Abstract
Petrochemical feedstocks are experiencing a fast growth in demand, which will further expand their market in the coming years. This is due to an increase in the demand for petrochemical-based materials that are used in households, hospitals, transportation, electronics, and telecommunications. Consequently, petrochemical industries rely heavily on olefins, namely propylene, ethylene, and butene, as fundamental components for their manufacturing processes. Presently, there is a growing interest among refineries in prioritising their operations towards the production of fuels, specifically gasoline, diesel, and light olefins. The cost-effectiveness and availability of petrochemical primary feedstocks, such as propylene and butene, can be enhanced through the direct conversion of crude oil into light olefins using fluid catalytic cracking (FCC). To achieve this objective, the FCC technology, process optimisation, and catalyst modifications may need to be redesigned. It is helpful to know that there are several documented methods of modifying traditional FCC catalysts’ physicochemical characteristics to enhance their selectivity toward light olefins’ production, since the direct cracking of crude oil to olefins is still in its infancy. Based on a review of the existing zeolite catalysts, this work focuses on the factors that need to be optimized and the approaches to modifying FCC catalysts to maximize light olefin production from crude oil conversion via FCC. Several viewpoints have been combined as a result of this research, and recommendations have been made for future work in the areas of optimising the yield of light olefins by engineering the pore structure of zeolite catalysts, reducing deactivation by adding dopants, and conducting technoeconomic analyses of direct crude oil cracking to produce light olefins.
1. Introduction
The most common usage of fluid catalytic cracking (FCC) units is in transportation fuels’ (gasoline, diesel, etc.) production [1]. Additional fuels, like diesel, distillate fuels, and olefinic gases, are produced through FCC after atmospheric distillation or vacuum distillation, which are physical separation processes. Due to their ability to transform vacuum gas oils (VGOs), atmospheric residue (AR), heavy petrol oil (HGO) and other heavy bottoms into liquid fuel (such as petrol), light olefin gases (such as ethylene and propylene) and other products (such as light cycle oil (LCO)), FCC units have long been regarded as the most important unit in petroleum refineries [2,3]. Catalysts, most often zeolite, are employed in the FCC to convert large hydrocarbon molecules into smaller, more valuable ones like ethylene and propylene. It is imperative to acknowledge that operators of the FCC must remain abreast of evolving market dynamics and shifting environmental regulations. Notably, this includes the increasing need for light olefin gases, such as propylene. Moreover, the global demand for crude oil in 2017 consisted of 12.7% dedicated to petrochemical feedstocks, amounting to a daily consumption of 12 million barrels. China is expected to account for more than 70% of the growth in oil demand by 2023, and global demand will increase from 2.2 mb/d to 102.2 mb/d [4]. The projected level of demand is anticipated to reach 18 million barrels per day (b/d) by the year 2030 [5]. The global light olefin gases market will account for a compound annual growth rate (CAGR) of about 4.76%, to reach USD 348 billion in 2030, up from USD 240.1 billion in 2022 [6]. The worldwide need for greener and cleaner industrial fluids, as well as the more stringent emission controls in several developed and developing nations, are expected to drive the light olefins market significantly throughout the projected period.
With the rapid growth of the world population, there is an increasing demand for food packaging materials, lubricants, and so forth. This increase in the rise in the production of petrochemicals is being driven by an increase in the demand for feedstocks across a variety of industries, including households, hospitals, electronics, transportation and telecommunications materials [7]. Light olefin gases, namely propylene, ethylene, butene, and butadiene, serve as fundamental components in the petrochemical sector, which is experiencing growth in its markets. Petrochemical products that are derived from these light olefin gases include polyethylene, polypropylene, polyvinyl chloride, acrylonitrile, styrene and ethylene glycol [8]. The principal aim of traditional crude oil refineries is to manufacture fuels for transportation purposes such as gasoline, with petrochemical feedstocks produced as by-products. As a by-product of gasoline production, FCC produces about 35% of the world’s propylene [9]. At present, the steam cracking of naphtha is industrially used to supply most of the light olefins for the petrochemical industries. The yields of propylene production via ethane-based steam cracking are much lower than those from naphtha-based steam cracking [7,8]. Approximately 60% of the global propylene demand is met through the steam cracking of naphtha and gas oils. With FCC, product selectivity can be better controlled as compared to noncatalytic processes like steam cracking and thermal cracking. Light olefin gases are usually produced by the FCC of vacuum gas oils (VGOs), heavy petrol oil (HGO), and other similar crude oil refinery residues (atmospheric distillation residue), which provide insufficient amounts to fulfil the ever-increasing worldwide market demand and yield inadequate quantities to meet the ever-increasing global market demand. Increasing olefin gas production might be best accomplished by directly cracking crude oil in an FCC unit, which is the fundamental goal of this research. This approach is expected to reduce the cost of refining since some of the costly processes will be avoided. As a result of bypassing some expensive refining processes (e.g., distillation), the direct cracking of crude oil into light olefin gases can also be considered cost-effective [10]. However, crude oil that is converted directly into light olefins via the FCC is regarded as one of the most cost-effective and steadfast means of producing primary feedstocks (e.g., ethylene, propylene and butene) for petrochemicals [7,11]. Crude oil that has been directly cracked into light olefins such as ethylene, propylene, 1-butene, and 2-butene via catalytic cracking is shown in Figure 1.
Figure 1.
Illustration of the catalytic cracking of crude oil into petrochemical feedstock.
The Fluid Catalytic Cracking Unit (FCCU) has the ability to crack a wide variety of low-value fuels, including unrefined crude oil [12]. According to projections, the demand for olefin gases like ethylene and propylene will rapidly increase by 2025, reaching over 200 and 140 million metric tons, respectively, with annual rates of growth of around 3.6% and 4.0% [8]. In 2022, the size and share of the olefins market in terms of revenue was valued at USD 240.1 billion worldwide. Based on the most recent research data, a compound annual growth rate (CAGR) of 4.76% has been predicted for the global olefins market from 2023 to 2030, which would increase market revenue to over USD 348 billion [13]. This suggests that light olefin gases will continue to be in demand, and that production needs to be expanded. The worldwide polymers industry is driving this growth in demand. Notwithstanding the diminishing crude oil reserves, the petroleum-refining industry is investing relatively little in alternative technologies and feedstocks (such as biomass-derived olefin gases) due to their current lack of economic viability in a volatile commodities market [14]. The proposal to address the increasing demand for petrochemical feedstocks, particularly light olefin gases, involves the utilisation of FCC for directly cracking crude oil [8,10]. Therefore, via modification of the catalyst and operating conditions, FCC has traditionally been used to crack low-value heavy oils, but has more recently been used to process more unconventional feedstocks, such as shale oils, pyrolysis oil [15], and bio-oils [16]. The maximisation of olefin gas yield in FCC units can be achieved through catalyst engineering, the introduction of additives, and operation at high severity levels with short contact times. The research findings indicate that the utilisation of the catalytic cracking of crude oil directly has the potential to generate a greater quantity of petrochemical feedstocks, specifically ethylene and propylene, which are currently experiencing significant demand in the market. Al-Khattaf and Ali., [12] conducted experiments to crack Arab Super Light crude oil using USY zeolite and MFI-based zeolite catalysts at temperatures of 500–575 °C to produce light olefin (gases) in a riser simulator reactor. They showed that an increment in the residence time or temperature of the reactor had a greater influence on propylene production compared to ethylene. Furthermore, the researchers demonstrated that the USY zeolite catalyst exhibited higher yields of propylene and ethylene in comparison to the MFI-based zeolite catalyst. This finding was attributed to the USY zeolite’s increased acidity, better shape selectivity, and improved hydrogen transferability. According to the results, the yield of light olefin gases produced by the FCC unit is largely reliant on both the characteristics of the feedstock and the properties of the catalyst. The experimental findings indicate that the formation of naphtha predominantly occurs through the process of cracking heavy fractions of crude oil. Conversely, the production of gaseous products within the C1–C4 range is primarily attributed to the cracking of fractions within the naphtha range. Usman et al., [9] conducted a comprehensive investigation into the cracking properties of three distinct light crude oils, namely Arab super-light, Arab extra-light, and Arab light. The investigation was performed utilising an equilibrated MFI zeolite catalyst within a micro-activity test (MAT) unit, operating at a temperature of 550 °C. Their studies produced a light olefin yield in the range 10–13% and naphtha in the range 50–60% for the three oils at 60% conversion of the feed oil. Comparisons of the authors’ results suggests that lighter crude oils yielded more naphtha, by about 8 wt% (weight %), depending on the difference in the gravity acting on the crude oil from the American Petroleum Institute (API).
A comprehensive evaluation was conducted to determine the crackability of different oil feedstocks, such as crude oils, as well as the effectiveness of FCC catalysts. This assessment involved the utilisation of various testing techniques, including a fixed-bed MAT, a fluidized-bed advanced cracking evaluation (ACE), a CREC Riser Simulator reactor, a micro-downer, and a Davidson Circulating Riser (DCR) pilot plant [5,9]. An investigation of the process of directly cracking crude oil was reported using Bach Ho crude oil, which possesses an American Petroleum Institute (API) gravity of 41°. This investigation was conducted using an equilibrium FCC catalyst (E-cat), an improved FCC catalyst (HT-FCC), and a ZSM-5 additive. The experiments are carried out in an MAT unit under specific conditions, including a temperature range of 620–650 °C, a catalyst-to-oil ratio of 1.5–2.5 (g/g), and a contact time of 12 s [17]. In contrast to conventional FCC catalysts, the conversion of crude oil may be limited as a result of the inclusion of lighter fractions, especially gas oil, naphtha, etc. The addition of ZSM-5 was found to enhance the efficiency of converting gasoline into light olefins. It is therefore necessary to modify standard FCC catalysts to overcome this challenge, using either catalyst additives or activation techniques. This can be achieved through the incorporation of catalyst additives and activation techniques, as well as by operating under high-severity conditions or integrating other process units [12]. It has been determined that the FCC process and catalyst technology will have a significant impact on the direct cracking of crude oil for the production of light olefin feedstocks in the petrochemical industry. A scholarly publication in 2017 presented a comprehensive analysis of the direct conversion of crude oil into olefins, focusing specifically on the application of FCC-type technology [10]. In a study conducted in 2018, the analysis of the use of light paraffinic crude oil for catalytic cracking into light olefin gases was performed using a fixed-bed microactivity test (MAT) and a fluidized-bed advanced cracking evaluation unit. The catalysts employed in this investigation included MFI zeolite (ZSM-5), an equilibrated FCC catalyst (E-Cat), and a mixture of E-Cat and MFI (referred to as E-Cat/MFI) at a constant catalyst-to-oil ratio of 4. The experiments were conducted at temperatures of 550 and 600 °C [12]. In contrast to the MAT unit, the fluidized-bed advanced-cracking evaluation unit demonstrated a significantly higher rate of coke production. At a temperature of 600 °C, the highest yield of light olefins (29 wt%) was achieved using the E-Cat/MFI catalyst, surpassing the yields obtained with MFI (23 wt%) and E-Cat (21 wt%) catalysts. The elevated temperature conditions, nevertheless, led to an increase in dry gas production due to thermal cracking. In terms of methodology, both the MAT and fluidized-bed advanced cracking evaluation unit yielded similar catalyst rankings, with the E-Cat catalyst performing the best, followed by the E-Cat/MFI catalyst, and then the MFI catalyst. This can be attributed to the diffusion restriction caused by the pore size of the MFI catalyst.
ZSM-5 is an additive used in modern fluid catalyst cracking plants to boost the production of light olefins like propylene [18,19]. Zeolite ZSM-5 catalyst pore sizes may be too small to crack direct crude oil. The main components of an FCC catalyst are zeolite Y and a matrix that consists of alumina, silica-alumina, silica, filler (for example, clay such as kaolinite), and a binder. The acid sites and zeolite pore structure control the cracking of hydrocarbon feedstock on FCC catalysts [20]. The large hydrocarbon C-C bonds in crude oils must be broken down into smaller fragments by the catalysts’ acid sites. The ultra-stable faujasite Y (USY) continues to be the key component of industrial FCC catalysts, despite the fact that the FCC process made use of a wide range of zeolites [21,22]. Consequently, the incorporation of zeolite Y into FCC catalysts leads to the generation of Brønsted acid sites and readily accessible Lewis acid sites. These sites are accountable for the cleavage of C–C bonds. Furthermore, the FCC catalyst requires a porous matrix to work well, since the pores of zeolite (USY) are too tiny, limiting the mass transport of large hydrocarbons and their access to the acid sites. Hence, it is imperative to engage in the development and alteration of zeolite-based catalysts with the aim of enhancing the yield of propylene and light olefins through direct crude oil cracking. In contrast to the use of thermal cracking as a standalone process, the utilisation of catalytic cracking in conjunction with E-Cat/ZSM-5 catalysts resulted in enhanced conversion rates, a doubling of light olefin yields (specifically ethylene and propylene), and an elevated concentration of aromatics within the naphtha fraction [23]. Although the conversion of crude oil to light olefins is still in its infancy, it is useful to be aware of the many documented ways of modifying catalyst characteristics in a traditional FCC process. The various techniques used to modify the zeolite-based catalyst to optimise the conversion of crude oil to light olefin gas production include: (1) particle size and acidity modification, (2) the creation of a mesoporous/hierarchical structure, (3) phosphorus treatment, and (4) the inclusion of additives such as alkali metals, ZSM-5, certain transition metals, and the incorporation of rare-earth metal [18]. The conversion of crude oil into transportation fuels and light olefins through the employment of FCC technology requires the optimisation of process variables and the modification of zeolite catalysts. The catalyst that cracks crude oil into light olefins is one of the most critical factors in the crude-oil-to-chemical switch of FCC technology. This review elucidates the roles these factors play in direct crude oil cracking in FCC to match existing refinery systems. The work highlights new perspectives and provides data to support further research in the field. The development of robust and active catalysts which are favourably selective towards light olefins’ production from crude oil cracking via FCC will be cost-effective and improve flexibility.
Based on this body of literature, the view is that, when the right catalyst and reactor configuration are developed, and the conditions of the various process factors are optimized, it is obvious that crude oil can be transformed into light olefins in a one-step process. For this reason, maximizing the yield of light olefins in the FCC direct cracking of crude oil will require the development of novel catalysts or modifications to existing zeolite-based catalysts, as well as the optimization of process factors, other processes, and a product evaluation parameter. As such, Section 2 of this review examines the process factors that need optimization and how a critical evaluation of experimental studies describes their effects on olefin production, whereas Section 3 will focus on the necessary catalyst modifications based on a critical analysis of the literature, and how these impact olefin yields. Section 4 discusses the use of kinetic models to understand the crude oil cracking mechanism and process factors that are critical to process technology development, catalyst design, and process factor optimization. Model-based optimization and process simulation depend on the development of an appropriate kinetic model.
2. Operating Conditions Optimization
Due to the FCC’s versatility under various reaction conditions, direct crude oil cracking can bridge the supply–demand gap of light olefins by maximising their production via optimisation. As part of this study, it is essential to establish the catalytic cracking process of crude oil and identify the key process variables that have a significant impact on the yields of light olefins, particularly propylene and ethylene, from this process. It is possible to appropriately optimise the FCC process and maximise olefin production by integrating catalyst modification, reaction residence time, temperature, hydrocarbon partial pressures and coke formation using a synergistic approach [24]. In order to maximise the production of propylene and other light olefins, it is crucial to use an appropriate zeolite-based catalyst, a well-designed reactor, and optimal operating conditions. The factors that influence the cracking process include the catalyst-to-oil ratio (CTO), the reaction temperature, the feedstock characteristics, the partial pressures of hydrocarbons, and the hydrogen transfer index. These process factors determine conversion and product distribution. When determining how best to run the FCC technology, several variables are considered. These include the reaction temperature, riser reactor, contact time, crude oil vaporisation, hydrocarbon partial pressure, CTO ratio, crude oil composition, and naphtha recycling [1,19]. In the FCC, propylene production can be maximized by changing the operation conditions and adding additives containing a ZSM-5 catalyst. To maximise the production of light olefins from direct crude oil cracking via FCC, it is also imperative to review the basic limitations of conventional zeolite catalysts, olefins’ processing, and catalysis technologies. There are a number of important technical advantages to the FCC process compared to other processes, including its continuous operation and the heat supply it provides for hydrocarbon cracking. Some of the coke material formed during the cracking reactions deposits on the catalyst surface, causing the catalyst to rapidly deactivate. It is relatively easy to burn off the deposited coke in the regenerator, continuously reactivating the catalyst.
2.1. Contact Time
In the FCC reactor, crude oil catalytically cracks into different products once it comes into contact with the hot, zeolite-based catalysts. An illustration of the typical catalytic cracking of hydrocarbons and the pathway to olefin production is shown in Figure 2. Figure 2a is long chain hydrocarbons that crack into straight and branched chain alkanes and alkenes, while Figure 2b is zeolite Y with high Si/Al ratio indicating more active and stable sites for hydrocarbon cracking. When discussing the FCC riser reactor, the term “contact time” refers to the amount of time that the oil vapour typically spends in close proximity to the catalyst. Therefore, it is worth analysing the influence of contact and residence time on product yield and distribution during direct crude oil cracking in FCC. This may be achieved by thinking about how long the reactants are in touch with one another. It is essential to avoid secondary reactions involving hydrogen transfer reactions by keeping the contact time short.
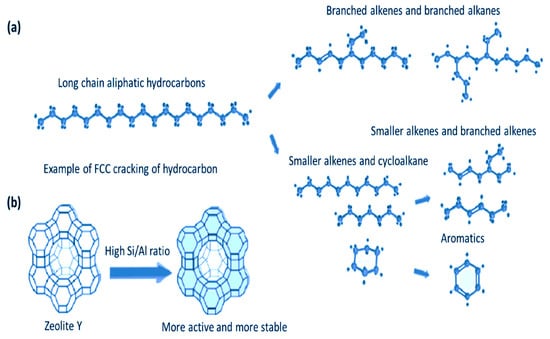
Figure 2.
Typical catalytic cracking of hydrocarbons once in contact with the hot catalyst in the FCC reactor [25].
Overcracking will also be controlled through reactions with short contact durations. Intermediate products of zeolite of crude oil catalytic cracking in FCC through the carbonium ion mechanism include light olefins such as butylene and propylene. These light olefins may easily undergo secondary processes such as hydrogen transfer, cracking, and aromatization [26]. It is, therefore, critical to optimize the contact time between the crude oil and catalyst to maximize these light olefin yields. The formation of C1–C4 gaseous products, the reduction in naphtha content, and the heavy fraction of the crude oil are both proportional to the contact time [12]. It was found that the heavy fraction of the crude oil was primarily converted to naphtha during the shortest residence time of 1 s. Thus, beyond 1 s contact time, the cracking of both primary and secondary naphtha fractions produces gaseous products in most cases [12]. According to the experimental data, the propylene yield increase became more significant as the contact time increased (from 1 to 10 s) than the ethylene yield increase when light crude oil was cracked over USY zeolite and MFI zeolite catalysts in the temperature range from 500 °C to 575 °C [12]. By increasing the contact times of the oil and catalyst, the catalytic cracking of large hydrocarbons becomes more thorough, which, in turn, increases the extent of cracking.
2.2. Effect of Temperature
Light olefins, naphtha, and other cracked products can be studied to determine how reaction temperatures affect conversion and yields. Thermal cracking rates and catalytic cracking increase with an increase in the FCC unit reaction temperature, leading to deeper cracking and a greater yield of light products. In an FCC conversion unit, the reaction temperature determines between catalytic cracking and thermal cracking to produce light olefins from crude oil. When temperatures rise over a certain point, catalytic cracking mechanisms become less active, while thermal cracking processes become more prevalent. As a result, FCC relies on two distinct mechanisms for its catalytic cracking reactions: carbonium ion and free radicals [26,27]. The proportional contributions of the two cracking mechanisms are currently unclear. Thermal cracking causes bond fission across multiple bonds between carbons, which ultimately results in the production of free radicals, which, in turn, leads to the production of ethylene through beta-scission reactions [5]. Thermal cracking predominantly promotes the production of ethylene, while catalytic cracking enhances the output of propylene. Therefore, through the process of catalysing the β-scission of long chain alkanes, propylene can be generated, leading to the formation of short-chain hydrocarbons. It is significant that the zeolite catalysts, possessing dual acidic sites, significantly influence the yield, distribution, and selectivity of products through the involvement of both the carbenium ion mechanism and the free radical mechanism. Elevated reaction temperatures were found to enhance the conversion of crude oil and the production of C2–C4 light olefins, liquefied petroleum gas (LPG), dry gas, and coke, while reducing the yields of naphtha, light-cycle oil (LCO), and heavy-cycle oil (HCO) in both thermal and catalytic cracking processes [23]. Temperature-related thermal cracking occurs at high temperatures, resulting in high dry-gas yields, which is an indication that pyrolytic cracking greatly contributes to this [5]. With an increased temperature, the yields of coke and dry gas will increase, since they are end products of the catalytic cracking of crude oil. However, LPG and light fractions (gasoline, diesel oil, etc.) which are intermediate products, decline after attaining optimum yields. Their decrease can be attributed to secondary reactions.
Thus, overcracking occurs during the catalytic cracking of crude oil due to the higher temperatures and longer reaction times that occur during this process. Secondary cracking is essential for light olefin production, because the fractions of FCC naphtha that are produced are also subject to secondary cracking. However, the operating conditions of the FCC must be balanced with the deactivation of the catalyst. In general, complete hydrocarbon conversion, with a boiling point of 343 °C or above, rose as the temperature increased [28]. It has been shown that, in addition to the yields of butylene, propylene, and overall light olefins passing through the maxima between 640 °C and 700 °C, the selectivity of total light olefins also becomes optimal in this temperature range [26]. Accordingly, optimization studies are necessary to determine the temperature at which light olefin yields can be maximized in direct crude oil FCC cracking. It has been observed that direct crude oil cracking over E-Cat caused the dry gas yield to rise from 1.9 to 13.7 wt% at a temperature range of from 550 to 650 degrees Celsius, owing to increases in ethylene, methane, and ethane yields [5]. Thermal cracking is the predominant mechanism responsible for the production of ethylene, with a limited occurrence of secondary reactions as it approaches the end product state. Consequently, increasing the temperature of the reaction would result in an increase in the production of ethylene, which is considered the most basic component of the light olefins. As the temperature rises above 716 °C, the ethylene yield approaches and exceeds the propylene yield [26]. Both an increase in the reaction temperature and the extension of the contact time have the potential to speed up the occurrence of secondary reactions involving intermediate products. The production of light olefins, specifically propylene and ethylene, is a key objective in the catalytic cracking of crude oil through the employment of an FCC unit. According to available reports, it has been observed that catalytic cracking becomes the dominant process at lower temperatures within the range of 500–550 °C. This particular phenomenon leads to a comparatively higher yield of propylene in comparison to ethylene [12], while thermal cracking alone resulted in a greater ethylene output [8]. According to the experimental data, the temperature range of 500–680 °C is identified as the optimal cracking temperature for the direct conversion of crude oil, as it enables the attainment of maximum yields of light olefins while minimising energy consumption.
2.3. Catalyst-to-Oil Ratio
The FCC riser is a complex reactor with multivariable interactions of hydrodynamics, mass and heat transfer, reactions, and operational limitations, making process modelling and simulation challenging [29]. Additionally, the yield of light olefins is influenced by the cracking temperature, catalyst-to-oil (CTO) ratio, residence time, characteristics of feedstock, and catalyst properties. It uses a finely crushed solid cracking zeolite catalyst with an average particle size of between 60 and 75 µm. The catalyst behaves as a fluid moving in a closed flow loop between the riser reactor and the regenerator when thoroughly mixed with crude oil droplets. Hot catalyst particles make contact with the sprayed crude oil in the riser reactor, induce the necessary cracking reactions, and deposit coke on the catalyst. Therefore, changing the catalyst mass flow rate changes the CTO ratio at a constant oil flow rate, which requires optimization to maximize light olefin gas yields from direct crude oil cracking in FCC. The mass flow rates of catalyst and crude oil are normally within the standard range of an industrial FCC unit, with a CTO ratio of 4:1–10:1 according to weight [29,30].
The study aims to investigate the impact of the CTO weight ratio on the production of light olefins via direct crude oil cracking in an FCC reactor by varying the mass of the catalyst that is fed into the reactor. Direct crude oil cracking in FCC encompasses the occurrence of catalytic cracking on zeolite catalyst surfaces, as well as thermal cracking processes on particle surfaces, and between catalyst particles and the reactor wall. Using equilibrium FCC catalysts (E-Cats) with ZSM-5 additives, catalytic cracking tests were conducted over 30 s, with catalyst-to-oil ratios ranging from 2 to 6 g/g. When the CTO ratio increased from 2 to 6 g/g, the production rates of C2–C4 light olefins, coke, LPG, and dry gas went up. However, the yields of naphtha, LCO and HCO decreased [23]. An increase in catalyst active sites per unit mass is likely to be responsible for the observed increase, as well as the increased contact between oil and catalyst with the increasing catalyst-to-oil ratio [31]. This suggests that catalytic cracking is more selective to the production of light olefin from the cracking of direct crude oil than thermal cracking alone. However, when the catalyst loading increases, catalytic cracking becomes more noticeable, and the impact of thermal cracking is reduced. Increasing the CTO ratio increases the catalytic cracking rate and the output of light olefins, particularly propylene [18]. This reduces the amount of coke that can be produced. Light fractions are often produced at the cost of the cracking fractions, which are heavy yielding gases, with coke as a by-product. The light olefin yields for catalytic cracking of heavy crude oil have been shown to marginally improve with increasing CTO weight ratios in experimental tests [26]. Reports show that utilising an MAT unit, an equilibrium FCC catalyst (E-cat), and a commercial MFI catalyst at a temperature of 650 °C enhances the conversion of AL crude oil from 1 to 6 g/g of CTO [8]. As a result, a rise in CTO often results in a more suitable environment for the generation of light olefins. It is important to keep in mind that the CTO ratio is established according to the restrictions imposed by the thermal balance of the FCC unit [24]. In most of the reviewed studies, the CTO ratio was in the range of 4:1–10:1, which is typical for industrial FCC units. Although increasing the CTO ratios increases conversion, doing so is difficult since this ratio is not an independent variable in an industrial FCC unit.
2.4. Characteristics of the Crude Oil
Crude oil can be classified into super-light oil, conventional light oil, medium oil, heavy oil, and extra heavy oil based on its API gravity [27]. The naphtha fraction significantly decreases as the crude oil becomes heavier [23]. As a result, the ability to manufacture light olefins may be limited by the amount of light fraction accessible from the crude oil. The heavy crude oils have high proportion of aromatic hydrocarbons, which gave them a low hydrogen content, making conversion difficult at typical FCC residence times. It has been reported that, as the crude oil’s H/C mole ratio increases and the aromatic hydrocarbon content decreases, the yields of light olefins increase [23,26]. Consequently, the propensity of the crude oil to produce coke and deactivate the catalyst increases as the crude oil becomes heavier [32]. The amount of Conradson carbon becomes coke; thus, as the Conradson carbon of the crude oil increases, it reduces the yield of light olefins that can be produced from directly cracking crude oil in FCC [24]. Despite this, there is little difference between ethylene yields for different crude oil feedstocks [26]. On the other hand, crude oils that contain lots of hydrogen donors, such as naphthene, also exhibit higher hydrogen transfer rates. Because of the possible hydrogenation to comparable paraffin, light olefin yields are anticipated to decrease. The production of light olefins from the following crude oils has been reported in the literature: Arab super-light crude oil, Arab extra-light crude oil, and Arab light crude oil with a characteristic API gravity of 51.3°, 39.3°, and 34°, and heavy oil (343 °C+) fraction of 27 wt%, 38 wt%, and 42 wt%, 2, respectively, was studied over E-cat at 550 °C [9]. The results showed that the yield of light olefins (C2–C4) increases as the crude oil heavy oil (343 °C+) fraction increases, while the naphtha and middle distillate fractions decrease.
A review of the direct production of olefin via the thermal and catalytic cracking of hydrocarbons has been published in the literature [33]. Olefins are aliphatic hydrocarbons with a C=C double bond and a general formula of CnH2n. They can be produced from an array of feedstocks, such as liquefied petroleum gas (LPG), light and heavy naphtha, gasoil, vacuum gas oils (VGOs), and directly from crude oil through either thermal or catalytic cracking. Furthermore, bio-derived olefins have been reported following the catalytic pyrolysis of biomass. With the growing awareness of climate change, a future research direction could be olefin production from biomass, which is a carbon-neutral feedstock, unlike fossil crude oil. In the refinery, the majority of the light olefins come from FCC, with minor contribution from the coking process, which is a thermal cracking method. On the other hand, a study demonstrated the production of light olefins from bio-oil, which is a product of biomass pyrolysis, through selective catalytic cracking using the La-modified HZSM-5 catalyst [34]. The maximum yield of light olefin observed from the catalytic cracking was about 0.28 kg olefins/kg bio-oil. Adding La to the zeolite catalyst (HZSM-5) was found to alter the acidity and strength of the acid sites, while tuning the acid sites by increasing the strength of the medium acid site improved the selectivity toward light olefin production and suppressed coke formation (which is a sign of reduced catalyst deactivation).
2.5. Hydrogen Transfer Index (HTI)
The hydrogen transfer reactions in the FCC process consume light olefins. As a result, the number of light olefins produced during direct crude oil cracking in an FCC unit could change based on the presence or absence of hydrogen transfer. Indications of the FCC catalyst’s hydrogen transfer activity from crude oil composition can be obtained using the hydrogen transfer index (HTI). To quantify the extent of the hydrogen transfer process, we may define an HTI by dividing the sum of C2–C4 paraffins by the amount of C2–C4 olefins in the produced gas. While olefins undergo hydrogenation to become paraffins, naphthenes receive hydrogen from them to transform into aromatic hydrocarbons. Also, higher rates of hydrogen transfer occur in crude oils containing a lot of hydrogen donors, such as naphthenes. In other words, HTI is a function of catalyst and feedstock composition. This occurrence of this phenomenon causes a drop in light olefin production. The longer contact/residence periods and greater back-mixing in the FCC unit increase the frequency of hydrogen transfer reactions. For crude oil cracking over MFI-zeolite, it has been shown that the HTI drops as the reaction temperature rises from 500 °C to 575 °C [12]. The HTI decreased in the following order for the zeolite catalysts that were studied: USY > Beta > MCM-22 > ZSM-5, based on the experimental data [24]. Several strategies can be used to modify catalyst qualities in order to decrease the hydrogen transfer processes. To minimise hydrogen transport, it is crucial to optimise the optimal density and dispersion of zeolite acid sites. Table 1 summarises the findings of the published research on the topic of crude oil’s conversion into light olefins, including details on the catalysts that were used and the operation conditions that yielded the desired results. In order to limit the hydrogen transfer activity of Zeolite Y, this is dealuminated [35]. In this approach, the tendency for FCC catalyst deactivation due to coke is also reduced [36].

Table 1.
Selected crude oil feedstocks, catalyst used, and achieved results.
The direct production of petrochemical feedstock such as light olefins (e.g., ethylene, propylene, butenes, butadiene) via cracking crude oil could be more economical than the steam cracking of ethane and gasoil. The direct cracking of crude oil into light olefin struggles with coking issues and catalyst deactivation. Corma et al. [41] modelled the direct thermal cracking of crude oil into light olefins and aromatics using an inert solid as a heat carrier, providing heat for feed vaporization and thermal cracking at a temperature range of 650–725 °C and residence time range 0.3–5 s. The result revealed that the solid-to-oil ratio had a negligible impact on the yield of light olefins. This observation reinforced the critical role played by the catalyst, especially zeolite, in improving the selectivity of the cracking process toward light olefins. The Py-GC/MS and fixed-bed reactor were used to directly investigate the catalytic pyrolysis of heavy crude oil, using calcium aluminate base catalysts and ZSM-5 acid zeolites to elucidate irs synergetic effect on the production of light olefins (C2–C4) and aromatics [42]. It was found that ZSM-5 with a Si/Al ratio of 40 produced a maximum selectivity of 65% toward light olefins, demonstrating a superior performance to zeolite catalysts with Si/Al ratios of 80 and 200. The inclusion of calcium aluminate 30 wt% in ZSM-5 further increased the selectivity toward light olefins in the range of 72–80%. This suggests that, in the design and modification of zeolite Y for the direct cracking of crude oil into light olefins via FCC technology, the ratio of Si/Al, and the acid–base catalyst coupling could play a significant role in improving the yield of light olefins.
3. Catalysts Modification
In recent years, refiners’ primary goal has been to maximize the production of petrochemical feedstocks at the expense of fuel [19,21,43]. The catalyst formulation and composition are crucial in this conversion [22]. In FCC technology, the process of selecting and constructing an appropriate catalyst for crude oil cracking into light olefins is an important part of the conversion of crude oil to light olefins from crude oil. Two properties of zeolites lead to their inclusion in the initial preparation of active material FCC catalysts: (1) they are cation exchangers, enabling different cations to be introduced, with differing catalytic properties; and (2) their pores have the same dimensions as simple molecules. As a result of their molecular sieving properties, they have the ability to induce shape and size selectivity relative to specific pores, which have a direct impact on the reactions, controlling reactant, and product accessibility. Figure 3 shows some selected zeolite 3D framework structures that are commonly modified and used in FCC technology and other catalytic reactions. Zeolite Socony Mobil-5 (ZSM-5, SiO2/Al2O3 = 30), which is a high-silica zeolite, was developed in 1972 by Mobil Oil Company with a micropore size in the range of from 0.3 nm to 1.2 nm. The MOR represents a mordenite zeolite structure with one-dimensional channels, a uniform and small pore size, a high internal surface area, and SiO2/Al2O3 ratios ranging from 9 to 20. The intricate structure of beta zeolite is made up of the intergrowth of two different structures, known as polymorphs A and B, each of which has a three-dimensional network of 12-ring pores (see Figure 1). The large, generally spherical internal cavities of the Y zeolite (FAU type) are tetrahedrally linked by pore apertures of approximately 0.8 nm and are defined by rings of twelve oxygen atoms. Typical zeolite Y is the core catalytic material for FCC; it contains aluminium, silicon, and oxygen within its regular structure, and tis a 3D, microporous, crystalline solid [21,22,44]. A zeolite catalyst is an aluminosilicate mineral consisting of an interconnected tetrahedra of alumina (AlO4) and silica (SiO4).
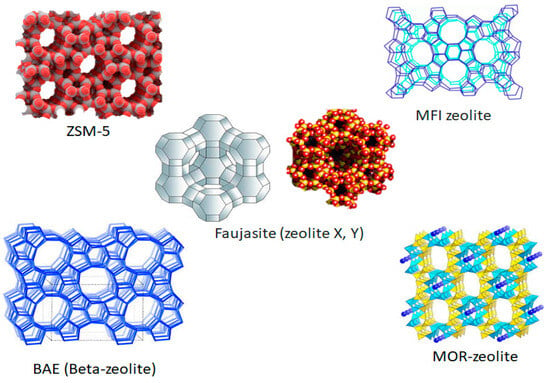
Figure 3.
Some selected zeolite 3D framework structures [21,22,44].
One of the critical variables to consider is the catalyst that is responsible for cracking crude oil in FCC to preferentially boost the production of light olefins. The cracking catalyst makes FCC units flexible. ZSM-5 is the second commonly utilised zeolite in FCC-catalytic cracking, after USY. The USY zeolite is the most common active FCC catalyst component that is necessary for cracking VGO and HGO into gasoline-range hydrocarbons, while ZSM-5, based on its pore size, induces shape selectivity for the conversion of naphtha fractions into light olefins. The schematic in Figure 4 shows the composition of a typical FCC catalyst and the cracking activities that occur when it interacts with crude oil.
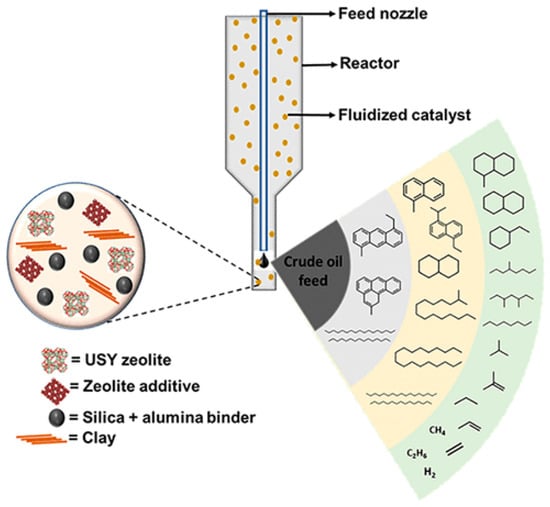
Figure 4.
Schematic diagram of the FCC catalyst’s composition and cracking activities [7].
The catalyst design presents considerable challenges when it comes to achieving maximum light olefins and conversion from a range of crude oil qualities. Therefore, the modification of the FCC catalyst for the cracking of direct crude oil will include zeolite component alterations, zeolite porosity tuning, and the inclusion of additives in the matrix materials. The hydrocarbon molecules in crude oil display a broad array of molecular weights and sizes, making zeolite catalyst pore-size-tuning a crucial factor for the successful conversion of crude oil into light olefins via FCC technology. Notably, ZSM-5 also functions as a molecular sieve. It is easy to modify the properties of zeolites in order to achieve the desired performance and selectivity. When compared to the traditional FCC catalyst, which produces about 4–6 wt% of propylene, the addition of ZSM-5 boosts propylene yield by around 1–5 wt%. There have been various modifications to the composition of ZSM-5 that resulted in improved yields of light olefins, particularly propylene and butene [1,7]. The active sites (valence electrons, acidity, strength, etc.), chemical composition, inclusion of particle size, phosphorus, rare-earth elements and pore size distribution, mechanical strength, and hydrothermal stability of the FCC catalyst all play a role in the catalyst’s capacity to optimise light olefins’ production [2,7]. By adjusting the mole ratio of the SiO2/Al2O3 components, it is possible to mechanically alter the density, acidity, and strength of the zeolite catalyst components, with the aim of promoting selectivity towards light olefins’ production, which implies that aromatic hydrocarbons yields could be favoured. However, the phosphorus stabilisation of the FCC catalyst has been demonstrated to increase not only the mechanical strength, attrition resistance, and hydrothermal stability, but also to minimise hydrogen transfer activity.
3.1. Zeolite Composite as an FCC Catalyst
To improve FCC catalyst performance, as well as maximising olefins’ production, silica and alumina can be added to zeolite Y or USY, or two different zeolites can be blended. The conventional USY catalyst was modified by the addition of MFI zeolites, which selectively crack naphtha to create light olefins [8]. For instance, when different types of crude oil feedstock were cracked, the selectivity to light olefins with a combination of ZSM-5 and E-Cat catalysts was demonstrated to be greater than when either ZSM-5 or E-Cat was used alone as the catalyst, in both fixed, fluidized-bed, advanced catalytic evaluation (ACE) and MAT units [23]. The composite nature of the catalyst suggests that the E-Cat medium-pore-size zeolite successfully cracked the heavy hydrocarbons in different crude oils into a range of gasoline hydrocarbons, which were then selectively cracked by the small-pored ZSM-5 zeolite component into light olefins. A commercially available additive based on ZSM-5 was used in the FCC process to improve the octane number of the gasoline. The yield of light olefins (such as propylene and butene) is increased when using it as an additive in the design of an FCC catalyst [1,7,24,45]. Zeolite ZSM-5 has an MFI-type crystal structure, and it has pores that are between 0.51 and 0.56 nm in diameter [1]. This explains why linear- and naphtha-range hydrocarbons are readily and selectively cracked into light olefins (C2–C4). In addition, when a ZSM-5 zeolite is added to an FCC catalyst, the naphtha fractions produced due to cracking can readily access its acid sites based on the pore size and hydrocarbon molecule size, resulting in the secondary cracking of naphtha fractions of crude oil into light olefins. It is for this reason that ZSM-5 leads to an increase in the yield of light olefins. The light olefins generated by this process are not consumed in significant quantities, despite their minimal hydrogen transfer activity [7], whereas hydrogen transfer reactions and bimolecular cracking are enhanced by the wide pores of the HY zeolite, leading to the manufacture of low-light olefins [24].
It has been proven that adding ZSM-5 (up to 25 wt%) to a fluidized catalytic cracking (FCC) catalyst can greatly increase the olefin yields of C3–C5, in addition to an increase in temperature [42,46]. The experimental results have shown that the addition of ZSM-5 to a zeolite Y FCC catalyst is an effective pathway to increasing the olefin production from hydrocarbon feedstocks. Thus, optimizing the incorporation of ZSM-5 into the design of the zeolite Y catalyst for the FCC of direct crude oil is another research direction toward maximizing light olefins’ production. This addition to the zeolite Y and modification of the pore size and structure architecture offer guidance in the development of next-generation catalysts for FCC, especially for the direct production of light olefins from crude oil.
3.2. Pore Size Modification
USY and ZSM-5 are zeolite-based catalysts used in the FCC process. As the principal active component, zeolite Y plays a major role in the gasoline yield, while ZSM-5 works as a propylene multiplier additive. In the catalytic cracking of crude oil through FCC, the pore size and topology of the zeolite are also crucial catalyst characteristics. A micropore provides high activity and selectivity towards light olefins, and influences the product distribution as well as shape-selectivity, while a mesopore provides better access to active sites for large hydrocarbon molecules [2,47]. In a nutshell, mesopores are more suitable for boiling point hydrocarbons, while the micropores are more accessible for hydrocarbon molecules in the gasoline range. As crude oil contains a wide variety of hydrocarbons, catalytic cracking will be difficult, since most catalytic reactions take place on the active sites or the zeolite acid, which tend to be located inside the cavities of zeolites or interior pores. Thus, micropores will confer mass transport limitations in the diffusion of large-molecular-weight species. Meanwhile, macropores and wider mesoporous zeolite cavities, due to their accessible exit pathways, can easily diffuse large molecular compounds, and can easily move out of zeolite cavities, which may prevent the hydrocarbons from staying in the zeolite cavity long enough for full cracking to occur [7]. Recently, hierarchical zeolites have been studied as catalysts possessing micro- and meso-porous frameworks to overcome mass transport limitations and improve the accessibility of the active site by large hydrocarbon molecules during the FCC process. Researchers have looked at large-pore zeolites like mordenite and beta and compared them to medium-pore ZSM-5 in an attempt to overcome the diffusion limitations [48]. It is possible to create hierarchical pore systems by incorporating an additional mesopore into microporous FCC catalysts. Various approaches can be followed to improve the zeolites’ pore characteristics in order to achieve selectivity toward light olefins to directly crack crude oil via FCC technology. The process of synthesizing hierarchical FCC zeolites includes two methods: bottom-up (e.g., hard templating and soft templating), and top–bottom (e.g., post-treatment of existing zeolites, such as dealumination and desilication) [48,49]. Porosity may be directly induced during the synthesis of a mesoporous zeolite using the bottom-up method. This is accomplished by introducing a templating agent to the blending of the zeolite precursors (for example, carbon or another substance). The zeolite framework is calcined at high temperatures to remove both the soft and hard templates. In the post-treatment approach, a variety of methods can be used to create mesoporosity in zeolite frameworks, including calcination, steaming (i.e., dealuminate), acid treatment (i.e., dealuminate), alkaline treatment (i.e., desilication), and chemical treatment [35,36,50]. The presence of high-acid/alkali solutions has been associated with the unpredictable distribution of pore size, as well as the collapse of the structure. It has been reported that mesopores and macropores can readily be created in ZSM-5 zeolites with lower SiO2/Al2O3 ratios (25–50) [51].The post-treatment strategy has significant benefits, such as increasing the hydrothermal and thermal stability of zeolite catalysts. Other advantages include the capacity to reduce waste and save money. In the catalytic cracking of crude oil via FCC, the introduction of an array of mesopores to zeolites may minimize the diffusion limitations, thereby improving the catalytic cracking efficiency and light olefins’ production. A detailed review has been published on hierarchical zeolites [49].
The crystal structure of the zeolite determines the sizes and shapes of the micropores and cavities [52]. As a result of the channels found in zeolites, molecules may diffuse differently, resulting in shape-selective catalysis and a visible difference in molecular transportation [53], despite the fact that these micropores confer exceptional catalytic activity and selectivity. This shape selectivity can be attributed to the zeolite shape and crystal size, resulting in micropores, particularly ZSM-5, whose pore size spans from 0.3 nm to 1.2 nm [54,55]. There is a probability that the zeolite catalyst is underutilised, and catalytic rates are reduced as a result of the micropore size and extended diffusion path length [44]. The design aspects of the hierarchical pore structure zeolites for catalytic applications aim to establish synthesis–property–structure–function performance relationships. In other words, the performance of a zeolite catalyst is a function of the synthesis method, property, and pore structure. Zeolites with hierarchical structures contain both mesopores and micropores.
3.3. Tuning Acidity
The physical, chemical, and textural properties of zeolite-based catalysts that promote their use in refining and fine chemical industries are their high activity, low propensity for coke formation, high organic nitrogen levels and high NH3 resistance, ease of regeneration, and molecular shape selectivity [56]. The distinctive shape selectivity, high intrinsic acidity, and high stability of zeolites make them an important heterogeneous catalyst in the chemical, oil refining, and petrochemical industries. The regular crystalline structure and acidity of zeolites are the distinctive properties responsible for their capacity to crack the C-C bonds of hydrocarbon molecules [57]. Thus, proper zeolite acidity adjustment and tuning are crucial for regulating its catalytic behaviour. Interestingly, the zeolite pore structure contains most of the active sites. There are two acid sites in the aluminosilicate zeolite structure: the Brønsted acid site and the Lewis acid site.
However, the number, strength, and distribution of acid sites within the pores of the zeolite catalyst are critical to the catalytic behaviour and the yield of olefin gas. The IR spectroscopy technique with adsorbed pyridine is a common approach to determining the acidity of catalysts and distinguishing between the two acidic surface sites [57]. the strength and number of acid sites of on Y zeolite catalysts can be determined using the temperature-programmed desorption (TPD) of ammonia methodologies [58]. When compared to the Lewis acid site, increasing the strength of the Brønsted acid sites enhances the cracking activity of the catalyst (the octane cracking rate constant increased from 33 s−1 (USY) to 102 s−1 (Na2H2-EDTA-treated USY)) [1,58,59]. This demonstrates that treating the USY zeolite catalyst with Na2H2-EDTA (ethylenediaminetetraacetic acid) increases the Brønsted acid strength and the catalytic activity. When combining zeolite USY/Y with ZSM-5-based additive modification to improve the light olefins’ yields, it is essential to take into account the acidity of the modified catalyst. An investigation was carried out in order to determine whether the ratio of SiO2 to Al2O3 has a substantial effect on the generation of light olefin. The density of both the Brönsted acid sites and the Lewis acid sites decreased as the SiO2/Al2O3 ratios increased from 33, 266, and 487 for the USY-based catalyst and ZSM-5 additive, respectively [60]. This suggests that the greater the SiO2/Al2O3 ratio (lower acidity), the less coke forms during the direct FCC cracking of crude oil, which is a trade-off for the increase in catalyst lifespan. Zeolites’ SiO2/Al2O3 ratio is discovered to impact the production of light olefins and catalytic performance [61]. The yields of light olefin gases are as follows: 45.94 wt%, 50.22 wt%, 41.93 wt%, 42.68 wt%, and 41.81 wt%, when the SiO2/Al2O3 ratio increases in the order of 32, 36, 38, 98, and 217 [61]. It has also been reported that changes in the Si/Al ratio in the FCC catalyst can affect the production of light olefins [24]. Hence, it is necessary to optimize the SiO2/Al2O3 ratio of the FCC catalyst regarding the yield of olefin. In the FCC catalyst, varying the Si/Al ratio changes the cracking rate. This is due to the fact that the SiO2/Al2O3 ratio is what determines the acid site strength, also known as its acidity. This is controlled using the amount of alumina (Al2O3). High acidity implies low Si/Al ratios. The SiO2/Al2O3 framework ratio in USY zeolite, a key active material in FCC catalysts, is about 5. The light olefins’ selectivity could be higher (around 3–8 wt%) in FCC catalysts with moderate acid sites, but the conversion rate may be lower (about 4–10%) because the reduction in acidity would restrict the secondary reaction between the primary products [24]. This is because the FCC catalyst’s cracking ability is embedded in its acidity. Research has provided evidence that an increased Si/Al ratio, indicating reduced acidity, leads to a reduction in coke formation. This reduction in coke formation has the potential to prolong the lifespan of the catalyst.
3.4. The Integration of Rare-Earth Metal, Alkali-Earth Metals and Transition Metals
The integration of phosphorus into the Al ion lattice stabilization and the basicity of the zeolite can be modified by incorporating a rare-earth metal; similarly, the addition of alkali earth decreases the intensity of the acid sites, and, consequently, new Lewis acid sites are created by including transition metals in the zeolite-blend catalysts. The introduction of these dopants or promoters has been demonstrated to increase the olefin selectivity in crude oil cracking via FCC [7]. By using modified HZSM-5 catalysts with alternating Si/Al2 ratios and alkaline treatment, light olefin yields were increased when Mn and alkaline treatment were applied as additives in FCC catalysts [62]. The high yield of light olefins is achieved due to the reduced re-adsorption of butene, ethylene, and propylene, which are fundamental compounds, as a consequence of the incorporation of rare-earth elements [24]. Conventionally, the yield of light olefins from the FCC cracking of crude ranges from 10 to 15 wt% of the feedstock; however, with the proper modification of the catalyst and optimal operating conditions, the FCC yield of light olefin could be further enhanced to 25–30 wt% [63]. It is for this reason that FCC catalysts have been modified, and their processes have been optimized to maximize light olefin yield from direct crude oil cracking. These factors could be extensively studied through simulation to obtain optimal FCC factors that maximize olefin production. As a result, it seems more plausible that adding rare-earth metals to a zeolite-mix FCC catalyst could improve selectivity (by about 6–10%) in the production of light olefins from crude oil cracking through an FCC unit [24]. The addition of rare-earth metals promotes selectivity toward olefin production. In 2011, a review article on the catalytic cracking of several industrial feedstocks using ZSM-5 zeolites that are modified for the manufacturing of light olefins, including heavy hydrocarbons and ethane, was published [63]. The study investigates the effects of common boosters, such as phosphorus, rare-earth elements, alkali and alkaline earths (Pr, Nd, Sm, Eu, La, Gd, Ce, etc.), transition metals (such as Ag, Fe, Cu, Zn, and Cr), and rare-earth elements on the chemical properties of modified ZSM-5, and their effectiveness in enhancing light olefin selectivity. Similar to this, the literature has studied and reported on the functions of the addition of rare-earth metals to the zeolite catalyst employed in FCC [64].
4. Kinetic Modelling
An understanding of the reaction mechanism and development of the kinetic model of the direct catalytic cracking of crude oil in the FCC is critical in process technology development, catalyst design, and process factors’ optimization. With the large amount of data that are available today, simulation would play a crucial role in achieving these objectives in an economic and timely manner.
Models of kinetic reactions are useful for evaluating the significance of various reactions for process simulation, development, and design. There are thousands of hydrocarbon species in crude oils, each with a wide boiling temperature distribution. The kinetic model is simplified by grouping hydrocarbons into crude oil and products based on their boiling points, as the use of individual component equations would be complex. As a consequence of this, the kinetic modelling of crude oil catalytic cracking may be accomplished by utilising a technique known as lumping. Using this method, hydrocarbon species that have a comparable boiling range and comparable characteristics are grouped together, which results in a smaller number of “pseudo” species. As part of the process simulation and development, kinetic modelling is important as it provides a quantitative assessment of several reactions being undertaken by the lumps within the crude oil. Using a riser simulator reactor, Al-Khattaf and Ali studied the kinetics of Arab super-light crude oil, utilising the simplest crude oil cracking model, a three-lump model that includes heavy fraction, C1–C4 gaseous products, and naphtha [12]. Based on the lumping strategy, the crude-oil-heavy fraction contains hydrocarbons with boiling temperatures greater than 220 °C, naphtha contains hydrocarbons between C5 and 220 °C, and the gas lump contains C1–C4 gases, including light olefins like butene, ethylene, and propylene, as shown in Figure 5a. The reaction network ignores coke production since its rate is low. Another piece of research used a four-lump model to represent the catalytic cracking of Arab light crude oil into naphtha and light olefins [9]. The crude oil four lumps are heavy-cycle oil (HCO) plus light-cycle oil (LCO), gas, naphtha, and coke. In the four-lump model, naphtha is generated from the cracking of (LCO + HCO), the catalytic cracking of LCO + HCO also resulted in the formation of coke and gas, while the cracking of naphtha only leads to the formation of gas, as shown in Figure 5b. The direct catalytic cracking of crude oil to chemicals was simulated using a four-lump kinetic model, as depicted in Figure 5c [65]. In the model, the process of cracking crude oil resulted in the production of several distinct products. These products include heavy oil, which refers to the heavier fraction obtained from the cracking process. Additionally, light oil was produced, consisting of lighter fractions, such as alkanes, diesel, gasoline, and coke. Furthermore, the cracking process yielded olefins and aromatic products, specifically propylene, ethylene, butylene, xylene, benzene, and toluene.
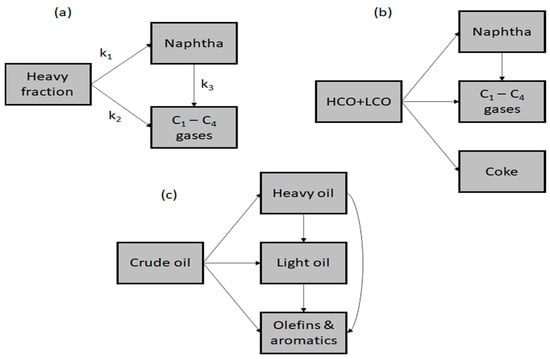
Figure 5.
Selected lump kinetic model for direct crude oil cracking [9,12,65].
In the kinetic equations, the catalyst deactivation can be accounted for using the deactivation function. The catalyst deactivation function, is defined by Equation (1) [66,67,68,69]. The ratio of deactivation rate to that of a fresh catalyst is shown in Equation (2), while the rate of disappearance or formation of any lump is given by Equation (3).
where denotes the catalyst deactivation function, denotes the decay order, denotes the kinetic deactivation constant, may be either TOS or COC, V denotes the reactor volume, WC denotes the catalyst weight, C denotes the molar concentration of the lump fraction, n denotes the number of lumps, and t denotes the time. Subscript 0 represents the reaction rate on a fresh catalyst, suggesting that the presence of coke (c) controls both the rate of the initial reaction (r0) and the rate of the subsequent production of coke itself (rc).
5. Conclusions
At present, the refining industry is primarily focused on fuel production, alongside a small amount of by-products, such as petrochemical feedstocks, especially propylene. Due to the increase in demand in the light olefins market, a potential approach is to directly produce petrochemical building blocks, such as ethylene, propylene, and butene, from crude oil through FCC technology. This method has emerged to meet the growing demands. As a result of the design and development of an active and appropriate pore structure of a modified zeolite catalyst, light olefins can be produced with greater selectivity from the direct cracking of crude oil in FCC. An overview of the zeolite catalyst modification techniques is presented in the following categories: zeolite blend aspects, modifications to the catalyst pore size and structure to improve selectivity, the incorporation of other elements, and the modulation of acidity. Regarding the production of light olefins through crude oil cracking, ZSM-5 has the desired shape selectivity, while zeolite USY is the zeolite that is most commonly used to optimize the cracking hydrocarbon blend. The work also explored and highlighted the key process factors that require optimization to maximize light olefins’ production. However, there is need to conduct a technoeconomic analysis of the concept of direct crude-oil-to-light-olefins conversion. A life-cycle assessment method can be used to compare the direct catalytic cracking process via FCC to convert crude oil light olefins based on its economic, social, and environmental performance. This suggests that process modelling and simulation could prove significant in achieving the optimum process operation variables that maximize light olefins’ yield in the direct conversion of crude oil to olefins using FCC technology. This overview combined several viewpoints, resulting in the synthesis of future research aiming to optimise light olefin yields through engineering zeolite catalyst pore structures, reducing deactivation through dopant addition, and conducting a technoeconomic analysis of crude oil cracking for light olefin production. Likewise, improving the pore structure to enhance accessibility, controlling the acid site strength, and diminishing the diffusional constraints for hydrocarbon macromolecules could increase the activity and selectivity of the zeolite-based catalyst, as well as increasing the yield of light olefins. If the right catalyst is developed and the reactor configuration is selected, it is possible to directly convert crude oil into light olefins. Thus, it is still possible to improve the catalyst’s performance and selectivity through the optimization of catalyst formulation and pore structure. Thus, the direct transformation of crude oil to light olefins via a one-step process is possible when the right catalyst and reactor configuration are developed.
Author Contributions
Conceptualization, R.E.E., R.P., I.M.M. and Y.M.J.; methodology, R.E.E. and Y.M.J.; software, R.E.E.; validation, R.E.E. and Y.M.J.; formal analysis, R.E.E., R.P., I.M.M. and Y.M.J.; investigation, R.E.E. and Y.M.J.; resources, R.E.E., R.P., I.M.M. and Y.M.J.; data curation, R.E.E.; writing—original draft preparation, R.E.E., writing—review and editing, R.E.E., R.P., I.M.M. and Y.M.J.; visualization, R.E.E.; supervision, R.P., I.M.M. and Y.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The main author, Ruth Ekperiware Emberru (R.E.E), expresses gratitude to the Tertiary Education Trust Fund (TETFund) of Nigeria for providing funding for her PhD research studies.
Conflicts of Interest
There is no conflict of interest regarding the publication.
References
- Suganuma, S.; Katada, N. Innovation of catalytic technology for upgrading of crude oil in petroleum refinery. Fuel Process. Technol. 2020, 208, 106518. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Ihli, J.; Jacob, R.R.; Holler, M.; Guizar-Sicairos, M.; Diaz, A.; da Silva, J.C.; Sanchez, D.F.; Krumeich, F.; Grolimund, D.; Taddei, M.; et al. A three-dimensional view of structural changes caused by deactivation of fluid catalytic cracking catalysts. Nat. Commun. 2017, 8, 809. [Google Scholar] [CrossRef] [PubMed]
- IEA. Oil Market Report; IEA: Paris, France, 2023. [Google Scholar]
- Al-Khattaf, S.; Saeed, M.R.; Aitani, A.; Klein, M.T. Catalytic Cracking of Light Crude Oil to Light Olefins and Naphtha over E-Cat and MFI: Microactivity Test versus Advanced Cracking Evaluation and the Effect of High Reaction Temperature. Energy Fuels 2018, 32, 6189–6199. [Google Scholar] [CrossRef]
- Zion Market Research Olefins Market Size, Share, Growth Report 2030. Available online: https://www.zionmarketresearch.com/report/olefins-market (accessed on 19 June 2023).
- Tanimu, A.; Tanimu, G.; Alasiri, H.; Aitani, A. Catalytic Cracking of Crude Oil: Mini Review of Catalyst Formulations for Enhanced Selectivity to Light Olefins. Energy Fuels 2022, 36, 5152–5166. [Google Scholar] [CrossRef]
- Al-Absi, A.A.; Al-Khattaf, S.S. Conversion of Arabian Light Crude Oil to Light Olefins via Catalytic and Thermal Cracking. Energy Fuels 2018, 32, 8705–8714. [Google Scholar] [CrossRef]
- Usman, A.; Siddiqui, M.A.B.; Hussain, A.; Aitani, A.; Al-Khattaf, S. Catalytic cracking of crude oil to light olefins and naphtha: Experimental and kinetic modeling. Chem. Eng. Res. Des. 2017, 120, 121–137. [Google Scholar] [CrossRef]
- Corma, A.; Corresa, E.; Mathieu, Y.; Sauvanaud, L.; Al-Bogami, S.; Al-Ghrami, M.S.; Bourane, A. Crude oil to chemicals: Light olefins from crude oil. Catal. Sci. Technol. 2017, 7, 12–46. [Google Scholar] [CrossRef]
- Krumeich, F.; Ihli, J.; Shu, Y.; Cheng, W.; Bokhoven, J.A. Van Structural Changes in Deactivated Fluid Catalytic Cracking Catalysts Determined by Electron Microscopy. ACS Catal. 2018, 8, 4591–4599. [Google Scholar] [CrossRef]
- Al-Khattaf, S.S.; Ali, S.A. Catalytic Cracking of Arab Super Light Crude Oil to Light Olefins: An Experimental and Kinetic Study. Energy Fuels 2018, 32, 2234–2244. [Google Scholar] [CrossRef]
- Louisville, K. Global Olefins Market Size Expected to Acquire USD 348 Billion by 2030, At a CAGR of 4.76%. 2023. Available online: https://www.globenewswire.com/en/news-release/2023/4/17/2647686/0/en/Global-Olefins-Market-Size-Expected-to-Acquire-USD-348-Billion-by-2030-At-a-CAGR-of-4-76.html (accessed on 19 June 2023).
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Naik, D.V.; Karthik, V.; Kumar, V.; Prasad, B.; Garg, M.O. Kinetic modeling for catalytic cracking of pyrolysis oils with VGO in a FCC unit. Chem. Eng. Sci. 2017, 170, 790–798. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Lappas, A.A. Co-processing bio-oil in the refinery for drop-in biofuels via fluid catalytic cracking. WIREs Energy Environ. 2018, 7, e281. [Google Scholar] [CrossRef]
- Le-Phuc, N.; Tran, T.V.; Phan, T.T.; Ngo, P.T.; Luong, T.N. Efficient processing of crude oil using direct cracking at high temperatures over modified FCC catalysts. Pet. Sci. Technol. 2022, 41, 2391–2401. [Google Scholar] [CrossRef]
- Alotibi, M.F.; Alshammari, B.A.; Alotaibi, M.H.; Alotaibi, F.M.; Saeed, M.A.; Navarro, R.M.; Fierro, J.L.G. ZSM—5 Zeolite Based Additive in FCC Process: A Review on Modifications for Improving Propylene Production. Catal. Surv. Asia 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, Z.; Yan, H.; Feng, X.; Zhao, H.; Liu, Y.; Chen, X. Produce petrochemicals directly from crude oil catalytic cracking, a techno-economic analysis and life cycle society-environment assessment. J. Clean. Prod. 2021, 308, 127283. [Google Scholar] [CrossRef]
- Hussain, A.I.; Aitani, A.M.; Kubu, M. Catalytic cracking of Arabian Light VGO over novel zeolites as FCC catalyst additives for maximizing propylene yield. Fuel 2016, 167, 226–239. [Google Scholar] [CrossRef]
- Alabdullah, M.A.; Gomez, A.R.; Vittenet, J.; Bendjeriou-Sedjerari, A.; Xu, W.; Abba, I.A.; Gascon, J. A Viewpoint on the Refinery of the Future: Catalyst and Process Challenges. ACS Catal. 2020, 10, 8131–8140. [Google Scholar] [CrossRef]
- Alabdullah, M.A.; Shoinkhorova, T.; Dikhtiarenko, A.; Ould-chikh, S.; Rodriguez-gomez, A.; Chung, S.; Alahmadi, A.O.; Hita, I.; Pairis, S.; Hazemann, J.; et al. Understanding catalyst deactivation during the direct cracking of crude oil. Catal. Sci. Technol. 2022, 12, 5657–5670. [Google Scholar] [CrossRef]
- Al-absi, A.A.; Aitani, A.M.; Al-khattaf, S.S. Thermal and catalytic cracking of whole crude oils at high severity. J. Anal. Appl. Pyrolysis 2020, 145, 104705. [Google Scholar] [CrossRef]
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Appl. Petrochem. Res. 2015, 5, 377–392. [Google Scholar] [CrossRef]
- Miteva, A.; Stoyanova, V. Zeolites application in terrestrial and space industry—A review. Aerosp. Res. Bulg. 2020, 32, 209–223. [Google Scholar] [CrossRef]
- Meng, X.; Xu, C.; Gao, J.; Li, L. Studies on catalytic pyrolysis of heavy oils: Reaction behaviors and mechanistic pathways. Appl. Catal. A Gen. 2005, 294, 168–176. [Google Scholar] [CrossRef]
- Hart, A. Advanced Studies of Catalytic Upgrading of Heavy Oils. Ph.D Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Hart, A.; Shah, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of the CAPRI process for heavy oil upgrading: Effect of hydrogen and guard bed. Ind. Eng. Chem. Res. 2013, 52, 15394–15406. [Google Scholar] [CrossRef]
- John, Y.M.; Patel, R.; Mujtaba, I.M. Maximization of propylene in an industrial FCC unit. Appl. Petrochem. Res. 2018, 8, 79–95. [Google Scholar] [CrossRef]
- Sadeghbeigi, R. Fluid Catalytic Cracking Handbook: An Expert Guide to the Practical Operation, Design, and Optimization of FCC Units, 3rd ed.; Elsevier/BH: Amsterdam, The Netherlands; Boston, MA, USA, 2012; ISBN 978-0-12-386965-4. [Google Scholar]
- Hart, A.; Greaves, M.; Wood, J. A comparative study of fixed-bed and dispersed catalytic upgrading of heavy crude oil using-CAPRI. Chem. Eng. J. 2015, 282, 213–223. [Google Scholar] [CrossRef]
- Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Down-hole heavy crude oil upgrading by CAPRI: Effect of hydrogen and methane gases upon upgrading and coke formation. Fuel 2014, 119, 226–235. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of hydrocarbons for the production of olefins: A state-of-the-art review I: Thermal cracking review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Gong, F.; Yang, Z.; Hong, C.; Huang, W.; Ning, S.; Zhang, Z.; Xu, Y.; Li, Q. Selective conversion of bio-oil to light olefins: Controlling catalytic cracking for maximum olefins. Bioresour. Technol. 2011, 102, 9247–9254. [Google Scholar] [CrossRef]
- Agudelo, J.L.; Hensen, E.J.M.; Giraldo, S.A.; Hoyos, L.J. In fluence of steam-calcination and acid leaching treatment on the VGO hydrocracking performance of faujasite zeolite. Fuel Process Technol. 2015, 133, 89–96. [Google Scholar] [CrossRef]
- Talebian-kiakalaieh, A.; Tarighi, S. Synthesis of hierarchical Y and ZSM-5 zeolites using post-treatment approach to maximize catalytic cracking performance. J. Ind. Eng. Chem. 2020, 88, 167–177. [Google Scholar] [CrossRef]
- Alabdullah, M.; Rodriguez-Gomez, A.; Shoinkhorova, T.; Dikhtiarenko, A.; Chowdhury, A.D.; Hita, I.; Kulkarni, S.R.; Vittenet, J.; Sarathy, S.M.; Castaño, P.; et al. One-step conversion of crude oil to light olefins using a multi-zone reactor. Nat. Catal. 2021, 4, 233–241. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, P.; Wang, J.; Gao, H.; Guo, J.; Wang, J.; Wang, Y.; Tian, Y.; Qiao, Y. Catalytic dehydrogenation cracking of crude oil to light olefins by structure and basicity/acidity adjustment of bifunctional metal/acid catalysts. Fuel 2023, 334, 126808. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, P.; Li, J.; Wang, C.; Qiao, Y.; Tian, Y. Fundamental studies and pilot verification of an olefins/aromatics-rich chemical production from crude oil dehydrogenation catalytic pyrolysis process. Fuel 2022, 310, 122435. [Google Scholar] [CrossRef]
- Alotaibi, F.M.; González-Cortés, S.; Alotibi, M.F.; Xiao, T.; Al-Megren, H.; Yang, G.; Edwards, P.P. Enhancing the production of light olefins from heavy crude oils: Turning challenges into opportunities. Catal. Today 2018, 317, 86–98. [Google Scholar] [CrossRef]
- Corma, A.; Sauvanaud, L.; Mathieu, Y.; Al-Bogami, S.; Bourane, A.; Al-Ghrami, M. Direct crude oil cracking for producing chemicals: Thermal cracking modeling. Fuel 2018, 211, 726–736. [Google Scholar] [CrossRef]
- Niwamanya, N.; Zhang, J.; Barigye, A.; Gao, C.; Sekyere, D.T.; Sun, H.; Chen, Y.; Msale, L.O.; Tian, Y. Study on acid-base coupled catalytic pyrolysis of heavy crude oil to light olefins and aromatics by Py-GC/MS and fixed bed reactor. J. Anal. Appl. Pyrolysis 2023, 169, 105835. [Google Scholar] [CrossRef]
- Alabdullah, M.; Shoinkhorova, T.; Rodriguez-gomez, A.; Dikhtiarenko, A.; Vittenet, J.; Ali, O.S.; Morales-Osorio, I.; Xu, W.; Gascon, J. Composition-performance Relationships in Catalysts Formulation for the Direct Conversion of Crude Oil to Chemicals. ChemCatChem 2021, 13, 1806–1813. [Google Scholar] [CrossRef]
- Bai, P.; Etim, U.J.; Yan, Z.; Mintova, S. Fluid catalytic cracking technology: Current status and recent discoveries on catalyst contamination. Catal. Rev. 2019, 61, 333–405. [Google Scholar] [CrossRef]
- Degnan, T.F.; Chitnis, G.K.; Schipper, P.H. History of ZSM-5 fluid catalytic cracking additive development at Mobil. Microporous Mesoporous Mater. 2000, 35–36, 245–252. [Google Scholar] [CrossRef]
- Buchanan, J.S. The chemistry of olefins production by ZSM-5 addition to catalytic cracking units. Catal. Today 2000, 55, 207–212. [Google Scholar] [CrossRef]
- Zheng, Q.; Huo, L.; Li, H.; Mi, S.; Li, X.; Zhu, X.; Deng, X.; Shen, B. Exploring structural features of USY zeolite in the catalytic cracking of Jatropha curcas L. seed oil towards higher gasoline / diesel yield and lower CO2 emission. Fuel 2017, 202, 563–571. [Google Scholar] [CrossRef]
- Zhao, S.; Collins, D.; Wang, L.; Huang, J. Influence of ZSM-5 porosity and binder introduction on the coke formation in the cracking of 1, 3, 5-triisopropylbenzene. Catal. Today 2021, 368, 211–216. [Google Scholar] [CrossRef]
- Čejka, J.; Millini, R.; Opanasenko, M.; Serrano, D.P.; Roth, W.J. Advances and challenges in zeolite synthesis and catalysis. Catal. Today 2020, 345, 2–13. [Google Scholar] [CrossRef]
- Agudelo, J.L.; Hensen, E.J.M.; Giraldo, S.A.; Hoyos, L.J. Effect of USY Zeolite Chemical Treatment with Ammonium Nitrate on Its VGO Hydrocracking Performance. Energy Fuels 2016, 30, 616–625. [Google Scholar] [CrossRef]
- Aloise, A.; Marino, A.; Dalena, F.; Giorgianni, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Bonura, G.; Frusteri, F.; Giordano, G. Desilicated ZSM-5 zeolite: Catalytic performances assessment in methanol to DME dehydration. Microporous Mesoporous Mater. 2020, 302, 110198. [Google Scholar] [CrossRef]
- Egeblad, K.; Christensen, C.H.; Kustova, M.; Christensen, C.H. Templating Mesoporous Zeolites. Chem. Mater. 2008, 20, 946–960. [Google Scholar] [CrossRef]
- Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Confinement in a Zeolite and Zeolite Catalysis. Acc. Chem. Res. 2021, 54, 2894–2904. [Google Scholar] [CrossRef]
- Van Donk, S.; Janssen, A.H.; Bitter, J.H.; De Jong, K.P. Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Mintova, S.; Grand, J.; Valtchev, V. Nanosized zeolites: Quo Vadis? C. R. Chim. 2016, 19, 183–191. [Google Scholar] [CrossRef]
- Maxwell, I.E. Zeolite catalysis in hydroprocessing technology. Catal. Today 1987, 1, 385–413. [Google Scholar] [CrossRef]
- Tonetto, G.; Atias, J.; De Lasa, H. FCC catalysts with different zeolite crystallite sizes: Acidity, structural properties and reactivity. Appl. Catal. A Gen. 2004, 270, 9–25. [Google Scholar] [CrossRef]
- Katada, N.; Kageyama, Y.; Takahara, K.; Kanai, T.; Ara Begum, H.; Niwa, M. Acidic property of modified ultra stable Y zeolite: Increase in catalytic activity for alkane cracking by treatment with ethylenediaminetetraacetic acid salt. J. Mol. Catal. A Chem. 2004, 211, 119–130. [Google Scholar] [CrossRef]
- Katada, N.; Nakata, S.; Kato, S.; Kanehashi, K.; Saito, K.; Niwa, M. Detection of active sites for paraffin cracking on USY zeolite by 27Al MQMAS NMR operated at high magnetic field 16 T. J. Mol. Catal. A Chem. 2005, 236, 239–245. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Jia, M.; Gao, X.; Yu, J. ZSM-5 zeolites with different SiO2/Al2O3 ratios as fluid catalytic cracking catalyst additives for residue cracking. Chin. J. Catal. 2015, 36, 806–812. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, J.; Xu, C.; Shen, B. Alkali-treatment of ZSM-5 zeolites with different SiO2/Al2O3 ratios and light olefin production by heavy oil cracking. Fuel Process. Technol. 2011, 92, 414–420. [Google Scholar] [CrossRef]
- Awayssa, O.; Aitani, A. Modified HZSM-5 as FCC additive for enhancing light olefins yield from catalytic cracking of VGO. Appl. Catal. A Gen. 2014, 477, 172–183. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Akah, A. Application of rare earths in fluid catalytic cracking: A review. J. Rare Earths 2017, 35, 941–956. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Jiao, N.; Gong, M.; Zhang, X. Simulation of Hydrodynamics of the Riser Reactor for Catalytic Cracking of Crude Oil to Chemicals. Ind. Eng. Chem. Res. 2023, 62, 5646–5657. [Google Scholar] [CrossRef]
- Corella, J.; Bilbao, R.; Molina, J.A.; Artigas, A. Variation with Time of the Mechanism, Observable Order, and Activation Energy of the Catalyst Deactivation by Coke in the FCC Process. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 625–636. [Google Scholar] [CrossRef]
- Corella, J.; Monzón, A. Modeling of the Deactivation Kinetics of Solid Catalysts by Two More Simultaneous and Different Causes. Ind. Eng. Chem. Res. 1988, 27, 369–374. [Google Scholar] [CrossRef]
- Fernandes, J.L.; Domingues, L.H.; Pinheiro, C.I.C.; Oliveira, N.M.C.; Ramôa, F. Influence of different catalyst deactivation models in a validated simulator of an industrial UOP FCC unit with high-efficiency regenerator. Fuel 2012, 97, 97–108. [Google Scholar] [CrossRef]
- Selalame, T.W.; Patel, R.; Mujtaba, I.M.; John, Y.M. A Review of Modelling of the FCC Unit—Part I: The Riser. Energies 2022, 15, 308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).