Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution
Abstract
:1. Introduction
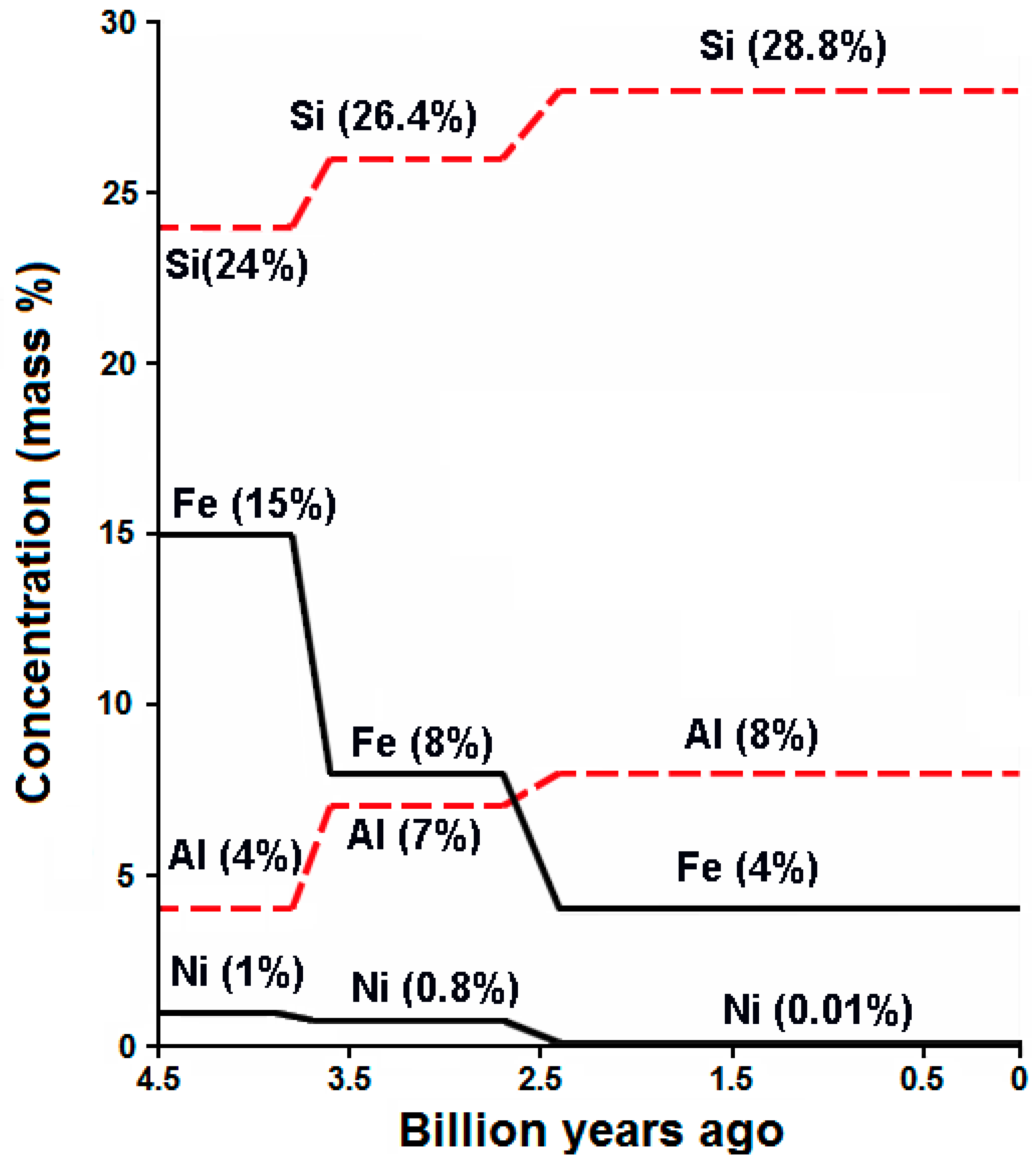
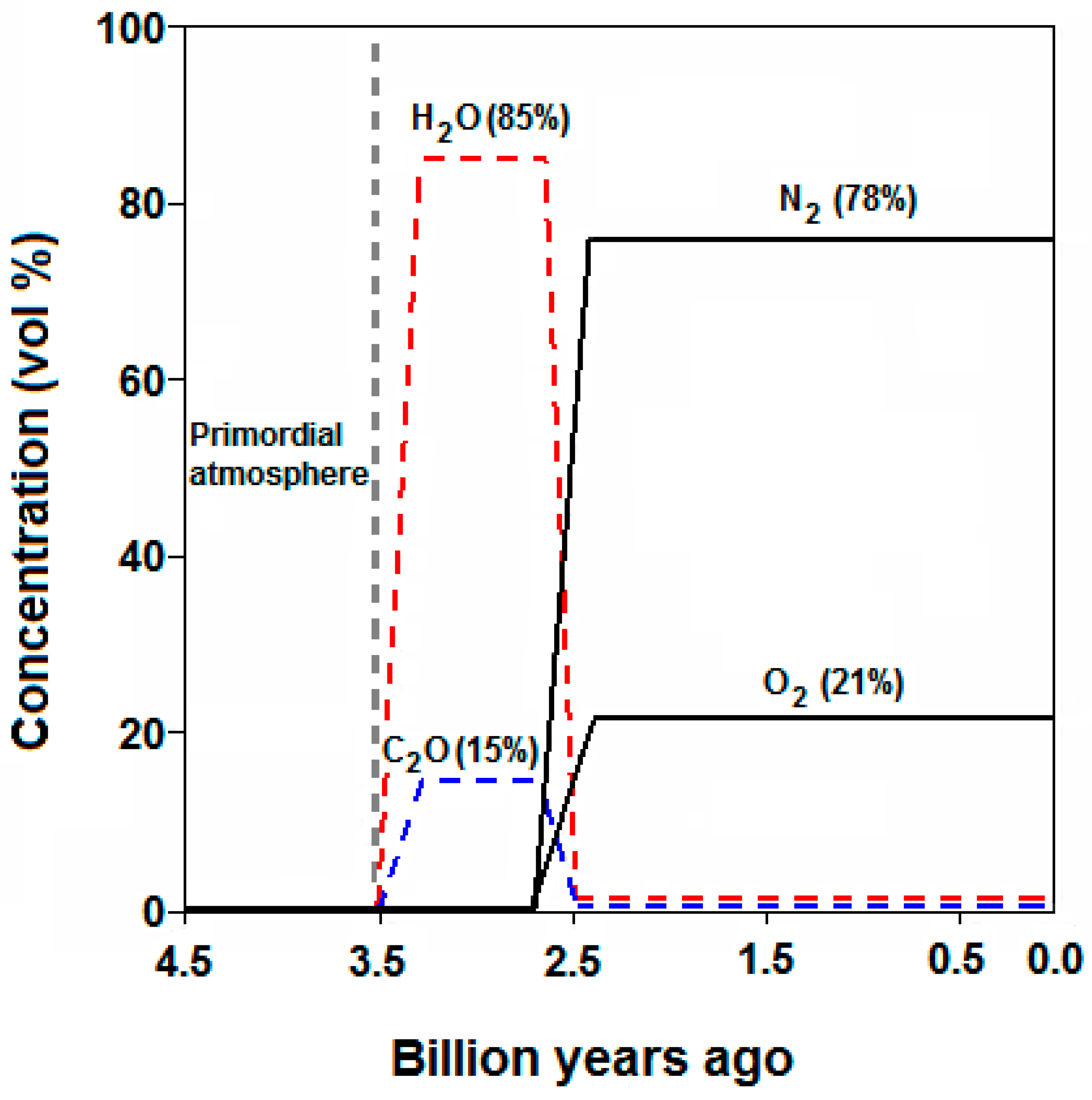
2. Atmospheric CO2 and the Carbon Cycle
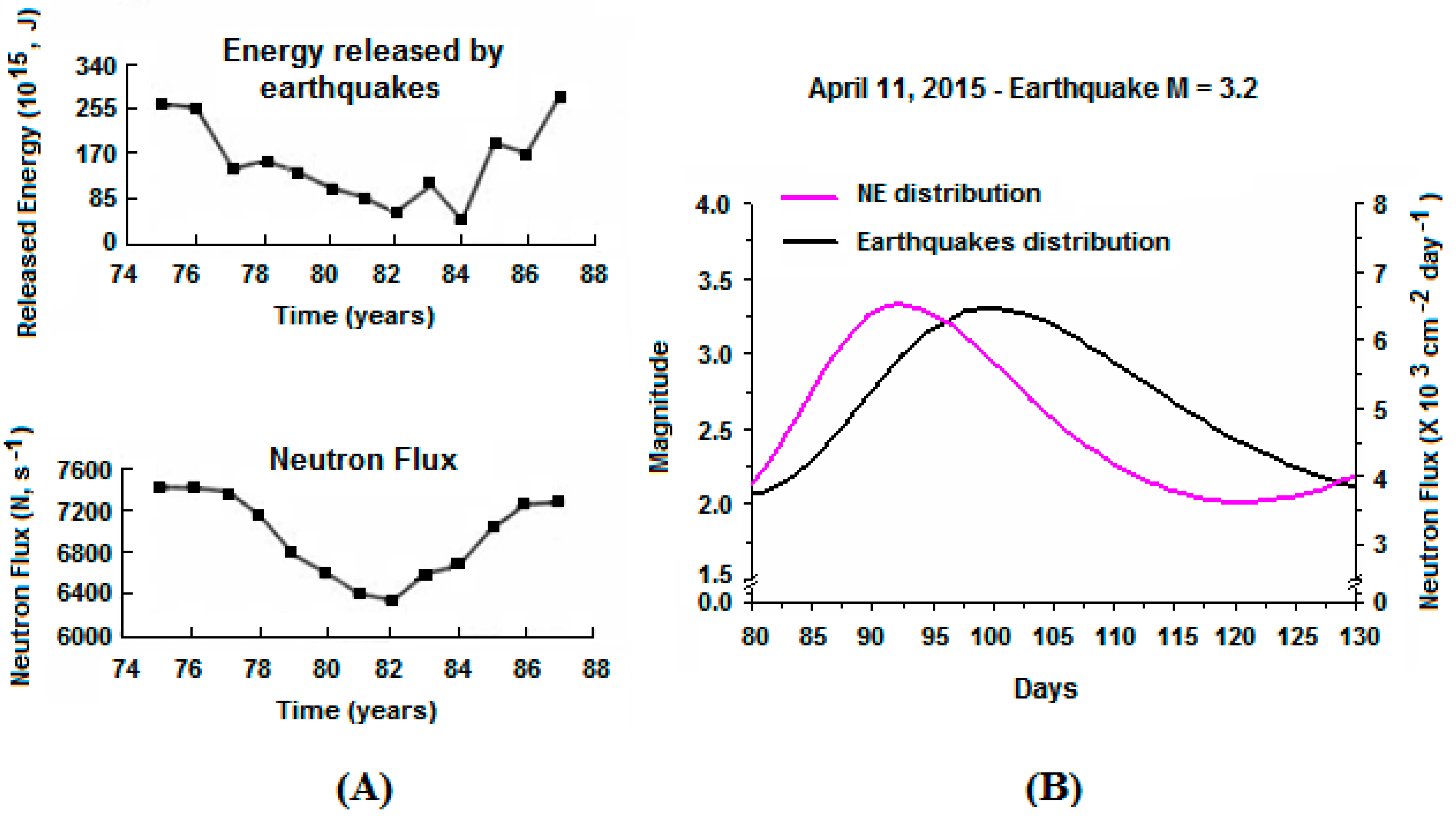
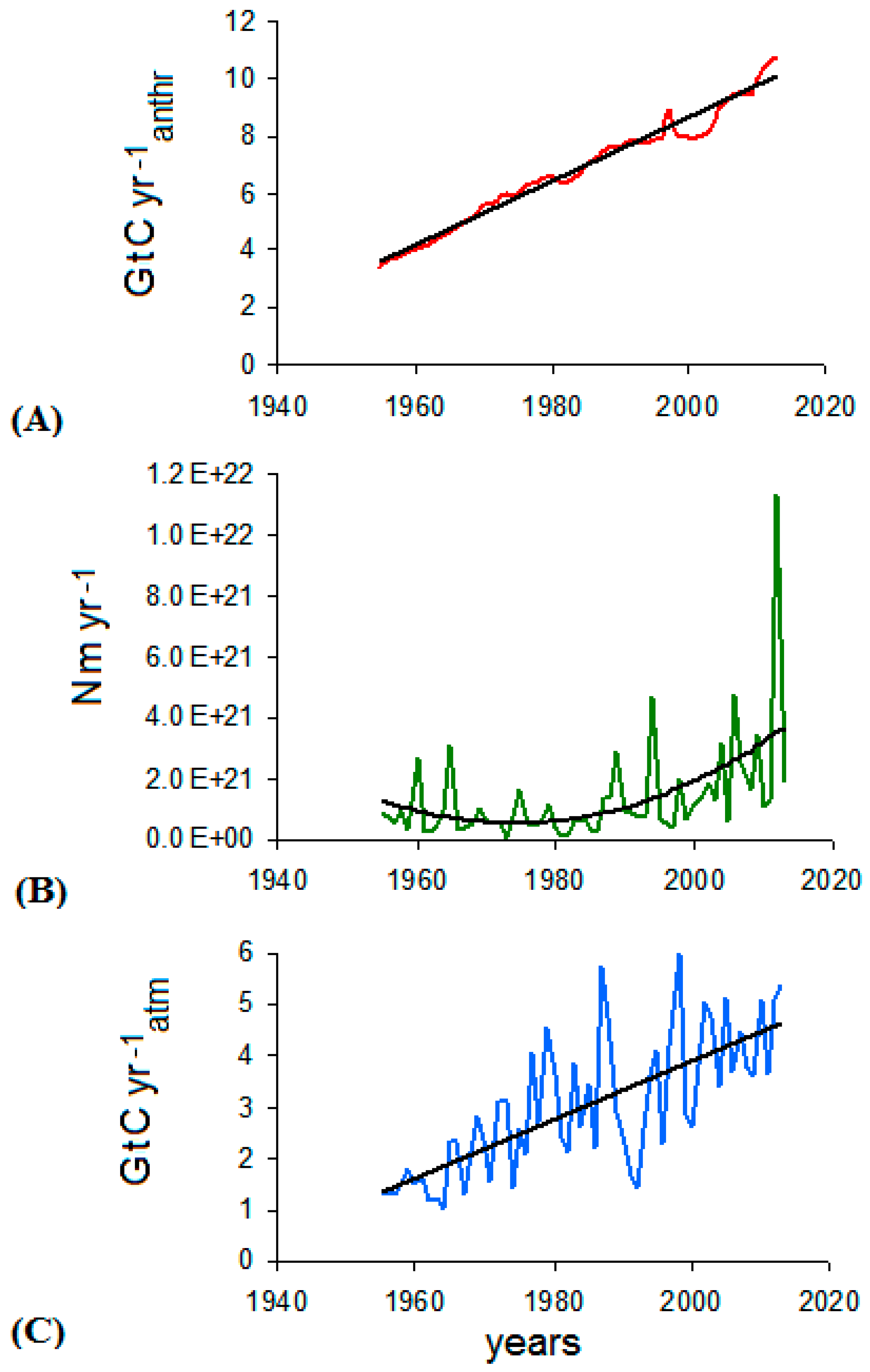
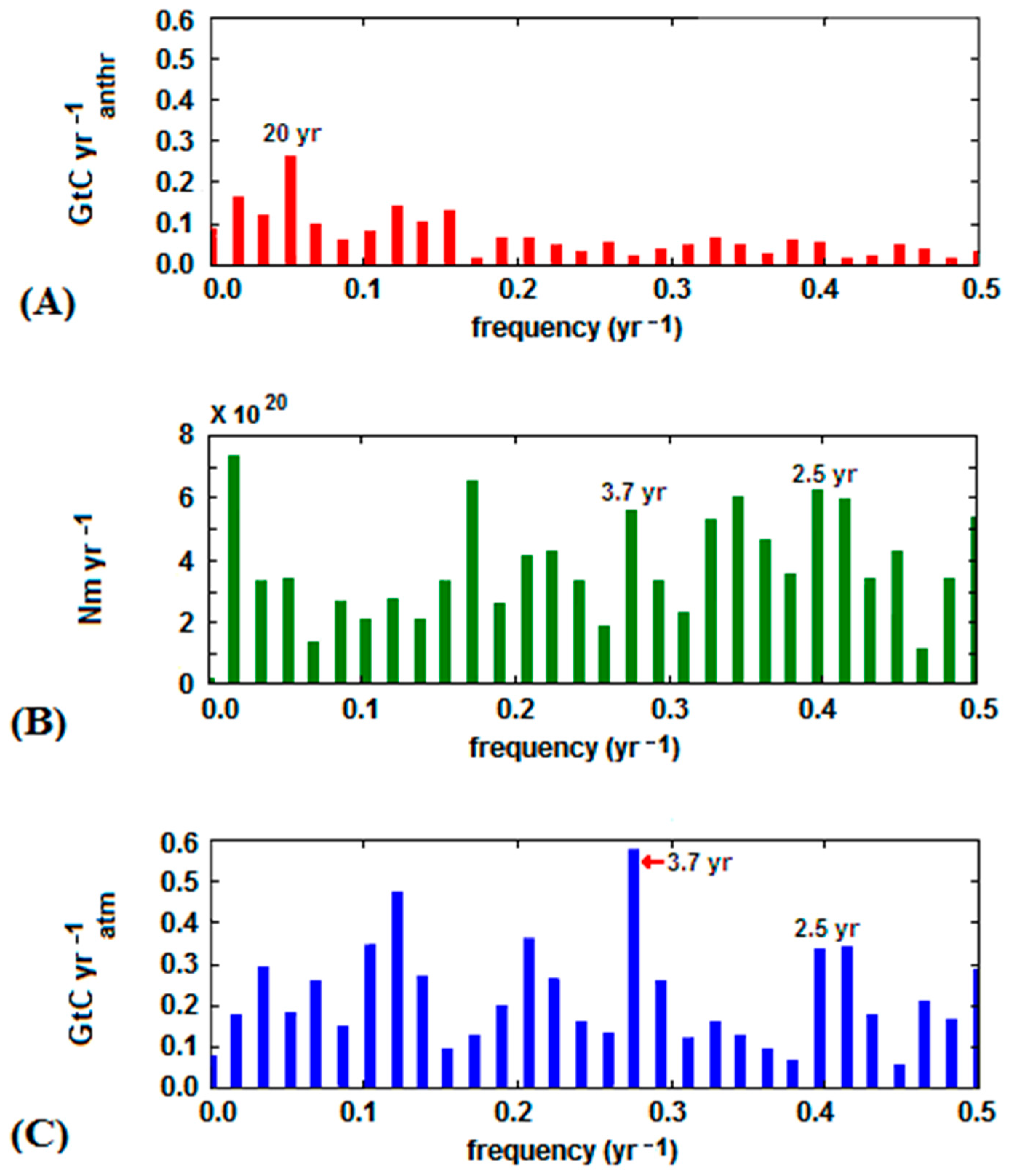
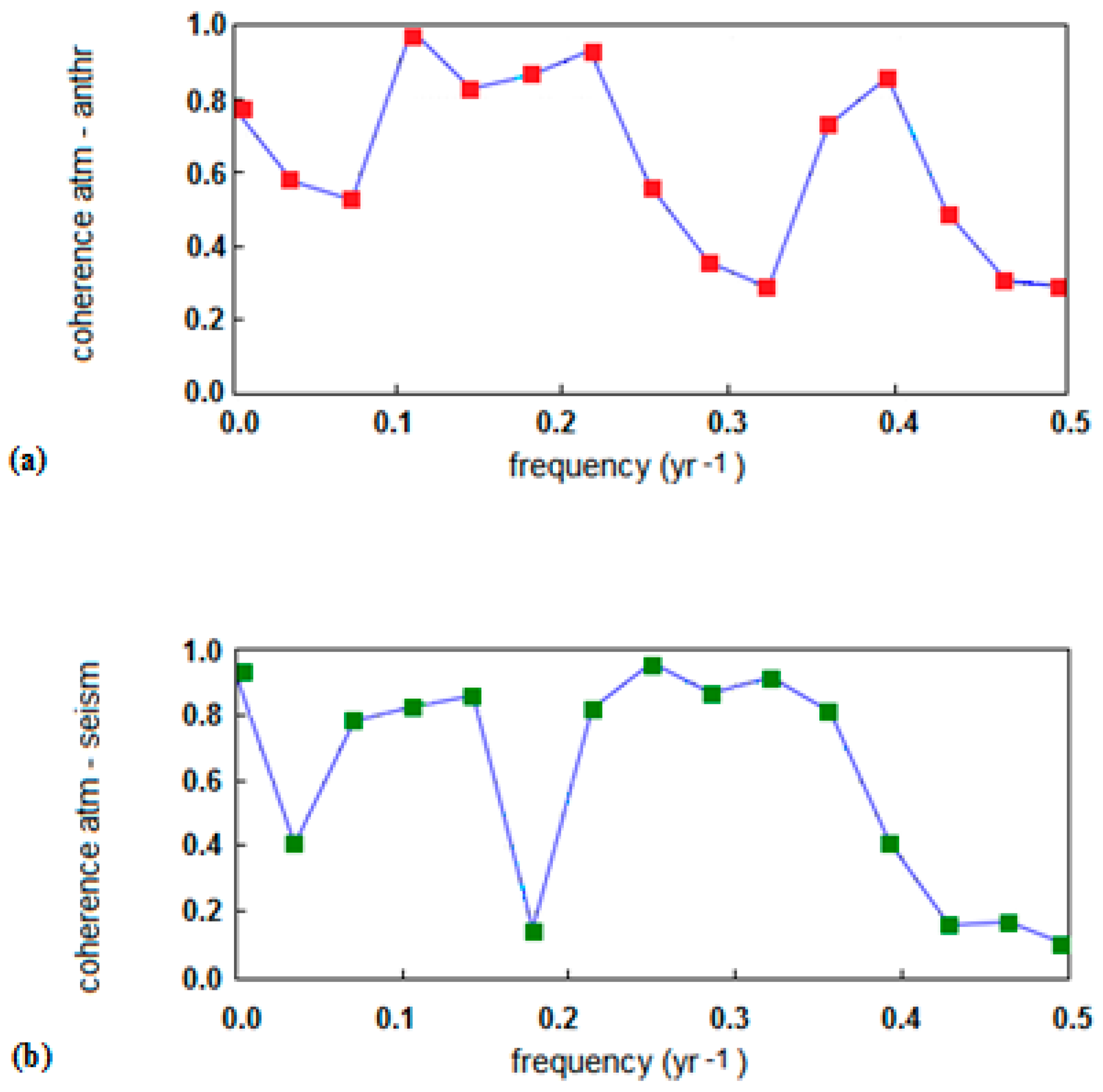
3. Data Analysis
- The significant acceleration in the anthropogenic emission rate, largely due to China’s contribution, observed since 2002 was not reflected in the analogous trend of atmospheric CO2, and yet continued its steady growth;
- the downward trend of atmospheric CO2 was seen in some periods, especially from 1988 to 1993, despite the continuous growth of the anthropogenic emissions; and
- intensive cyclic fluctuations around the trend line were observed for the atmospheric CO2 growth rate whereas the increase of the anthropogenic CO2 emission rate was smoother.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L. The inception of the oceans and CO2-atmosphere in the early history of the Earth. Earth Planet. Sci. Lett. 2004, 227, 179–184. [Google Scholar] [CrossRef]
- Weast, R.C. Handbook of Chemistry and Physics, CRC Handbook of Chemistry and Physics; CRC Press: New York, NY, USA, 1980. [Google Scholar]
- Garrison, T.S. Oceanography: An Invitation to Marine Science; Thompson Brooks Cole: Belmont, CA, USA, 2005. [Google Scholar]
- Schopf, J. Earth’s Earliest Biosphere: Its Origin and Evolution; Princeton University Press: Princeton, NJ, USA, 1983. [Google Scholar]
- Kolb, E. Blind Watchers of the Sky: The People and Ideas that Shaped Our View of the Universe; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Kolb, E.W.; Matarrese, S.; Notari, A.; Riotto, A. Primordial inflation explains why the universe is accelerating today. arXiv, 2005; arXiv:hep-th/0503117. [Google Scholar]
- Williams, R.P.; Da Silva, F.J.R. Evolution was chemically constrained. J. Theor. Biol. 2003, 220, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Buesseler, K.O.; Doney, S.C.; Karl, D.M.; Boyd, P.W.; Caldeira, K.; Chai, F.; Coale, K.H.; de Baar, H.J.; Falkowski, P.G.; Johnson, K.S.; et al. Ocean iron fertilization moving forward in a sea of uncertainty. Science 2008, 319, 162. [Google Scholar] [CrossRef] [PubMed]
- Holland, H.D. The oxygenation of the atmosphere and oceans. Philos. Trans. R. Soc. Lond. Ser. B 2006, 361, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.; Burgess, L.; Longhi, J.; Smith, W.H. An empirical thermal history of the Earth’s upper mantle. J. Geophys. Res. 1994, 99, 835–850. [Google Scholar] [CrossRef]
- Kasting, J.F.; Ackerman, T.P. Climatic consequences of very high carbon dioxide levels in the Earth’s early atmosphere. Science 1986, 234, 1383–1385. [Google Scholar] [CrossRef]
- Yung, Y.L.; De More, W.B. Photochemistry of Planetary Atmospheres; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Ahrens, T.J. The state of mantle minerals. Technophysics 1971, XX, 189–219. [Google Scholar]
- Ringwood, A.E. The chemical composition and the origin of Earth. In Advance in Earth Science; Hurley, P.M., Ed.; MIT PRESS: Cambridge, UK, 1962. [Google Scholar]
- Gaillard, F.; Scaillet, B. A theoretical framework for volcanic degassing chemistry in a comparative planetology perspective and implications for planetary atmospheres. Earth Planet. Sci. Lett. 2014, 403, 307–316. [Google Scholar] [CrossRef]
- Tera, F.; Papanastassiou, D.A.; Wasserburg, G.J. Isotopic evidence for a terminal lunar cataclysm. Earth Planet. Sci. Lett. 1974, 22, 1–21. [Google Scholar] [CrossRef]
- Cohen, B.A.; Swindle, T.D.; Kring, D.A. Support for the Lunar Cataclysm Hypothesis from Lunar Meteorite Impact Melt Ages. Science 2000, 290, 1754–1755. [Google Scholar] [CrossRef]
- Saito, M.A. Less nickel for more oxygen. Nature 2009, 458, 714–715. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.E.; Kirschvink, J.L.; Hilburn, I.A.; Nash, C.Z. The Paleoproterozoic snowball Earth: A climate disaster triggered by the evolution of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 11131–11136. [Google Scholar] [CrossRef] [PubMed]
- Konhauser, K.O.; Pecoits, E.; Lalonde, S.V.; Papineau, D.; Nisbet, E.G.; Barley, M.E.; Arndt, N.T.; Zahnle, K.; Kamber, B.S. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature 2009, 458, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Jobstraibizer, P. The distribution of aluminum in the Earth: From cosmogenesis to Sial evolution. Coord. Chem. Rev. 1996, 149, 367–400. [Google Scholar] [CrossRef]
- Taylo, S.R.; McLennan, S.J. Planetary Crusts: Their Composition, Origin and Evolution; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Hawkesworth, C.I.; Kemp, A.I.S. Evolution of the continental crust. Nature 2006, 443, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Doglioni, C. Interno della Terra. In Enciclopedia Scienza e Tecnica; Treccani: Milano, Italy, 2007; pp. 595–605. [Google Scholar]
- Rudnik, R.L.; Fountain, D.M. Nature and composition of the continental crust: A lower crustal perspective. Rev. Geophys. 1995, 33, 267–309. [Google Scholar] [CrossRef]
- Yaroshevsky, A.A. Abundances of chemical elements in the Earth’s crust. Geochem. Int. 2006, 44, 54–62. [Google Scholar] [CrossRef]
- Carpinteri, A.; Manuello, A. Evolution and fate of chemical elements in the Earth’s Crust, Ocean, and Atmosphere. In Acoustic, Electromagnetic, Neutron Emissions from Fracture and Earthquakes; Carpinteri, A., Lacidogna, G., Manuello, A., Eds.; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Carpinteri, A.; Manuello, A. Reply to “Comments on ‘Geomechanical and Geochemical Evidence of Piezonuclear Fission Reactions in the Earth’s Crust’ by A. Carpinteri and A. Manuello” by U. Bardi and G. Comoretto. Strain 2013, 49, 548–551. [Google Scholar] [CrossRef]
- Carpinteri, A.; Borla, O.; Lacidogna, G.; Manuello, A. Neutron emissions in brittle rocks during compression tests: Monotonic vs. cyclic loading. Phys. Mesomech. 2010, 13, 264–274. [Google Scholar] [CrossRef]
- Carpinteri, A.; Manuello, A. Geomechanical and geochemical evidence of piezonuclear fission reactions in the Earth’s crust. Strain 2011, 47, 282–292. [Google Scholar] [CrossRef]
- Carpinteri, A.; Lacidogna, G.; Manuello, A.; Borla, O. Piezonuclear fission reactions: Evidences from microchemical analysis, neutron emission, and geological transformation. Rock Mech. Rock Eng. 2012, 45, 445–459. [Google Scholar] [CrossRef]
- Carpinteri, A.; Manuello, A.; Negri, L. Chemical evolution in the Earth’s mantle and its explanation based on piezonuclear fission reactions. In Acoustic, Electromagnetic, Neutron Emissions from Fracture and Earthquakes; Carpinteri, A., Lacidogna, G., Manuello, A., Eds.; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Sobolev, G.A.; Shestopalov, I.P.; Kharin, E.P. Implications of Solar Flares for the Seismic Activity of the Earth. Izvestiya. Phys. Solid Earth 1998, 34, 603–607. [Google Scholar]
- Volodichev, N.N. Lunar periodicity of the neutron radiation burst and seismic activity on the Earth. In Proceedings of the 26th International Cosmic Ray Conference, Salt Lake City, UT, USA, 17–25 August 1999. [Google Scholar]
- Sigaeva, E.; Nechaev, O.; Panasyuk, M.; Bruns, A.; Vladimirsky, B.; Kuzmin, Y. Thermal neutrons’ observations before the Sumatra earthquake. Geophys. Res. Abstr. 2006, 8, 00435. [Google Scholar]
- Carpinteri, A.; Borla, O. Fracto-emissions as seismic precursors. Eng. Fract. Mech. 2017, 177, 239–250. [Google Scholar] [CrossRef]
- Carpinteri, A.; Lacidogna, G.; Manuello, A.; Niccolini, G.; Borla, O. Time correlation between acoustic, electromagnetic and neutron emissions in rocks under compression. In Proceedings of the Conference & Exposition on Experimental and Applied Mechanics (SEM), Costa Mesa, CA, USA, 11–14 June 2012; Chapter N. 50. pp. 387–393. [Google Scholar]
- Carpinteri, A.; Lacidogna, G.; Borla, O.; Manuello, A.; Niccolini, G. Electromagnetic and neutron emissions from brittle rocks failure: Experimental evidence and geological implications. Sadhana 2012, 37, 59–78. [Google Scholar] [CrossRef]
- Borla, O.; Lacidogna, G.; Di Battista, E.; Niccolini, G.; Carpinteri, A. Electromagnetic emission as failure precursor phenomenon for seismic activity monitoring. In Proceedings of the Conference & Exposition on Experimental and Applied Mechanics (SEM), Greenville, SC, USA, 2–5 June 2014; Volume 5, pp. 221–229. [Google Scholar]
- Lacidogna, G.; Borla, O.; Niccolini, G.; Carpinteri, A. Correlation between acoustic and other forms of Energy emissions from fracture phenomena. In Acoustic, Electromagnetic, Neutron Emissions from Fracture and Earthquakes; Carpinteri, A., Lacidogna, G., Manuello, A., Eds.; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Manuello, A.; Sandrone, R.; Guastella, S.; Borla, O.; Lacidogna, G.; Carpinteri, A. Neutron emissions and compositional changes at the compression failure of Iron-rich natural rocks. In Acoustic, Electromagnetic, Neutron Emissions from Fracture and Earthquakes; Carpinteri, A., Lacidogna, G., Manuello, A., Eds.; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Girault, F.; Bollinger, L.; Bhattarai, M.; Koirala, B.P.; France-Lanord, C.; Rajaure, S.; Gaillardet, J.; Fort, M.; Sapkota, S.N.; Perrier, F. Large-scale organization of carbon dioxide discharge in the Nepal Himalayas. Geophys. Res. Lett. 2014, 41, 6358–6366. [Google Scholar] [CrossRef]
- Rothman, D.H. Atmospheric carbon dioxide levels for the last 500 million years. Proc. Natl. Acad. Sci. USA 2001, 99, 4167–4171. [Google Scholar] [CrossRef]
- Royer, D.L. Atmospheric CO2 and O2 during the Phanerozoic: Tools, patterns, and impacts. In Treatise on Geochemistry; Turekian, K., Holland, H., Eds.; Elsevier Science: New York, NY, USA, 2014; pp. 251–267. [Google Scholar]
- Bergman, N.M.; Lenton, T.M.; Watson, A.J. Copse: A new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci. 2004, 301, 182–204. [Google Scholar] [CrossRef]
- CDIAC, 2016: Global Fossil-Fuel CO2 Emissions. Available online: http://cdiac.ornl.gov/trends/emis/tre_glob_2013.html (accessed on 14 October 2017).
- Luthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.-M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K.; et al. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 2008, 453, 379–382. [Google Scholar]
- Global Carbon Budget. Available online: http://www.globalcarbonproject.org/carbonbudget/ (accessed on 16 October 2017).
- Intergovernmental Panel on Climate Change (IPPC). Intergovernmental Panel on Climate Change; AR4-WG1; IPPC: Geneva, Switzerland, 2007. [Google Scholar]
- Lewis, S.L.; Lopez-Gonzalez, G.; Sonké, B.; Affum-Baffoe, K.; Baker, T.R.; Ojo, L.O.; Phillips, O.L.; Reitsma, J.M.; White, L.; Comiskey, J.A.; et al. Increasing carbon storage in intact African tropical forests. Nature 2009, 457, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, J. Counting carbon in the Amazon. Nature 2009, 461, 1048–1052. [Google Scholar] [CrossRef]
- Schuur, E.A.; Vogel, J.G.; Crummer, K.G.; Lee, H.; Sickman, J.O.; Osterkamp, T.E. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature 2009, 459, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Salby, M.L. Physics of the Atmosphere and Climate; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Oliver, J.G.J. Trends in Global CO2 Emissions: 2015 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2015. [Google Scholar]
- Courtney, R.S. Limits to Existing Quantitative Understanding of Past, Present and Future Changes to Atmospheric Carbon Dioxide Concentration; International Conference on Climate Change: New York, NY, USA, 2008. [Google Scholar]
- King, C.H. Gas Geochemistry Applied to Earthquake Prediction: An Overview. J. Geophys. Res. 1986, 91(B12), 12269–12281. [Google Scholar] [CrossRef]
- Pierotti, L.; Botti, F.; D’Intinosante, V.; Facc, G. Anomalous CO2 content in the Gallicano thermo-mineral spring (Serchio Valley, Italy) before the 21 June 2013, Alpi Apuane Earthquake (M = 5.2). Phys. Chem. Earth 2015, Parts A/B/C, 85–86. [Google Scholar]
- Gheradi, F.; Pierotti, L. The suitability of the Pieve Fosciana hydrothermal system (Italy) as a detection site for geochemical seismic precursors. Appl. Geochem. 2018, 92, 166–179. [Google Scholar] [CrossRef]
- Search Earthquake Catalog. Available online: https://earthquake.usgs.gov/earthquakes/search/ (accessed on 16 October 2017).
- Padron, E.; Melian, G.; Marrero, R.; Nolasco, D.; Barrancos, J.; Padilla, G.; Hernandez, P.A.; Perez, N.M. Changes in the diffuse CO2 emission and relation to seismic activity in and around El Hierro, Canary Islands. Pure Appl. Geophys. 2008, 165, 95–114. [Google Scholar] [CrossRef]
- Keeling, C.D.; Whorf, T.P.; Wahlen, M.; Van der Plichtt, J. Interannual extremes in the rate of atmospheric carbon dioxide since 1980. Nature 1995, 375, 666–670. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpinteri, A.; Niccolini, G. Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution. Sci 2019, 1, 17. https://doi.org/10.3390/sci1010017
Carpinteri A, Niccolini G. Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution. Sci. 2019; 1(1):17. https://doi.org/10.3390/sci1010017
Chicago/Turabian StyleCarpinteri, Alberto, and Gianni Niccolini. 2019. "Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution" Sci 1, no. 1: 17. https://doi.org/10.3390/sci1010017
APA StyleCarpinteri, A., & Niccolini, G. (2019). Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution. Sci, 1(1), 17. https://doi.org/10.3390/sci1010017




