Abstract
Nizatidine is a gastroprotective drug with a short biological half-life and narrow absorption window. This study aimed at developing floating tablets of nizatidine using various HPMC viscosity grades, namely K4M, E4M, K15 and K200M. Directly compressed tablets revealed an excellent uniformity in hardness, thickness and weight and nizatidine was evenly distributed within the matrix floating tablets. Buoyancy study revealed floating lag time as low as 18–38 s, and tablets remain buoyant for upto 24 h. However, the later depended upon viscosity grade of HPMC and that the higher the viscosity, the less was the total floating time. In vitro dissolution indicated viscosity dependent nizatidine release from the floating tablets. HPMC K4M and E4M based floating tablets released almost 100% drug in 12 h, whilst higher viscosity polymers such as K15 and K200M only released 81.88% and 75.81% drug, respectively. The drug release followed non-Fickian diffusion from tablets formulated with K4M, K15 and K200M, whilst super case II transport was observed with E4M based tablets. More interestingly, K4M and E4M polymers have similar viscosity yet exhibited different drug release mechanism. This was attributed to the difference in degree of substitution of methoxyl- and hydroxypropoxyl- groups on polymer backbone.
Keywords:
buoyancy; dissolution; floating tablets; HPMC; nizatidine; substitution level; viscosity grades 1. Introduction
By far the most common route for drug administration is the oral route because of low-cost therapy, improved patient compliance and relatively less side effects [1]. However, dosage forms delivered orally may exit the stomach early due to faster gastric emptying rates, therefore, bioavailability of drugs may compromise because of low residence time [2]. An extended residence of drugs in the stomach is important if their local action is required, or drugs have poor solubility and stability in intestinal fluid, or the absorption of drugs occurs within a narrow region of gastrointestinal tract [3]. To counter these issues, gastroretentive drug delivery systems have been developed to prolong the gastric retention time of drugs [4,5]. Various types of gastroretentive systems have been proposed that include low- and high-density floating systems, bioadhesive floating systems, expandable and self-unfolding systems, and magnetic systems [6,7,8]. The low-density floating systems are advantageous owing to their low cost and easy manufacturing, and have the ability to remain buoyant in gastric media for an extended period of time [9].
Hydroxypropyl methylcellulose (HPMC) is the most common matrix-forming polymer used in formulating the low-density floating devices [6]. For example, Baumgartner et al. successfully developed floating matrix tablets of pentoxyfilline using HPMC K4M [3]. In another study, Shoufeng et al. prepared floating capsules with two viscosity grades of HPMC namely, K4M and K100LV, in combination with Carbopol 934P. This study revealed that low viscosity grade polymer (HPMC K100 LV) was more beneficial in formulating hydrodynamically controlled floating system as compared with relatively higher viscosity grade counterpart (K4M) [10]. Overall, effective floating of delivery system was achieved by swelling of HPMC along with the addition of gas generating agent. Several other studies have demonstrated the use of HPMC as matrix-forming polymer in formulating the floating gastroretentive systems of various drugs, including famotidine [11], dipyridamole [12], calcium chloride [13], berberine hydrochloride [14], and many more. Most of the studies utilised only one grade of HPMC, except from the study by Baumgartner et al. [3]; thus, a more rigorous study is warranted to investigate a more detailed role of viscosity grades in formulating floating tablets and subsequent dissolution of nizatidine from the dosage form.
Therefore, the present investigation was aimed at developing nizatidine floating tablets using different HPMC viscosity grades, namely K4M (3000–5600 cp), E4M (3000–5600 cp), K15 (11,250–21,000 cp) and K200M (150,000–258,000 cp) [15]. Nizatidine is a gastroprotective drug, belonging to H2-receptor antagonists, commonly administered for managing peptic ulcer and gastroesophageal reflux disorder [16]. Nizatidine is absorbed from the upper gastrointestinal tract for its local activity on parietal cells of gastric mucosa. It is susceptible to metabolism by colonic bacteria and has a relatively short biological half-life of 2 h with rapid clearance, which makes it a suitable candidate for floating sustained release system [17].
2. Materials and Methods
2.1. Materials
Strides Pharma Science Limited (Formerly Strides Shasun Limited, Bangalore, India) provided nizatidine as a gift sample. Colorcon UK generously gifted HPMC K4M, HPMC E4M, HPMC K15 and HPMC K200M. All other tableting excipient including carbopol 934P, sodium bicarbonate, magnesium stearate and lactose were of research grade and were sourced from BDH Laboratory (UK). Hydrochloric acid (37%) was sourced from Fisher Scientific (UK). All the experiments were performed using de-ionised water that was prepared at in-house facility.
2.2. Micromeritic Properties of Powder Blends
All the ingredients as given in Table 1 were passed through a 250 µm sieve before mixing them into homogenous powder blends. The micromeritic properties including bulk density, tapped density, repose angles, Hausner’s ratio and compressibility index of all powder blends were determined using methods described in the previous study [18].

Table 1.
Composition of nizatidine floating tablets.
2.3. Preparation of Floating Tablets of Nizatidine
Single punch tablet press equipped with 12 mm diameter die was used to compress each carefully weighed powder blend. The recipes of tablet formulations are presented in Table 1. The compression force of the tablet press was adjusted such that it would produce tablets with hardness between 4.0–4.5 kg/cm2 and total weight of 450 mg. The tablet hardness was confirmed using a Monsanto tablet hardness tester (Shanghai Huanghai drug test instrument Co., Ltd., China).
2.4. Physical Evaluation of Floating Tablets
The prepared tablets were physically evaluated for their weight variation, hardness, thickness and friability. For this purpose, twenty tablets were randomly selected for each test. Tablets were weighed carefully on a digital balance to obtain weight variation among each tablet batch. Hardness of tablets was evaluated using Monsanto hardness tester (Shanghai Huanghai drug test instrument Co., Ltd., China). Thickness of selected tablets was determined using a Vernier caliper. Finally, pre-weighed twenty tablets were evaluated for friability using Roche friabilator. The weight of the tablets was measured again after 4 min or completion of 100 revolutions.
2.5. Content Uniformity
Ten tablets were randomly chosen from each formulation and crushed in a pestle and mortar. A 150 mg drug equivalent powder was dissolved in 0.1N HCl solution in a 100 mL volumetric flask and the flask was rocked for half an hour. The solution was filtered using a 0.45 µm filter paper and analyzed spectrophotometrically at 325 nm after appropriate dilutions. The quantity was estimated using a calibration curve based on a series of dilutions. The calibration curve was linear in the concentration range of 15.62–500 µg/mL.
2.6. In Vitro Buoyancy Studies
In vitro buoyancy of floating tablets was determined by floating lag time method as described earlier [19]. For this purpose, one tablet was placed in a 100 mL beaker containing 0.1N HCl maintained at 37 °C and then the time taken by the tablet to rise and float at the surface of the media was noted. Total floating duration was observed until the tablet stopped floating. The experiment was repeated three times and the values are reported as mean and standard deviation.
2.7. In Vitro Dissolution Studies
The dissolution study of floating tablets was conducted using a 6 vessel USP type II dissolution apparatus containing 900 mL of freshly prepared 0.1N HCl maintained at pH 1.2 [20,21]. The bath temperature was set at 37 ± 0.5 °C. One tablet in each vessel was placed and the apparatus was started with a paddle speed of 50 rpm. After pre-determined interval of time (0.5, 1, 2, 3, 4, 5, 6, 7, 8, 12 h), 5 mL sample was withdrawn using a syringe fitted with a 0.45 µm Nylon syringe filter. The dissolution media was quickly replenished with the same amount of pre-warmed 0.1N HCl in order to maintain the sink conditions. The withdrawn samples were analyzed spectrophotometrically at 325 nm after appropriate dilutions, and the percent drug release was calculated using a calibration curve based on a series of dilutions in the concentration range of 15.62–500 μg/mL.
2.8. Drug Release Kinetics
The kinetics of drug release is an important tool to investigate the mechanism by which drug is released from the dosage form. The mechanism of drug release was evaluated by fitting dissolution data to zero-order, first-order, Higuchi model and Korsmeyer-Peppas model [22].
3. Results and Discussion
Here we report floating tablets formulated using different viscosity grades of HPMC in order to investigate the effect of polymer viscosity on the dissolution behaviour of nizatidine. To date no published report is available with this particular drug, which focused on comparing dissolution of nizatidine with viscosity of chosen HPMC grades.
3.1. Micromeritic Studies
Various preformulation parameters including bulk density, tapped density and flow properties were estimated for all four formulations and the results are presented in Table 2. The values of bulk density were ranged between 0.36 to 0.44 g/cm3 whilst the tapped densities were ranged between 0.41 to 0.50 g/cm3. Flow properties of all powder blends were estimated in order to assure consistent scale-up manufacturing of tablets. For this purpose, repose angles, Hausner’s ratio and Carr’s indices were assessed. Repose angles in the range between 25–30° indicate good powder flow properties [23]. Our results show that repose angles of all polymer blends were in the range of 25.31 to 27.60, thus confirming good powder flow properties that are suitable for compression into tablets. Hausner’s ratio below 1.25 indicates good flowability of powders [24]. The Hausner’s ratio for all four powder blends were less than 1.25, as presented in Table 2, therefore, deemed suitable for compression. Similarly, the Carr’s index, which is an indicator of compressibility of powders, showed good flow properties of all four formulations. It should be noted that the standard deviations for repose angles, Hausner’s ratio and Carr’s index were very small, suggesting a homogenous powder mixing with uniform particle size of components [25]. Overall, HPMC K4M based powder blend (F1) showed excellent flow properties as compared with other three viscosity grades of HPMC.

Table 2.
Flow properties of powder blends.
3.2. Post-Compression Parameters
The floating tablets of nizatidine were manufactured by directly compressing the powdered blends in a single-punch tablet compression machine. The formulated tablets were subsequently assessed for post-compression parameters including hardness, thickness, weight variation, friability and content uniformity as summarised in Table 3. It is previously reported that hardness of tablets greater than 5 kg/cm2 may prolong the floating lag time, which is unfavourable in case of low-density floating systems [26]. This is because higher compression force is required to compress powders, which results in highly compacted tablets with low porosity, consequently penetration of dissolution media in tablets becomes slow, thus delaying the rise of tablet to the surface of dissolution media [2]. In this study, the hardness of tablets ranged between 4.0 to 4.50 kg/cm2 for all formulations, as given in Table 3, indicating suitable hardness for floating tablets. Tablet thickness was ranged between 4.1–4.5 mm and no significant variation in thickness was observed in all tablet formulations. Furthermore, all tablet formulations (F1 to F4) exhibited less than 1% friability, thus confirming sufficient mechanical strength of tablets to withstand any wear and tear during handling and packaging.

Table 3.
Post-compression evaluation of nizatidine floating tablets.
The percent weight variation was ≤5%, which met the specification provided by the United States Pharmacopeia (USP) [27]. A low variation in tablet weight is often linked with the content uniformity. Difference in weight among tablets within a batch can result in variation in contents, especially the active pharmaceutical ingredient. Consequently, dosage forms with sub-therapeutic or toxic levels may result. Content uniformity test revealed 96.8–100% drug content in the tablet formulations thus meeting the USP standards for solid dosage forms.
3.3. In Vitro Buoyancy Studies
Floating drug delivery systems require a gas-generating agent such as sodium carbonate to be added in the formulation. The reaction between sodium bicarbonate and water produces carbon dioxide gas, which entraps within the polymers, thus slightly lowering the overall density of tablets. The subtle decrease in tablet density renders them to float upon the stomach’s gastric media. Thus, floating lag time determines how much time a tablet takes to rise upon the gastric media. Our results showed that the floating lag time was 18 s for F1, 22 s for F2, 27 s for F3 and 37 s for F4. From these results, HPMC viscosity appeared to directly influence the floating lag time with increasing polymer viscosity increased the lag time. Seeing the lag time results, one could expect HPMC viscosity dependent total floating time for all tablet formulations. F4 formulation consisted of HPMC K200, which has highest viscosity compared to its counterparts, showed lowest total floating time of 17 h as compared with 19 h for F3 (HPMC K15), 22 h for F2 (HPMC E4M) and 24 h for F4 (HPMC K4M). With decreasing viscosity of HPMC polymer, total floating time increased. The total floating lag time for HPMC K4M based tablets was slightly higher than that of the HPMC E4M based tablets. It is a well-established fact that water mobility and self-diffusion coefficient in the gel layer varies with degree of substitution of HPMC polymer and HPMC K4M based matrix tablets showed lowest self-diffusion coefficient among various HPMC viscosity grades [28]. Thus, inner regions of the gel show a restricted water diffusion, which may lead to a delay in complete hydration of tablets, therefore, a longer floating duration was observed.
3.4. In Vitro Dissolution Studies
In vitro dissolution studies were performed in order to investigate the dissolution of nizatidine from floating matrix tablets, and the dissolution profiles are presented in Figure 1. Pure nizatidine (150 mg in 900 mL dissolution media) dissolution was used as reference to calculate the percentage drug release from tablets. The main aim of the dissolution studies was to draw some relevancy between HPMC viscosity and the dissolution behaviour. No formulation showed burst effect upon immersing in the 0.1N HCl as dissolution media and the nizatidine was released in a more controlled fashion. The contributing factors were HPMC and Carbopol (used in fixed amount), both polymers can form a gel layer around the tablet, which allows for slow drug diffusion across the gel layer [29,30]. Absence of burst drug release also confirmed homogenous mixing of nizatidine within polymer matrix [31]. Interestingly, HPMC’s viscosity appeared to dictate the overall dissolution of nizatidine. The percentage drug released from HPMC K4M and E4M based tablets (F1 and F2) was about 100% in 12 h. We obtained similar dissolution of nizatidine from F1 and F2 formulation because of the fact that HPMC K4M and E4M shares similar viscosities (3000–5600 cp). The basic difference between K4M and E4M grades is the degree of substitution of methoxyl- and hydroxypropoxyl-groups within the polymer backbone [32]. However, degree of substitution did not appear to influence the overall dissolution of nizatidine, but it may possibly influence the drug release rate from the matrix tablets [33].
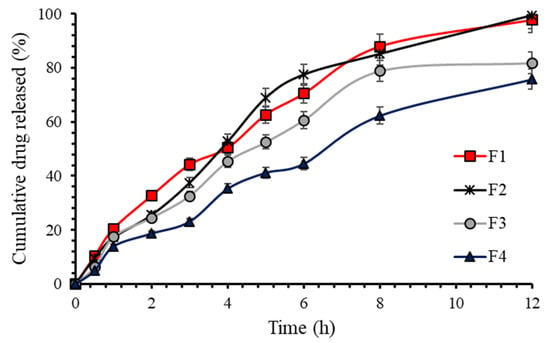
Figure 1.
Percentage nizatidine release from floating tablets.
For the HPMC grades with higher viscosity, the percentage drug release was decreased to 81.88% and 75.81% for F3 and F4, respectively. This decrease in overall dissolution was attributed to the higher viscosity of the HPMC polymers. It is a well-known fact that HPMC grades with higher viscosity tend to hydrate quickly and form gel layer at a faster rate [34,35]. This viscous gel layer in turn reduced the molecular mobility, which resulted in slower nizatidine diffusion from the matrix type floating tablets.
In order to get further insight into dissolution behaviour, a model independent approach was applied on dissolution profiles of all four formulations [36]. Model independent approach helps to find similarity (f2) and difference (f1) between dissolution curves of a reference (marketed product) and the test formulation. Since there is no availability of marketed nizatidine floating tablets, therefore it was not possible to compare our formulations with the reference. Thus, for the purpose of comparison, F2 (HPMC E4M) formulation which resulted in highest drug dissolution (99.5%) in 12 h was designated as the reference and the remaining formulations were compared for f1 and f2 factors. To ensure similarity in two dissolution profiles, f1 less than 10 and f2 greater than 65 is generally required [37]. Our results showed that the dissolution behaviour of F1 and F2 was similar (f1 = 8.00; f2 = 65.22), due to the fact that both formulations were composed of similar viscosity grade HPMC. The non-similar dissolution profiles were achieved for F3 (f1 = 15.66; f2 = 48.80) and F4 (f1 = 32.49; f2 = 35.12) when compared with the reference formulation (F2). Form the results; it is plausible to say that viscosity of HPMC largely influenced the dissolution behaviour of nizatidine from the floating tablets. The higher the viscosity of HPMC, the lower the dissolution of nizatidine.
3.5. Drug Release Kinetics
Kinetics of drug release is an important tool to describe an in vitro dissolution behaviour of drugs from the formulations, which may be correlated with their in vivo performance. For this purpose, nizatidine dissolution data were fitted to various kinetic equations based on zero-order, first-order, Higuchi and Korsmeyer-Peppas models [36]. All these kinetic models help to understand the release mechanism of drug under investigation from the formulation, and most suited model was selected based on the best fitting of the experimental results. The best-fit data based on R2 values are presented in Table 4, and the suitable model being the one with highest R2 values. The primary aim of sustained or controlled release products is to achieve a constant drug release rate in a zero-order release fashion. However, this is often not possible because one or more formulation factors may change during dissolution of the dosage form. Since floating tablets were composed of hydrophilic polymers, namely HPMC and Carbopol, both the polymers have the tendency to swell in an aqueous medium, thus altering the boundary conditions that should remain constant if a dosage form has to behave in zero-order manner. Such changes may promote pseudo first-order or first-order drug release rates. Our results show that first-order release rate was more favourable because of higher correlation (R2 value), which was followed by the Higuchi model and the zero-order model was least favourable in the given conditions. This is owing to polymer swelling which possibly had changed the overall thickness of the floating tablets, thus nizatidine diffusion was concentration dependent. As long as the mechanism of drug release is concerned, the dissolution data were fitted to Korsmeyer and Peppas model for further insights. Korsmeyer-Peppas equation (Mt/M∞ = Kptn) explains the drug release mechanism from hydrophilic matrix systems [38]. If the n value equals 0.45, then the drug release follows Fickian diffusion mechanism (case I). Non-Fickian diffusion or anomalous transport dominates if 0.45 < n < 0.89. If the n value equals 0.89, then the drug release follows Case II transport due to the relaxation of the polymeric chains in the matrix. A super case II transport is achieved if the n value is greater than 0.89. Super case II transport involves release of the drug through combined diffusion and polymer erosion mechanisms [38,39]. The corresponding n values for F1, F3 and F4 suggested a non-Fickian diffusion of nizatidine molecules from the polymer matrix. However, super case II transport was observed for F2 formulation. It is noteworthy that K4M and E4M grades have similar viscosity but have different degree of substitution [32], therefore, drug release mechanism may differ which was the case in our study. Chemically, E4M (2910) grade has more methoxy-groups as compared with K4M (2208) grade, and E4M polymer hydrates less efficiently to resist matrix disintegration, thus the gel layer strength was lower than that of the K4M grade HPMC [40]. Mitchell and coworkers previously described that HPMC gel strength also dictates the drug release rates in the rank order of methylcellulose > HPMC 2208 (K4M) > HPMC 2910 (E4M) > HPMC 2906 (F4M) [41]. Therefore, nizatidine release followed super case II transport as depicted by the release exponent (n) value of Korsmeyer-Peppas model in the present investigation.

Table 4.
Nizatidine release kinetics from floating tablets.
4. Conclusions
Our study concludes that viscosity of HPMC polymers is a key factor in controlling the performance of nizatidine floating tablets. The higher the viscosity of HPMC grade, the lower the total floating time and extent of nizatidine dissolution. HPMC grades with similar viscosities but different degree of substitution (E4M and K4M) resulted in different release mechanisms, which was attributed to the difference in the number of methoxy-groups attached with the HPMC. Current investigation also indicated that if the matrix forming polymers are judicially chosen, it is possible to achieve formulations that may exhibit controlled release at a desired rate.
Author Contributions
Y.S. was project administrator who conceptualized the study and methodology, and wrote the original draft article; N.I. executed the experiments and analyzed the data; T.H., A.M.Y., I.U.K. and S.A.A.R. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to thank Strides Pharma Science Limited (Formerly Strides Shasun Limited, Bangalore, India) for providing gift sample of nizatidine. The authors show gratitude to Colorcon UK for their generous support by providing HPMC polymers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, K.; Wen, H.; Yang, F.; Yu, Y.; Gai, X.; Wang, H.; Li, P.; Pan, W.; Yang, X. Study of controlled-release floating tablets of dipyridamole using the dry-coated method. Drug Dev. Ind. Pharm. 2018, 44, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Tadros, M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010, 74, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Kristl, J.; Vrečer, F.; Vodopivec, P.; Zorko, B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int. J. Pharm. 2000, 195, 125–135. [Google Scholar] [CrossRef]
- Pawar, V.K.; Kansal, S.; Garg, G.; Awasthi, R.; Singodia, D.; Kulkarni, G.T. Gastroretentive dosage forms: A review with special emphasis on floating drug delivery systems. Drug Deliv. 2011, 18, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Kumar, A.; Rath, G.; Goyal, A.K. Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit. Rev. Ther. Drug Carrier. Syst. 2014, 31, 531–557. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Gastroretentive drug delivery systems. Expert Opin. Drug Deliv. 2006, 3, 217–233. [Google Scholar] [CrossRef]
- Chavanpatil, M.D.; Jain, P.; Chaudhari, S.; Shear, R.; Vavia, P.R. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006, 316, 86–92. [Google Scholar] [CrossRef]
- Nayak, A.K.; Malakar, J.; Sen, K.K. Gastroretentive drug delivery technologies: Current approaches and future potential. J. Pharm. Educ. Res. 2010, 1, 1–10. [Google Scholar]
- Eisenächer, F.; Garbacz, G.; Mäder, K. Physiological relevant in vitro evaluation of polymer coats for gastroretentive floating tablets. Eur. J. Pharm. Biopharm. 2014, 88, 778–786. [Google Scholar] [CrossRef]
- Li, S.; Lin, S.; Daggy, B.P.; Mirchandani, H.L.; Chien, Y.W. Effect of hpmc and carbopol on the release and floating properties of gastric floating drug delivery system using factorial design. Int. J. Pharm. 2003, 253, 13–22. [Google Scholar] [CrossRef]
- Dureja, H.; Pandey, P.; Mittal, M. Gastroretentive delivery—Box-behnken-designed gastroretentive floating tablets of famotidine. Drug Dev. Deliv. 2015, 15, 247–256. [Google Scholar]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3d extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef]
- Sharma, B.G.; Khanna, K.; Kumar, N.; Nishad, D.K.; Basu, M.; Bhatnagar, A. Development and gamma scintigraphy evaluation of gastro retentive calcium ion-based oral formulation: An innovative approach for the management of gastro-esophageal reflux disease (gerd). Drug Dev. Ind. Pharm. 2017, 43, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; He, X.; Yang, X.L.; Du, W.J.; Cui, C.L.; Wang, L.; Wang, X.; Zhang, C.F.; Guo, C.R. The in vitro/vivo evaluation of prepared gastric floating tablets of berberine hydrochloride. AAPS PharmSciTech 2017, 18, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, N. Development of Nizatidine Floating Tablets; University of Central Punjab: Lahore, Pakistan, 2017. [Google Scholar]
- Jain, A.; Pandey, V.; Ganeshpurkar, A.; Dubey, N.; Bansal, D. Formulation and characterization of floating microballoons of nizatidine for effective treatment of gastric ulcers in murine model. Drug Deliv. 2015, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Balata, G. Design and evaluation of gastroretentive floating tablet of nizatidine: A trial to improve its efficacy. Int. J. Pharm. Pharm. Sci. 2014, 6, 423–429. [Google Scholar]
- Madan, J.R.; Kamate, V.J.; Dua, K.; Awasthi, R. Improving the solubility of nevirapine using a hydrotropy and mixed hydrotropy based solid dispersion approach. Polim. Med. 2017, 47, 83–90. [Google Scholar]
- Jiménez-Castellanos, M.R.; Zia, H.; Rhodes, C.T. Design and testing in vitro of a bioadhesive and floating drug delivery system for oral application. Int. J. Pharm. 1994, 105, 65–70. [Google Scholar] [CrossRef]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.-H.; Park, J.-B.; Kim, D.W. Fabrication of intragastric floating, controlled release 3D printed theophylline tablets using hot-melt extrusion and fused deposition modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef]
- Teaima, M.; Hamid, M.M.A.; Shoman, N.A.; Jasti, B.R.; El-Nabarawi, M.A. Promising swellable floating bupropion tablets: Formulation, in vitro/in vivo evaluation and comparative pharmacokinetic study in human volunteers. Drug Des. Dev. Ther. 2020, 14, 2741. [Google Scholar] [CrossRef]
- Qi, X.; Chen, H.; Rui, Y.; Yang, F.; Ma, N.; Wu, Z. Floating tablets for controlled release of ofloxacin via compression coating of hydroxypropyl cellulose combined with effervescent agent. Int. J. Pharm. 2015, 489, 210–217. [Google Scholar] [CrossRef]
- Riley, G.; Mann, S.; Jesse, R. Angles of repose of cohesive powders. J. Powder Bulk Solids Technol. 1978, 2, 15–18. [Google Scholar]
- Thalberg, K.; Lindholm, D.; Axelsson, A. Comparison of different flowability tests for powders for inhalation. Powder Technol. 2004, 146, 206–213. [Google Scholar] [CrossRef]
- Hwang, K.-M.; Cho, C.-H.; Tung, N.-T.; Kim, J.-Y.; Rhee, Y.-S.; Park, E.-S. Release kinetics of highly porous floating tablets containing cilostazol. Eur. J. Pharm. Biopharm. 2017, 115, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Gambhire, M.N.; Ambade, K.W.; Kurmi, S.D.; Kadam, V.J.; Jadhav, K.R. Development and in vitro evaluation of an oral floating matrix tablet formulation of diltiazem hydrochloride. AAPS PharmSciTech 2007, 8, E166–E174. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeia. 27, the National Formulary 22; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2004; Volume 20852, p. 1539. [Google Scholar]
- Rajabi-Siahboomi, A.R.; Bowtell, R.W.; Mansfield, P.; Davies, M.C.; Melia, C.D. Structure and behavior in hydrophilic matrix sustained release dosage forms: 4. Studies of water mobility and diffusion coefficients in the gel layer of HPMC tablets using NMR imaging. Pharm. Res. 1996, 13, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Mulye, N.; Inamdar, K. Sustained Release Tablet Containing Hydrocolloid and Cellulose Ether. U.S. Patent 6,416,786, 9 July 2002. [Google Scholar]
- Lee, P.I.; Peppas, N.A. Prediction of polymer dissolution in swellable controlled-release systems. J. Control. Release 1987, 6, 207–215. [Google Scholar] [CrossRef]
- Sharma, M.; Kohli, S.; Dinda, A. In-vitro and in-vivo evaluation of repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer. Saudi Pharm. J. 2015, 23, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Alderman, D. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int. J. Pharm. Tech. Prod. Mfr. 1984, 5, 1–9. [Google Scholar]
- Ghori, M.U.; Conway, B.R. Hydrophilic matrices for oral control drug delivery. Am. J. Pharmacol. Sci. 2015, 3, 103–109. [Google Scholar]
- Daly, P.B.; Davis, S.S.; Keimerley, J.W. The effect of anionic surfactants on the release of chlorpheniramine from a polymer matrix tablet. Int. J. Pharm. 1984, 18, 201–205. [Google Scholar] [CrossRef]
- Nakano, M.; Ohmori, N.; Ogata, A.; Sugimoto, K.; Tobino, Y.; Iwaoku, R.; Juni, K. Sustained release of theophylline from hydroxypropylcellulose tablets. J. Pharm. Sci. 1983, 72, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Patra, S.; Pani, N.R. Optimization of hpmc and carbopol concentrations in non-effervescent floating tablet through factorial design. Carbohydr. Polym. 2014, 102, 360–368. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.-P. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Shahzad, Y.; Saeed, S.; Ghori, M.U.; Mahmood, T.; Yousaf, A.M.; Jamshaid, M.; Sheikh, R.; Rizvi, S.A. Influence of polymer ratio and surfactants on controlled drug release from cellulosic microsponges. Int. J. Biol. Macromol. 2018, 109, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.L. Design and evaluation of hydroxypropyl methylcellulose matrix tablets for oral controlled release: A historical perspective. In Hydrophilic Matrix Tablets for Oral Controlled Release; Springer: New York, NY, USA, 2014; pp. 17–51. [Google Scholar]
- Mitchell, K.; Ford, J.L.; Armstrong, D.J.; Elliott, P.N.C.; Hogan, J.E.; Rostron, C. The influence of substitution type on the performance of methylcellulose and hydroxypropylmethycellulose in gels and matrices. Int. J. Pharm. 1993, 100, 143–154. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).