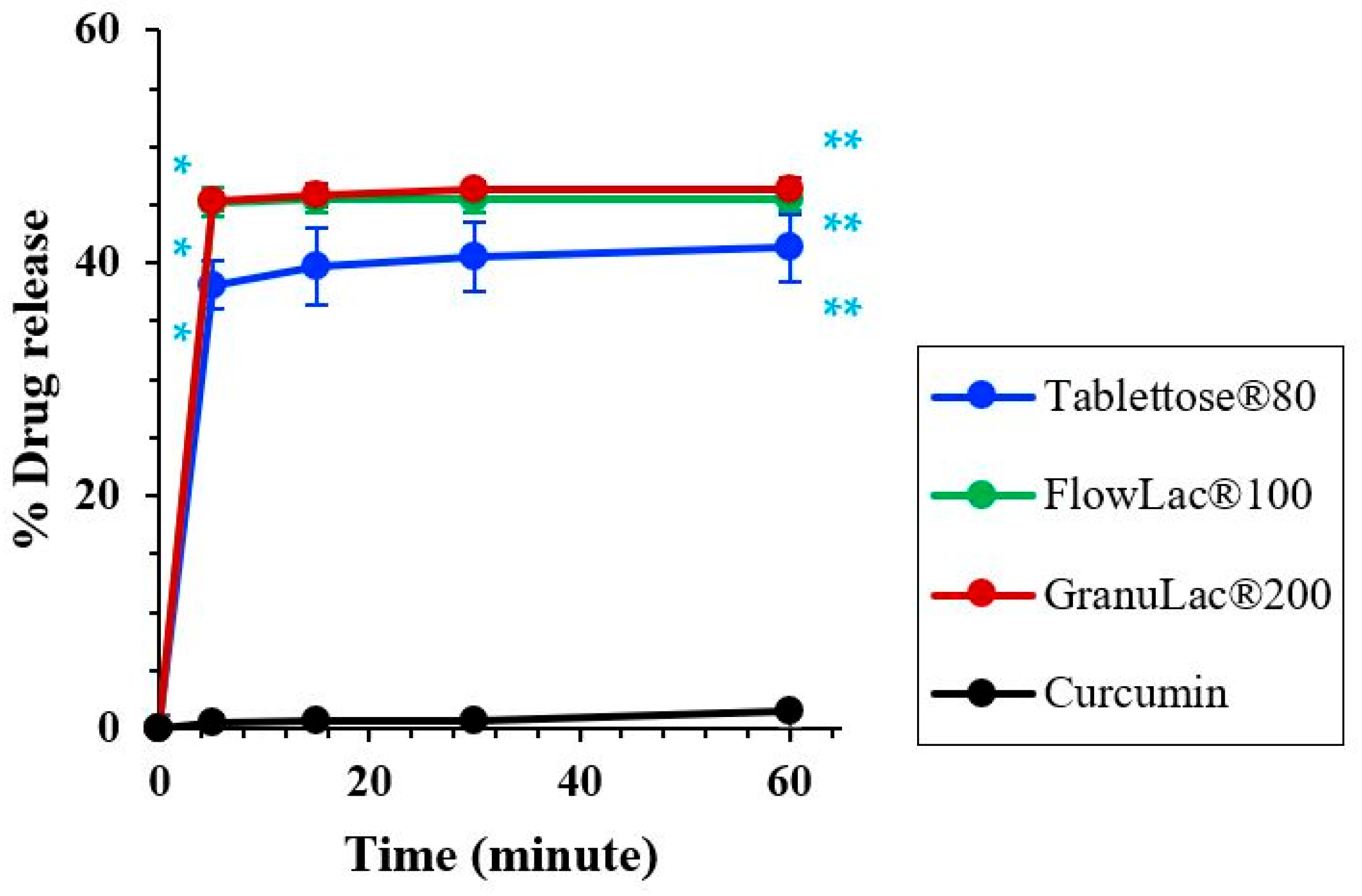
2.9. Dissolution Studies
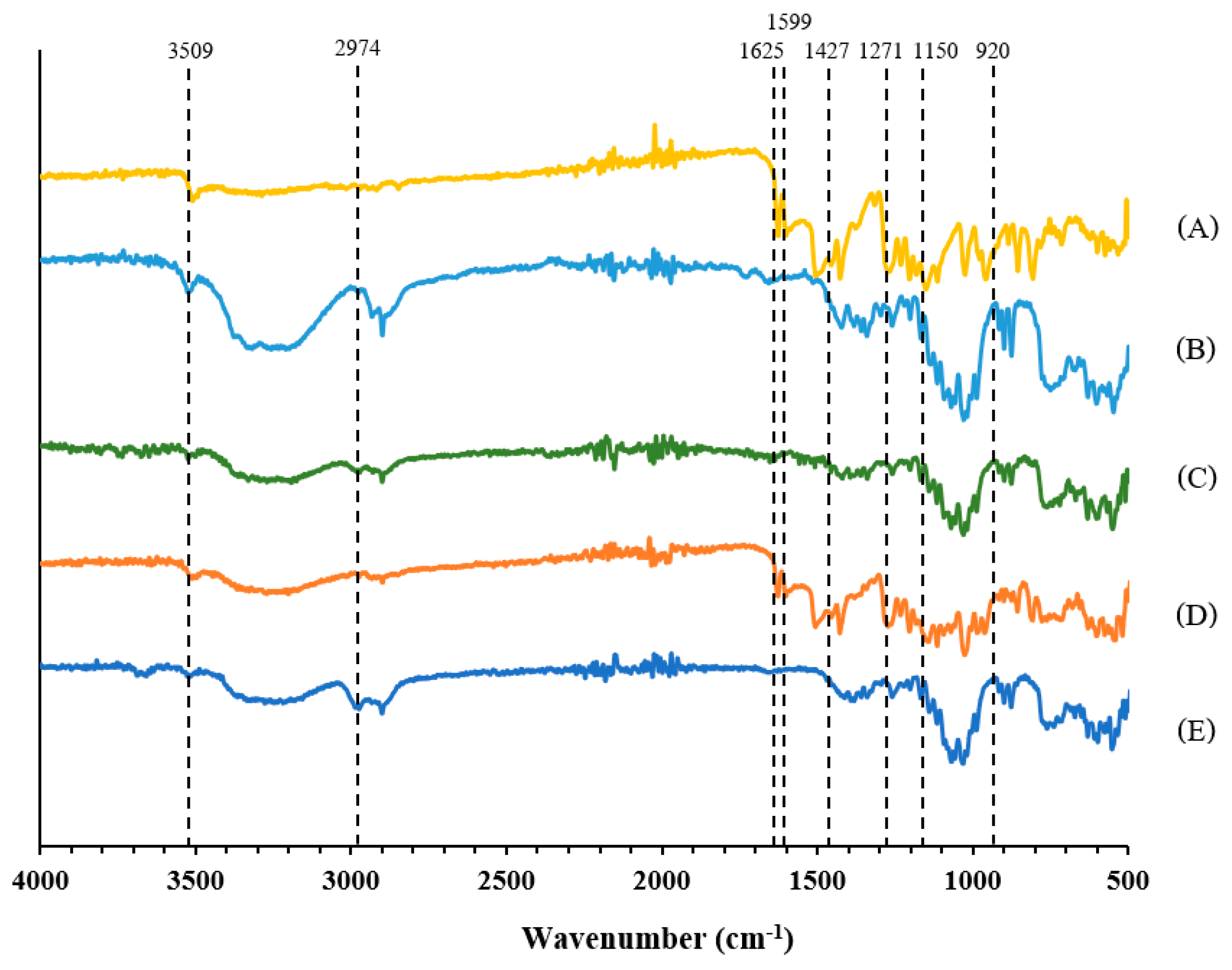
2.10. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.11. Differential Scanning Calorimetry (DSC)
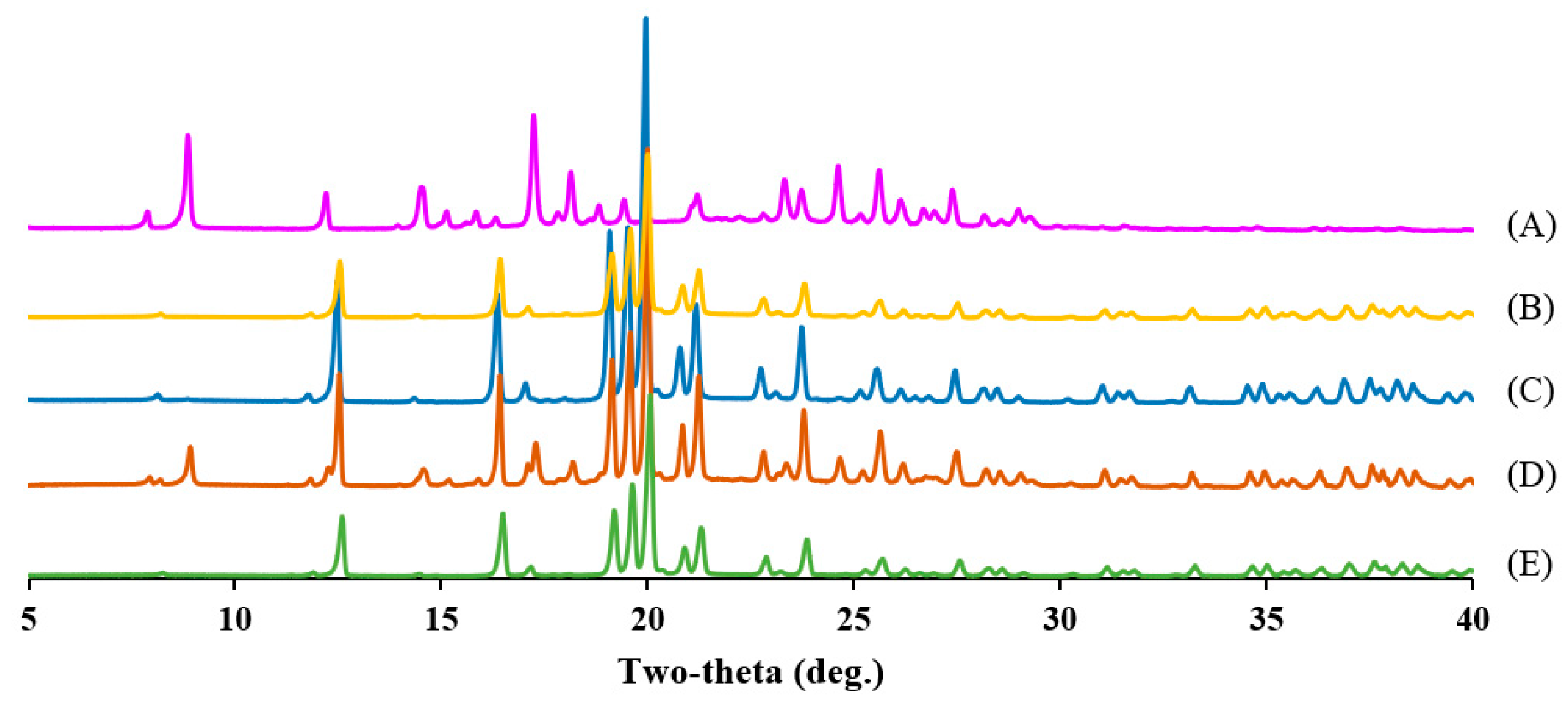
2.12. Powder X-Ray Diffraction (PXRD)
2.13. Stability Study
2.14. Statistical Analysis
3. Results and Discussion
3.1. Solubility Test
3.2. Ternary Phase Diagram of Microemulsion
3.3. Characterization of Microemulsion and L-SNEDDS
3.4. Characterization of Microemulsion and S-SNEDDS
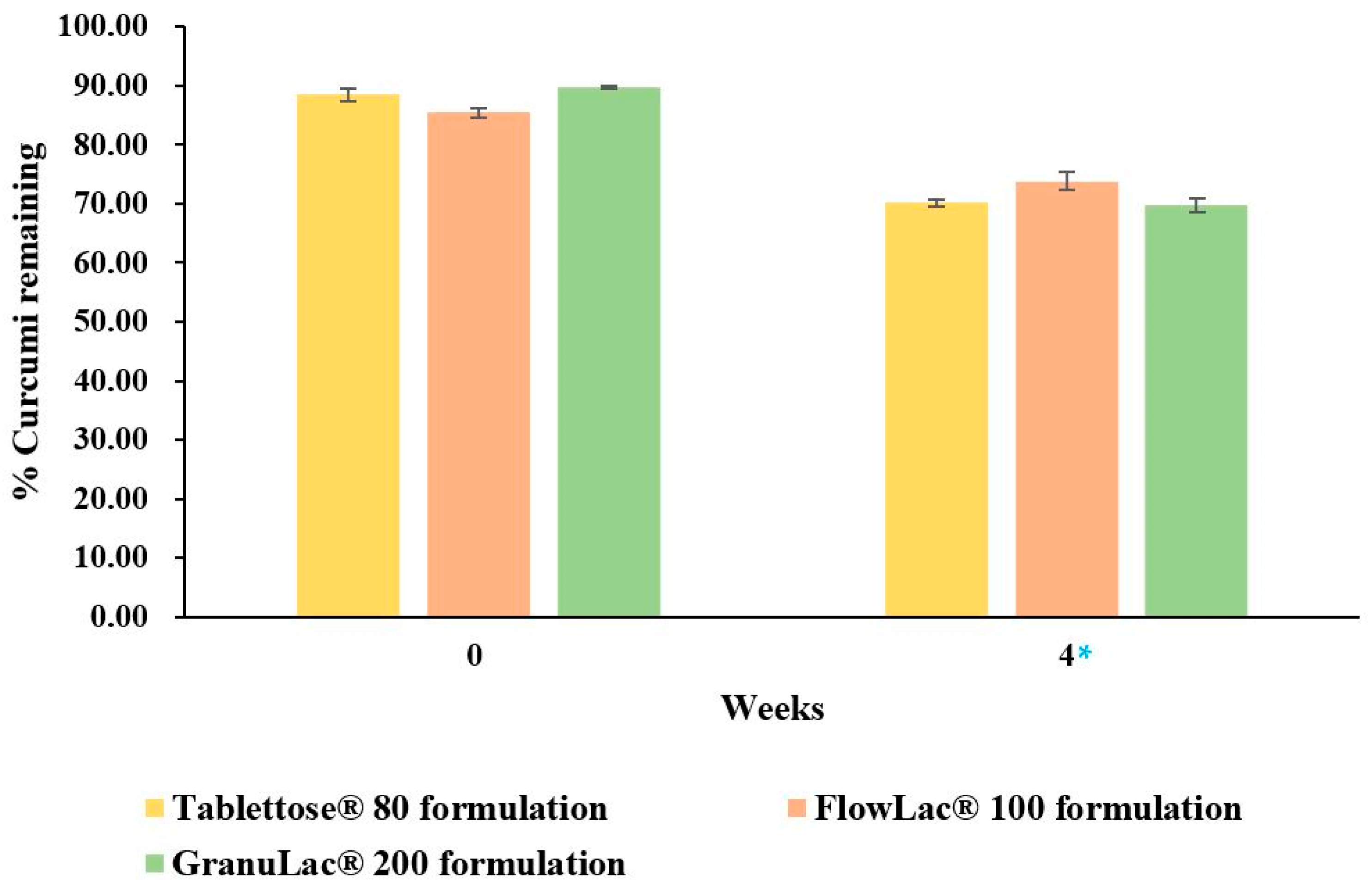
3.5. Stability Study
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent developments in formulation design for improving oral bioavailability of curcumin: A review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent advances to improve curcumin oral bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Kim, M.-K.; Choi, G.-J.; Lee, H.-S. Fungicidal Property of Curcuma longa L. Rhizome-Derived Curcumin against Phytopathogenic Fungi in a Greenhouse. J. Agric. Food Chem. 2003, 51, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Senft, C.; Polacin, M.; Priester, M.; Seifert, V.; Kögel, D.; Weissenberger, J. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer 2010, 10, 491. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef]
- Paolino, D.; Vero, A.; Cosco, D.; Pecora, T.M.; Cianciolo, S.; Fresta, M.; Pignatello, R. Improvement of Oral Bioavailability of Curcumin upon Microencapsulation with Methacrylic Copolymers. Front. Pharmacol. 2016, 7, 485. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef]
- Marques, M.S.; Cordeiro, M.F.; Marinho, M.A.G.; Vian, C.O.; Vaz, G.R.; Alves, B.S.; Jardim, R.D.; Hort, M.A.; Dora, C.L.; Horn, A.P. Curcumin-loaded nanoemulsion improves haemorrhagic stroke recovery in wistar rats. Brain Res. 2020, 1746, 147007. [Google Scholar] [CrossRef]
- Ahmad, S.; Hafeez, A. Formulation and Development of Curcumin-Piperine-Loaded S-SNEDDS for the Treatment of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 1067–1082. [Google Scholar] [CrossRef]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [PubMed]
- Venkata Ramana Rao, S.; Shao, J. Self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of protein drugs: I. Formulation development. Int. J. Pharm. 2008, 362, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Vanani, R.; Moezi, L.; Heli, H. In vivo evaluation of a self-nanoemulsifying drug delivery system for curcumin. Biomed. Pharmacother. 2017, 88, 715–720. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Kumar, B.; Wadhwa, S.; Gulati, M.; Anupriya, A.; Awasthi, A.; Vishwas, S.; Kaur, J.; Corrie, L.; et al. Self-nanoemulsifying composition containing curcumin, quercetin, Ganoderma lucidum extract powder and probiotics for effective treatment of type 2 diabetes mellitus in streptozotocin induced rats. Int. J. Pharm. 2022, 612, 121306. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Alanazi, F.K.; Abdel-Rahman, S.I. Stabilization benefits of single and multi-layer self-nanoemulsifying pellets: A poorly-water soluble model drug with hydrolytic susceptibility. PLoS ONE 2018, 13, e0198469. [Google Scholar] [CrossRef]
- Yi, T.; Wan, J.; Xu, H.; Yang, X. A new solid self-microemulsifying formulation prepared by spray-drying to improve the oral bioavailability of poorly water soluble drugs. Eur. J. Pharm. Biopharm. 2008, 70, 439–444. [Google Scholar] [CrossRef]
- Abdalla, A.; Mäder, K. ESR studies on the influence of physiological dissolution and digestion media on the lipid phase characteristics of SEDDS and SEDDS pellets. Int. J. Pharm. 2009, 367, 29–36. [Google Scholar] [CrossRef]
- Kim, J.S.; Din, F.u.; Cho, H.J.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Park, S.; Youn, Y.S.; Oh, K.T.; et al. Impact of carrier hydrophilicity on solid self nano-emulsifying drug delivery system and self nano-emulsifying granule system. Int. J. Pharm. 2023, 648, 123578. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Singh, H.P.; Patra Ch, N.; Rao, M.E. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech 2012, 13, 1416–1427. [Google Scholar] [CrossRef]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Physical stability of redispersible dry emulsions containing amorphous sucrose. Eur. J. Pharm. Biopharm. 2002, 53, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Zheng, J.; Chen, Z.; Shi, Q.; Liu, H. Development of a Solid Supersaturatable Self-Emulsifying Drug Delivery System of Docetaxel with Improved Dissolution and Bioavailability. Biol. Pharm. Bull. 2011, 34, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kang, J.H.; Oh, D.H.; Yong, C.S.; Choi, H.G. Development of novel flurbiprofen-loaded solid self-microemulsifying drug delivery system using gelatin as solid carrier. J. Microencapsul. 2012, 29, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Sirirak, T. Development and skin penetration pathway evaluation of microemulsions for enhancing the dermal delivery of celecoxib. Colloids Surf. B Biointerfaces 2020, 193, 111103. [Google Scholar] [CrossRef]
- Shah, D.S.; Moravkar, K.K.; Jha, D.K.; Lonkar, V.; Amin, P.D.; Chalikwar, S.S. A concise summary of powder processing methodologies for flow enhancement. Heliyon 2023, 9, e16498. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. A simple reversed phase high-performance liquid chromatography (HPLC) method for determination of in situ gelling curcumin-loaded liquid crystals in in vitro performance tests. Arab. J. Chem. 2017, 10, 1029–1037. [Google Scholar] [CrossRef]
- Nonsuwan, P.; Phiboonchaiyanan, P.P.; Hirun, N.; Kraisit, P. Curcumin-loaded methacrylate pullulan with grafted carboxymethyl-β-cyclodextrin to form hydrogels for wound healing: In vitro evaluation. Carbohydr. Polym. 2023, 321, 121294. [Google Scholar] [CrossRef]
- Tønnesen, H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Pharmazie 2002, 57, 820–824. [Google Scholar]
- Schmidt, M.; Huber, V.; Touraud, D.; Kunz, W. Aromas: Lovely to Smell and Nice Solvents for Polyphenols? Curcumin Solubilisation Power of Fragrances and Flavours. Molecules 2024, 29, 294. [Google Scholar] [CrossRef]
- Huber, V.; Schmidt, M.; Touraud, D.; Kunz, W. Towards a sustainable and green extraction of curcuminoids using the essential oil of Cinnamomum cassia. Sustain. Food Technol. 2023, 1, 319–327. [Google Scholar] [CrossRef]
- Engelberg, S.; Lin, Y.; Assaraf, Y.G.; Livney, Y.D. Targeted Nanoparticles Harboring Jasmine-Oil-Entrapped Paclitaxel for Elimination of Lung Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1019. [Google Scholar] [CrossRef] [PubMed]
- Weerapol, Y.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P. Self-nanoemulsifying drug delivery system of nifedipine: Impact of hydrophilic-lipophilic balance and molecular structure of mixed surfactants. AAPS PharmSciTech 2014, 15, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.R.; Pulikkal, A.K. Preparation, stability and biological activity of essential oil-based nano emulsions: A comprehensive review. OpenNano 2022, 8, 100066. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef]
- Bettie van der Walt, E. Evaluation and Comparison of the Physical Properties and Drug Release Characteristics of Directly Compressible Lactose-Based Filler/Binders; North-West University, Potchefstroom Campus: Potchefstroom, South Africa, 2010; Available online: https://dspace.nwu.ac.za/bitstream/handle/10394/4922/vanderwalt_erasmus_b.pdf?sequence=2 (accessed on 20 October 2024).
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) for Oral Delivery of Olmesartan Medoxomil: Design, Formulation, Pharmacokinetic and Bioavailability Evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Sargam, Y.; Wang, K.; Tsyrenova, A.; Liu, F.; Jiang, S. Effects of anionic and nonionic surfactants on the dispersion and stability of nanoSiO2 in aqueous and cement pore solutions. Cem. Concr. Res. 2021, 144, 106417. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Agmo Hernández, V. An overview of surface forces and the DLVO theory. ChemTexts 2023, 9, 10. [Google Scholar] [CrossRef]
- Józsa, L.; Vasvári, G.; Sinka, D.; Nemes, D.; Ujhelyi, Z.; Vecsernyés, M.; Váradi, J.; Fenyvesi, F.; Lekli, I.; Gyöngyösi, A.; et al. Enhanced Antioxidant and Anti-Inflammatory Effects of Self-Nano and Microemulsifying Drug Delivery Systems Containing Curcumin. Molecules 2022, 27, 6652. [Google Scholar] [CrossRef]
- Opustilová, K.; Lapčíková, B.; Lapčík, L.; Gautam, S.; Valenta, T.; Li, P. Physico-Chemical Study of Curcumin and Its Application in O/W/O Multiple Emulsion. Foods 2023, 12, 1394. [Google Scholar] [CrossRef]
- GombÁs, Á.; Szabó-Révész, P.; Kata, M.; Regdon, G.; Erős, I. Quantitative Determination of Crystallinity of α-Lactose Monohydrate by DSC. J. Therm. Anal. Calorim. 2002, 68, 503–510. [Google Scholar] [CrossRef]
- Larhrib, H.; Martin, G.P.; Prime, D.; Marriott, C. Characterisation and deposition studies of engineered lactose crystals with potential for use as a carrier for aerosolised salbutamol sulfate from dry powder inhalers. Eur. J. Pharm. Sci. 2003, 19, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Alshadidi, A.; Shahba, A.A.; Sales, I.; Rashid, M.A.; Kazi, M. Combined Curcumin and Lansoprazole-Loaded Bioactive Solid Self-Nanoemulsifying Drug Delivery Systems (Bio-SSNEDDS). Pharmaceutics 2021, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, Z.; Jafarizadeh, H. Photocatalytic and antibacterial activities study of prepared self-cleaning nanostructure surfaces using synthesized and coated ZnO nanoparticles with Curcumin nanodispersion. Z. Fur Krist. Mater. 2018, 234, 307–328. [Google Scholar] [CrossRef]
- Fagnani, R.; Battaglini, A.; Beloti, V.; Urbano, A.; Bronzol, J. Alcohol Stability of Milk from the Perspective of X-Ray Diffractometry. Food Biophys. 2016, 11, 198–205. [Google Scholar] [CrossRef]
- Ismail, E.; Sabry, D.; Mahdi, H.; Khalil, M. Synthesis and Characterization of some Ternary Metal Complexes of Curcumin with 1,10-phenanthroline and their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef]
- Balieiro, A.L.; Santos, R.A.; Pereira, M.M.; Figueiredo, R.T.; Freitas, L.S.; Alsina, O.L.S.d.; Lima, A.S.; Soares, C.M.F. Adsorption process of molecularly imprinted silica for extraction of lactose from milk. Braz. J. Chem. Eng. 2016, 33, 361–372. [Google Scholar] [CrossRef]
- Rajesh, S.Y.; Singh, S.K.; Pandey, N.K.; Sharma, P.; Bawa, P.; Kumar, B.; Gulati, M.; Jain, S.K.; Gowthamarajan, K.; Singh, S. Impact of various solid carriers and spray drying on pre/post compression properties of solid SNEDDS loaded with glimepiride: In vitro-ex vivo evaluation and cytotoxicity assessment. Drug Dev. Ind. Pharm. 2018, 44, 1056–1069. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Bera, B.; Khazal, R.; Schroën, K. Coalescence dynamics in oil-in-water emulsions at elevated temperatures. Sci. Rep. 2021, 11, 10990. [Google Scholar] [CrossRef]



| Oils | Solubility (mg/mL) | Surfactants | Solubility (mg/mL) |
|---|---|---|---|
| Chamomile | 0.31 ± 0.006 | Tween® 20 | 14.86 ± 0.07 |
| Clove | 2.14 ± 0.043 | Tween® 80 | 15.63 ± 0.03 |
| Rose | 0.2 ± 0.006 | Span® 20 | 3.08 ± 0.03 |
| Eucalyptus | 1.56 ± 0.026 | Span® 80 | 1.47 ± 0.01 |
| Jasmine | 7.28 ± 0.615 | Cremophor® EL | 15.08 ± 0.06 |
| Lavender | 2.41 ± 0.037 | Cremophor® RH40 | 27.12 ± 0.45 |
| Lemon | 0.3 ± 0.001 | ||
| Sunflower | 0.26 ± 0.011 |
| Order | Cremophor® RH40 (g) | Jasmine Oil (g) | Water (g) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 1 | 3.0 | 1.00 | 1.00 | 3.75 ± 0.52 * | 0.05 ± 0.01 ** | −0.68 ± 1.44 |
| 2 | 3.5 | 1.00 | 0.50 | 5.44 ± 0.90 * | 0.09 ± 0.04 ** | −0.80 ± 1.63 |
| 3 | 3.5 | 0.75 | 0.75 | 3.67 ± 0.51 * | 0.10 ± 0.06 ** | 0.35 ± 1.83 |
| 4 | 3.5 | 0.50 | 1.00 | 2.67 ± 1.16 * | 0.17 ± 0.08 | 0.43 ± 1.28 |
| 5 | 4.0 | 0.50 | 0.50 | 83.56 ± 61.77 | 0.18 ± 0.06 | −0.59 ± 2.92 |
| Cremophor® RH40 (g) | Jasmine Oil (g) | Water (g) | Curcumin (mg) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 3 | 1 | 1 | - | 3.75 ± 0.52 | 0.05 ± 0.01 | −0.68 ± 1.44 |
| 3 | 1 | - | 100 mg | 188.52 ± 26.21 | 0.06 ± 0.03 | 0.42 ± 1.07 |
| p-values | <0.001 | <0.166 | 0.085 |
| Formulations (Type of Solid Carriers) | Angle of Repose (Degrees) | Results |
|---|---|---|
| GranuLac® 200 | 25.95 ± 0.27 | Excellent |
| Tablettose® 80 | 38.77 ± 0.53 | Fair |
| FlowLac® 100 | 42.08 ± 0.97 | Passable |
| Formulations | Mean Droplet Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Tablettose® 80 | 75.27 ± 2.40 | 0.157 ± 0.02 | +0.31 ± 0.71 |
| FlowLac® 100 | 49.53 ± 6.79 * | 0.203 ± 0.04 | +1.25 ± 0.60 |
| GranuLac® 200 | 44.97 ± 4.80 * | 0.193 ± 0.06 | +1.20 ± 1.00 |
| Baseline | One Week | Two Weeks | Three Weeks | Four Weeks | ||
|---|---|---|---|---|---|---|
| Tablettose® 80 formulation | Size (nm) | 75.27 ± 2.40 | 92.57 ± 3.78 | 129.6 ± 4.77 * | 147.4 ± 4.54 * | 185.77 ± 21.91 * |
| PDI | 0.157 ± 0.02 | 0.153 ± 0.01 | 0.104 ± 0.01 a | 0.115 ± 0.01 a | 0.137 ± 0.01 | |
| FlowLac® 100 formulation | Size (nm) | 49.53 ± 6.79 | 68.03 ± 4.47 | 85.30 ± 3.03 ** | 92.70 ± 8.56 ** | 117.23 ± 14.14 ** |
| PDI | 0.203 ± 0.04 | 0.166 ± 0.01 | 0.111 ± 0.01 b | 0.120 ± 0.01 b | 0.095 ± 0.01 b | |
| GranuLac® 200 formulation | Size (nm) | 44.97 ± 4.80 | 59.00 ± 0.62 | 67.77 ± 7.67 *** | 65.50 ± 3.92 *** | 68.93 ± 8.95 *** |
| PDI | 0.193 ± 0.06 | 0.227 ± 0.02 | 0.141 ± 0.02 | 0.200 ± 0.03 | 0.159 ± 0.04 |
| Zeta Potential (mV) | |||||
|---|---|---|---|---|---|
| Baseline | One Week | Two Weeks | Three Weeks | Four Weeks | |
| Tablettose® 80 formulation | 0.31 ± 0.71 | −0.78 ± 0.24 | −0.74 ± 0.18 | −0.59 ± 0.44 | 2.29 ± 2.33 * |
| FlowLac® 100 formulation | 1.25 ± 0.60 | 1.02 ± 0.66 | 1.13 ± 0.63 | −0.66 ± 2.16 | −0.50 ± 0.59 |
| GranuLac® 200 formulation | 1.20 ± 1.00 | 1.64 ± 0.44 | 1.41 ± 0.41 | −0.85 ± 1.08 | 1.06 ± 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teerapipattanapong, P.; Jaikon, P.; Ningsanonda, N.; Yonemochi, E.; Furuishi, T.; Hirun, N.; Kraisit, P. Evaluating Various Lactose Types as Solid Carriers for Improving Curcumin Solubility in Solid Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDSs) for Oral Administration. Sci 2024, 6, 69. https://doi.org/10.3390/sci6040069
Teerapipattanapong P, Jaikon P, Ningsanonda N, Yonemochi E, Furuishi T, Hirun N, Kraisit P. Evaluating Various Lactose Types as Solid Carriers for Improving Curcumin Solubility in Solid Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDSs) for Oral Administration. Sci. 2024; 6(4):69. https://doi.org/10.3390/sci6040069
Chicago/Turabian StyleTeerapipattanapong, Panida, Pimrada Jaikon, Nichapa Ningsanonda, Etsuo Yonemochi, Takayuki Furuishi, Namon Hirun, and Pakorn Kraisit. 2024. "Evaluating Various Lactose Types as Solid Carriers for Improving Curcumin Solubility in Solid Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDSs) for Oral Administration" Sci 6, no. 4: 69. https://doi.org/10.3390/sci6040069
APA StyleTeerapipattanapong, P., Jaikon, P., Ningsanonda, N., Yonemochi, E., Furuishi, T., Hirun, N., & Kraisit, P. (2024). Evaluating Various Lactose Types as Solid Carriers for Improving Curcumin Solubility in Solid Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDSs) for Oral Administration. Sci, 6(4), 69. https://doi.org/10.3390/sci6040069







