Advances in Pulsed Liquid-Based Nanoparticles: From Synthesis Mechanism to Application and Machine Learning Integration
Abstract
1. Introduction
2. Applications
2.1. Biomedical
| Material (Nanomaterial) | Laser Type | Wavelength | Pulse | Fluence/Energy | Rep. Rate | Pulse Time | Medium | Size Range | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 nanoparticles | Nd:YAG | 1064 & 532 nm | 10 ns | 16–32 J/cm2 | 1 Hz | 100 pulses/5 min | DI water | 24 ± 6 nm | Drug delivery, MRI, hyperthermia | [50] |
| TeO2 nanoparticles | Nd:YAG | 1064 nm | 100 ns | ~284 J/cm2 | 1 kHz | 5 min | DI water | ~70 nm; aggl. ≤ 800 nm | Antibacterial, anticancer, cytocompatible | [51] |
| Gold nanoparticles (review) | Various | 193–1064 nm | fs/ns | 10–1000 J/cm2 | 1–10 Hz | 5–30 min | Water/saline/THF/ethanol | <5–100 nm | Molecular imaging, drug delivery | [52] |
| Gold NPs (bioconjugates, review) | Nd:YAG, fs Ti:Sapph, excimer | 193–1064 nm | fs/ns | 1–100 J/cm2 | Hz–kHz | min–h | Water/buffers | 10–80 nm | SERS biosensing, immunoassays, imaging | [53] |
| Y-PSZ dental ceramic (microchanneling) | DPSS Nd:YAG | 1064 nm | 120 ns | — | 5–100 kHz | seconds | Air/water film | μm-scale channels | Dental implants/tools | [54] |
| AuNPs; Au/CNT nanocomposites | Q-sw Nd:YAG | 1064/355 nm | 10 ns | 50/140 mJ/pulse | 10 Hz | 15–30 min | Water; CNT dispersion | Au 3–20 nm | Anticancer (HCT-116, HeLa) | [55] |
| Ferrite/ZnFe2O4 composites | Q-sw Nd:YAG | 532 nm | 9 ns | 300 mJ | 10 Hz | 40 min | Water | 55–88 nm (PLAL) | Drug delivery, hyperthermia, MRI | [56] |
| FexOy@Au core–satellite | Yb:KGW fs | 1025 nm | 420 fs | 50–100 µJ | 5–10 kHz | 20 + 15 min | 1 mM NaCl | Core 10–50 nm; Au 7.5 nm | Photo/magnetothermal therapy; MRI | [57] |
| Pd nanoparticles | Nd:YVO4 (diode-pumped) | 532 & 1064 nm | ns | 0.26/0.36 mJ | 20 kHz | — | Water, methanol | ~6–15 nm | Antimicrobial; cytocompatible (L929) | [58] |
| SnO2 nanoparticles | Q-sw Nd:YAG | 1064 nm | 10 ns | 400–800 mJ/pulse | 1 Hz | 300 pulses | DDW | 17.6–25.8 nm | Antibacterial; anticancer (A549) | [59] |
| Au nanowires (HC-PCF biosensor) | Nd:YAG (SHG/fund.) | 532 & 1064 nm | ns | 2 J (500 pulses) | — | seconds | Ethanol → PCF | ≤40 nm dia; ≤3 µm length | Colon biosensor (SPR) | [60] |
| Magnetic NPs (review) | Nd:YAG, Ti:Sapph, etc. | 193–1064 nm | fs/ps/ns | 0.1–10 J/cm2 | Hz–kHz | min–h | Water/organic | 5–100 nm | MRI, hyperthermia, delivery | [61] |
| Elemental boron NPs (PEGylated) | Yb:KGW fs | 1025–1030 nm | 270–480 fs | 350 µJ/pulse | 8 kHz | 7 h | Water | ~37–40 nm | BNCT, photoacoustic, photothermal | [62] |
| ZnO@NiO core–shell m, | Nd:YAG + plasma jet | 1064 nm | ns + plasma | 800 mJ/pulse | 7 Hz | 10 + 10 min | DI water | 20–100 nm | Antibacterial; cytotoxicity | [63] |
| SeO2 nanoparticles | Q-sw Nd:YAG | 1064 nm | ~100 ns | 400 mJ/pulse | 8 Hz | 200 pulses | DI water | XRD ~39 nm; AFM ~150 nm | Antibacterial; anticancer; aging effect | [64] |
| Au/ZnO nanocomposites | Nd:YAG (Au PLAL) + UV | 1064 & 355 nm | 10 ns | 70 mJ (Au); 140 mJ (UV) | 10 Hz | 30 + 30 min | Water | —(TEM uniform Au) | Anticancer (HCT116, HeLa), biocompatibility | [65] |
| AuNPs (solvent effect, anticancer) | Q-sw Nd:YAG | 532 & 1064 nm | 10 ns | — | 6 Hz | 300 pulses (~50 s) | DDDW/NaOH/DMEM | TEM 5–25 nm | Anticancer (Hepa 1–6), IC50 35–74 µg/mL | [66] |
| Au, Mg, Zn NPs (dental antibac.) | Nd:YAG (SISMA OEM Plus) | 1064 nm | ≈35 ns | 0.3 mJ; 8488 J/cm2 | 20 kHz | 15 & 30 min | DDW + SDS (0.025 M) | Au 5–7.5 nm; Mg 1.9–3.8; Zn 120 → 19.5 | Antibacterial vs. oral pathogens; biocompatible | [67] |
| Se NPs (amorph → trigonal) | Nd:YAG | 1064 nm | ~100 ns | 0.25–0.75 J/cm2 | ~3 kHz | ~5 min + autoclave | DI water | 70 ± 29 → 320 ± 28 nm | Broad-spectrum antibacterial | [68] |
| CQDs/GQDs (review) | Nd:YAG; Ti:Sapph | 355/532/800/1064 nm | fs → ns | Varies | 10 Hz–1 kHz | min–h | Water; organics; amines | CQDs 1–10 nm; GQDs < 100 nm | Bioimaging, PDT/PTT, delivery | [69] |
| Pd–Cu bimetal nanoparticles | Nd:YAG | 532 nm | 10 ns | 500 mJ/pulse | 4 Hz | Pd 2–5 min; Cu 1–4 min | DI water | FESEM 31–51 nm; XRD 3–16 nm | Antibacterial; antioxidant; no hemolysis | [70] |
| Metal-oxide core–shell NPs (mini-review) | Nd:YAG (typ.) | 532/1064 nm (typ.) | ns (fs/ps covered) | — | — | — | Liquids (water common) | Core 9–70 nm; shell 2–60 nm | Antibacterial, anticancer, imaging, delivery | [41] |
2.2. Catalysis and Energy
| Material (Nanomaterial) | Laser Type | Wavelength | Pulse | Fluence/Energy | Rep. Rate | Pulse Time | Medium | Size Range | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| TiO2, α-Fe2O3, TiO2–Fe2O3 | Q-sw Nd:YAG | 1064 nm | 8 ns | 3.5–17.7 J/cm2 | 6 Hz | 15 min | Water | 12–32 nm | Photocatalysis, PEC | [76] |
| Au/Ag core–shell series | Nd:YAG | 1064 nm | 6 ns | 60–90 mJ/pulse | 10 Hz | 10–15 min | DI water | 35–50 nm | Dye degradation catalysis | [77] |
| Ag–MWCNT composites | Nd:YAG | 1064 nm | 7 ns | ~100 mJ/pulse | 10 Hz | 15 min | MWCNT aqueous | Ag ~14 nm | 4-NP/MO/MB degradation | [78] |
| Au/Ag (alloy, core–shell) | Nd:YAG | 1064 nm | 7 ns | 60 mJ/pulse | 10 Hz | 7 min | Water/HAuCl4 | Ag 20 nm; comp. 34 nm | 4-NP catalytic reduction | [79] |
| Photocatalysis for wastewater (review) | Various | 248–1064 nm | fs/ns | 0.2–8 J/cm2; 40–350 mJ | 10 Hz–kHz | min–h | Water/EtOH/mixed | 5–100 nm | Pollutant degradation | [80] |
| Laser ablation in air (perspective) | ns/fs (various) | 355–1064 nm | ns–fs | Variable | Hz–kHz | min | Air | — | OER/HER. Li-ion | [81] |
| Cu nanoparticles (recycled vs. industrial) | Nd:YAG | 1064 nm | 10 ns | 13 J/cm2 | 10 Hz | 10 min | Methanol | ~2 & 30–100 nm | HER/OER on Ni foam | [82] |
| CuO@ZnO core–shell | Q-sw Nd:YAG | 1064 nm | 10 ns | 500–900 mJ | 1 Hz | 5 min (200 pulses) | Water | 19–70 nm | MB photocatalysis | [83] |
| Fe3O4 NPs (cat. + antibac.) | Nd:YAG | 532 & 1064 nm | 10 ns | 22–26 J/cm2 | 1 Hz | 5 min | Water | 30–65 nm (SEM) | MB degradation | [84] |
| Hydrogen gen./storage/detection (review) | Nd:YAG, fiber, Ti:Sapph, excimer | 193–1064 nm | fs/ps/ns | 0.1–100 J/cm2 | Hz–kHz | min–h | Water/solvents/gas | 2–100 nm | HER/PEC; sensing | [85] |
| PLAL nanocatalysts (review) | Various | 193–1064 nm | fs/ps/ns | 0.1–100 J/cm2 | Hz–kHz | min–h | Various | 2–100 nm | HER/ORR/CO2R/degradation | [86] |
| Laser-synthesized NPs (broad review) | Various | 193–1064 nm | fs/ps/ns | — | Hz–MHz | min–cont. | Water/organic | 2–200 nm | Catalysis, energy, photonics | [87] |
| FeNi alloy NPs (modeling) | ps laser (simulated) | 800 nm | 10 ps | 600–3000 J/m2 (absorbed) | — | ns–µs (model) | Water (CG-MD) | 4–12 nm | Defect-rich → catalysis | [88] |
| Rh–Ni@graphitic-carbon (HER) | Q-sw Nd:YAG | 1064 nm | 7 ns | 100 mJ (ablation); 90 mJ (dec.) | 10 Hz | 30 + 10 min | Acetonitrile → aqueous salts | GC shell 1.7–12.9 nm; Rh few-nm | HER: η10 ≈ 46 mV; Tafel 36 mV/dec | [89] |
| Liquid pulsed laser propulsion (review) | Nd:YAG, CO2, etc. | 532/1064/10.6 µm | ns/ps | 1–20 J/cm2 | Hz–kHz | pulses → cont. | Propellants (liq.) | — | Micro-thrusters/propulsion | [90] |
2.3. Sensors and Environmental Remediation
| Material (Nanomaterial) | Laser Type | Wavelength | Pulse | Fluence/Energy | Rep. Rate | Pulse Time | Medium | Size Range | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Polymer–Fe3O4 tablets | Nd:YAG (2ω) | 532 nm | 6 ns | 9 J/cm2 | 10 Hz | — | Air (solid tablet) | 400 nm–4 µm | Oil–water separation | [95] |
| Au–ZnO core–shell nanostructures | Nd:YAG | 1064 nm | ns | 2000 mJ; 200 pulses | — | — | Ethanol | <50 nm | Optical & biological sensors | [96] |
| LIG on polyimide | UV Nd:YVO4 | 355 nm | ns | 0.11–0.6 J/cm2 | — | scanning | Air (PI substrate) | Porous, layered | Humidity/ ion sensors | [97] |
| Ag nanoparticles (gas sensor) | Nd:YAG | 1064 nm | 10 ns | ~102 J/cm2 | 10 Hz | 20–80 min | AgNO3 + TSC (aq) | 5–31 nm | NH3/Ethanol gas sensing | [98] |
| Au nanoparticles (colorimetry) | Nd:YAG | 532 nm | 9 ns | 25–75 mJ | 10 Hz | 10–30 min | Milli-Q water | TEM ~26 ± 2 nm | LSPR colorimetric sensing | [99] |
| Scale-up perspective (green) | Nd:YAG, Ti:Sapph, fiber | 193–1064 nm | fs/ps/ns | — | Hz–MHz | min–h | Water/organics/flow | Broad | Sensors & remediation | [10] |
| ASS & synaptic devices (review) | Nd:YAG, Ti:Sapph, fiber | 355–1064 nm | fs/ps/ns | — | Hz–kHz | min–h | Water/EtOH | 2–100 nm | Artificial sensory systems; neuromorphic | [100] |
| MoS2/WS2–Ag nanocomposites | Q-sw Nd:YAG (Litron LPY 674G-10) | — | 8 ns | 26.3 J/cm2 | 10 Hz | 20 + 15 min | DI water | Ag 15–17 nm; few-layer TMDs | Dopamine/AA sensing; NLO limiting | [101] |
| B,N co-doped GO (BNG1/BNG2) | Nd:YAG (Quanta-Ray Pro 230-10) | 1064 nm | 10 ns | 625 mJ/pulse | 10 Hz | 60 min | Ethanol + NH3 + H3BO3 | 50–60 nm | Gas sensors (ethanol/acetone/NH3) | [102] |
| rGO, Co3O4, rGO/Co3O4 composite films | Q-sw Nd:YAG | 1064 nm | 10 ns | 140 mJ; 300–1000 pulses | 4 Hz | 75–250 s | DI water (sequential) | 23–51 nm (FE-SEM) | H2S/NO2 gas sensing | [103] |
| PLAL for sensing & photonics (review) | fs/ps/ns lasers | 355–1064 nm | fs/ps/ns | 0.08–1000 J/cm2 (varies) | Hz–kHz–MHz | min–h | Water, acetone, ethanol, chloroform, SDS/PVP | 3–200 nm; periodic 95–350 nm | SERS sensing (explosives, dyes, pesticides); NLO devices | [104] |
2.4. Electronics and Photonics
2.5. Case Studies Linking Synthesis Parameters, Defect Profiles, and Application Performance
3. Fundamentals of Pulsed Liquid-Based Synthesis
3.1. Definitions and Key Terminology
3.2. Key Parameters
3.2.1. Laser Wavelength and Pulse Duration
| Parameter | Effect on NP Formation | Example Materials | References |
|---|---|---|---|
| Short wavelength (266–532 nm) | Higher ablation efficiency, smaller NP size, narrow distribution | Cu, Ag, Pd, Au | [1,122,123] |
| Long wavelength (1064 nm) | Lower absorption, larger particles, higher ablation threshold | Au, Pd, Cu | [1,122] |
| Femtosecond/picosecond pulses | Non-thermal ablation, high supersaturation, monodisperse NPs | Au, Si, Ag | [1,124,125,126] |
| Nanosecond pulses | Thermal melting, ejection, bimodal distributions, larger aggregates | Au, Ag, Pd | [1,126] |
3.2.2. Laser Fluence and Repetition Rate
3.2.3. Role of Solvents and Surfactants
3.2.4. Surfactants and Chemical Additives
3.2.5. Target Composition and In Situ Doping
3.3. Size Control, Crystallinity, and Surface Chemistry
3.3.1. Plasma and Bubble Dynamics
3.3.2. Rayleigh–Plesset and Gilmore Models
- ρ = density of the liquid surrounding the liquid;
- (t) = Bubble radius at a time t;
- =: Rate of change of radius—how fast the bubble expands or contracts;
- = : Acceleration of the bubble wall;
- = Pressure inside the bubble;
- = Pressure inside the liquid far from the bubble (ambient pressure);
- μ = dynamic viscosity of the liquid;
- σ = surface tension at the liquid–gas interface.
- Maximum bubble radius;
- Collapse time;
- Interfacial pressure;
- Velocity profiles in the surrounding fluid.
- Understanding early-stage bubble growth;
- Analyzing laser–liquid interaction timescales;
- (t) = bubble radius as a function of time;
- = wall velocity;
- = = acceleration;
- c(t) = local speed of sound in the liquid;
- = pressure inside the bubble;
- = pressure in the liquid at the bubble;
- Ρ = density of surrounding liquid;
- = enthalpy at the bubble wall;
- H = enthalpy in the bulk fluid.
- Shockwave formation at collapse;
- Energy dissipation during rebounds;
- Variation in pressure transmission depending on compressibility gradients.
- In the context of PLAL, the Gilmore model is particularly valuable for simulating:
- Ultrashort laser pulse interactions where the plasma-induced shockwave propagates through the liquid at supersonic velocities;
- Asymmetric bubble collapses near surfaces or boundaries, which can create jetting and influence NP morphology;
3.3.3. Collapse-Driven Nucleation and NP Formation
3.3.4. Computational Insights
| Parameter | Typical Range | Effect on NP Properties | Representative Values & Sources |
|---|---|---|---|
| Plasma Temperature | 4000–7000 K | Higher temperatures enhance nucleation; optimal for small, spherical NPs. Lower temperatures favor larger, irregular particles. | At 6000 K, spherical Al NPs formed with narrow size distribution. |
| Plasma Electron Density | ~1025–1027 m−3 | High density favors electrostatic charging of NPs → colloidal stability, repulsion—based dispersion. | Electron charging occurs at ps scale when density ≈ 1026 m−3. |
| Plasma Lifetime | ~10–1000 ns (fs–ns pulses) | Long-lived plasma leads to coalescence; short-lived plasma leads to finer NPs. | Cooling rate ~10 K/ns observed within first 200 ns of LAL. |
| Bubble Maximum Radius | 2–4 mm (depends on solvent & pulse energy) | Larger bubble volume supports more particles; collapse pressure affects secondary NP formation. | Ag NPs released during 2–4 mm radius bubbles in water. |
| Bubble Lifetime | 200–600 μs | Longer lifetime → larger, more crystalline particles; short lifetime → metastable phases. | Ni NPs: hcp in ACN (shorter collapse), fcc in methanol (longer). |
| Collapse Pressure | Up to several hundred MPa | Sudden rise in temperature and pressure at collapse nucleates dense and crystalline particles via supersaturation. | The Rayleigh–Plesset & Gilmore models simulate collapse at high P, T (≥1000 K, ≥100 MPa). |
| Shockwave Velocity | 1500–2700 m/s | High velocity enhances compression of vapor phase, initiating particle nucleation and fragmentation. | 2600 m/s before 200 ns (Tsuji et al.), 1700 m/s after 500 μm. |
| Number of Laser Pulses per Bubble | 1–5 pulses (depends on repetition rate & bubble) | Multiple pulses inside bubble lifetime can cause reshaping, fragmentation, or secondary NP formation. | Enhanced NP ejection observed between bubble collapse and rebound phase. |
| NP Size (primary particles) | 10–30 nm | Small, monodisperse particles formed inside cavitation bubble center or early plasma stages. | Primary Ag NPs 10–20 nm under 4000–6000 K plasma. |
| NP Size (secondary particles) | >40–100 nm | Formed later by agglomeration, molten droplet coalescence, or during bubble rebound. | Secondary particles ~60–100 nm after bubble collapse. |
3.3.5. Post-Synthesis Modifications
Laser Fragmentation in Liquid (LFL)
Laser Melting in Liquid (LML)
Ligand-Assisted PLAL
Real-Time Feedback and Optical Diagnostics
Time-Resolved Spectroscopy
3.3.6. Plasma Diagnostics:
4. Defect Engineering via Pulsed Liquid Ablation
4.1. Types of Defects
4.2. Mechanism
5. Material Systems and Compositions
5.1. Metals
5.2. Metal Oxides & Chalcogenides
5.3. Emerging Materials
5.4. Representative Case Studies
5.4.1. Au–Ag Alloy and Core–Shell Nanoparticles
5.4.2. Comparison with Chemical Methods
5.4.3. Silver Nanoparticles
5.4.4. Oxidation Behavior of Ag NPs
5.4.5. Zeta Potential and Stability of Ag NPs
5.4.6. Size and Dispersion Characteristics of Ag NPs
5.4.7. Functional Implications of Ag NPs
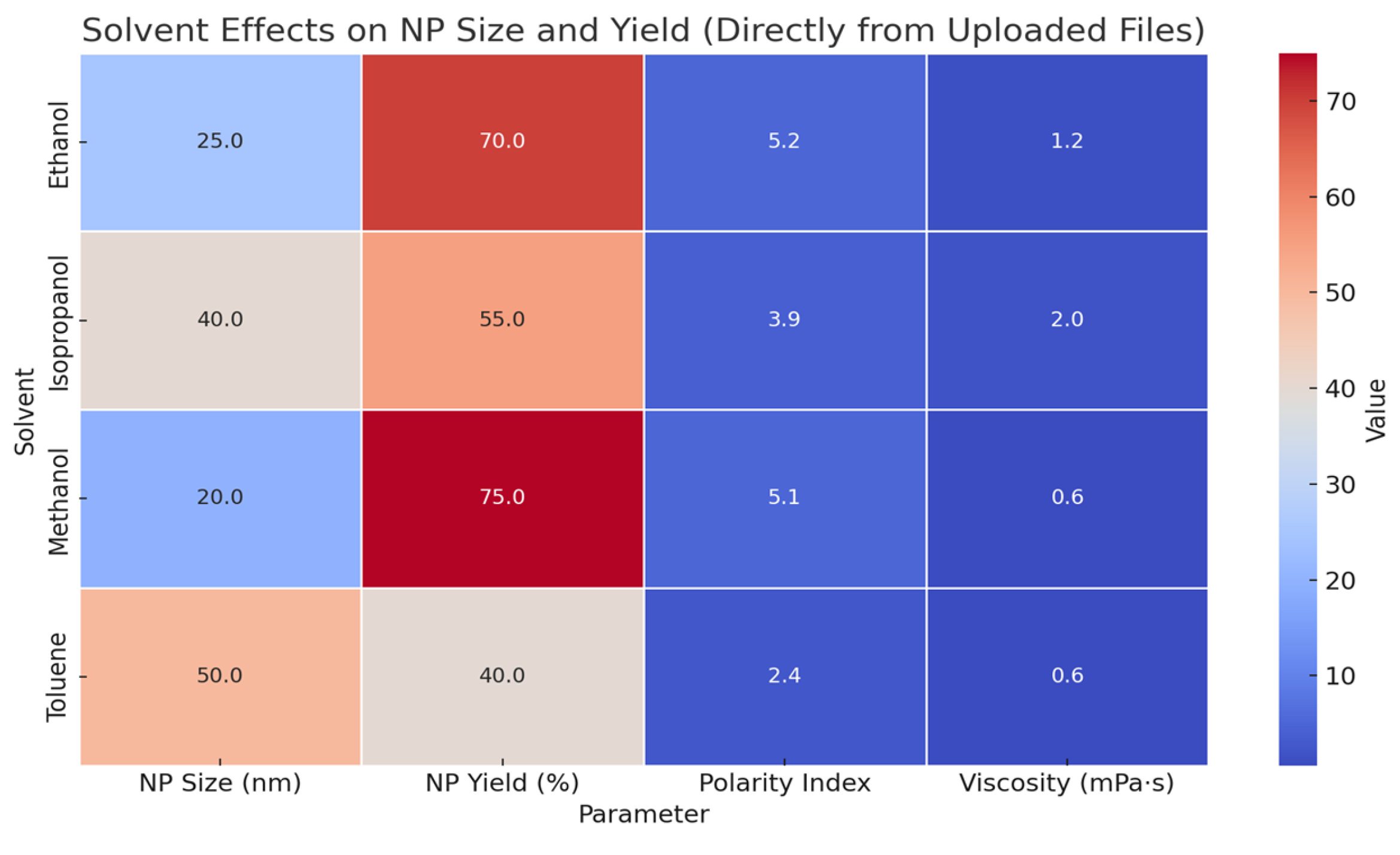
| Parameter | Ethanol | Deionized Water |
|---|---|---|
| Oxidation | Minimal Ag2O formation [122,196] | Progressive Ag2O formation [196] |
| Mean Particle Size | ~12 nm [1] | Up to 25+ nm over time [196] |
| Zeta Potential | −30 to −45 mV [1,129] | −10 to −20 mV [1] |
| Plasmon Peak | Stable at 400–410 nm [197] | Red-shifted and broadened [196] |
| Applications | SERS, antimicrobial, sensing [2,197] | Less ideal due to oxidation [196] |
5.4.8. Silicon-Based Nanoparticles
5.4.9. Oxide and Doped Nanoparticles
6. Electrical Discharge and Other Pulsed Energy Techniques
6.1. Spark Discharge Nanoparticle Synthesis
6.2. Arc Discharge Techniques
6.3. Nanosecond Pulsed Discharges and Microplasmas
6.4. Ultrasonics
6.5. Mechanistic Distinctions and Reaction Zones
- Hot Zone: Closest to the discharge plasma, this region reaches several thousand Kelvin, leading to the immediate vaporization and ionization of electrode material. Rapid cooling in this zone leads to the formation of small, spherical, and highly crystalline nanoparticles. For instance, Chang et al. reported the synthesis of ~7–8 nm TiO2 and CuO NPs in this region under spark discharge conditions [198].
- Intermediate Zone: Extending outward, this area contains partially ionized vapor where recombination reactions, radical-induced alloying, and ion–molecule collisions dominate. Here, processes such as core–shell formation or alloying (e.g., Sn/Zn and Cu/Zn) are facilitated by sufficient thermal energy and reactive species density [197].
- Cold Zone: Situated furthest from the plasma source, this region features low temperatures and energy densities. It promotes condensation-driven growth of larger particles, agglomeration, or surface oxidation. Material formed here often exhibits reduced crystallinity or porosity due to slower quenching dynamics.
6.6. Comparative Assessment with PLAL
6.7. Examples of Synthesized Materials
| Feature/Parameter | PLAL | Spark Discharge | Arc Discharge | Nanosecond/ Microplasma |
|---|---|---|---|---|
| Energy Source | Laser pulses (ns–fs) | High-voltage capacitor pulses | Continuous high-current arc | High-voltage nanosecond pulses |
| Plasma Duration | ~10−9–10−12 s | ~10−6–10−3 s | ~10−3–1 s | 1–1000 ns |
| Material Input | Solid target | Metal electrodes | Metal or carbon electrodes | Electrodes + liquid/gas |
| Control Over NP Size | High (via fluence, pulse width) | Moderate (via pulse energy/freq) | Low to moderate | High (via pulse duration/energy) |
| Surface Cleanliness | High (no stabilizers) | High | Moderate (risk of contamination) | High |
| Product Types | Metals, oxides, QDs | Metals, alloys, some oxides | Carbon NPs, metal carbides, alloys | Alloys, nitrides, reactive clusters |
| Throughput | Low–moderate | Moderate | High | Low–moderate |
| Scalability | Limited by laser power/optics | High (simple circuits) | Moderate (thermal management needed) | Moderate |
| Setup Cost | High | Low | Moderate | Moderate |
7. Characterization Techniques
7.1. Morphological Characterization
7.2. Structural Characterization
7.3. Chemical and Surface Characterization
In Situ and Real-Time Techniques
7.4. Cavitation Bubble Characterization
7.5. Plasma-Shockwave Characterization
7.6. Nanoparticle Growth Characterization
7.7. In Situ and Time-Resolved Characterization of Defects
7.8. Computational and Machine Learning Advances in Defect Engineering
7.9. Machine Learning for Defect Detection and Process Optimization
8. Challenges and Limitations
8.1. Scalability and Reproducibility
8.2. Incomplete Mechanistic Understanding
8.3. Environmental and Safety Considerations
8.4. Equipment and Cost Barriers
9. Future Perspectives
9.1. Integration with Artificial Intelligence (AI)/Machine Learning (ML) for Process Optimization
9.2. Hybrid Techniques and Combinatorial Approaches
9.3. Towards Greener and Sustainable Synthesis
- Using renewable or benign solvents (e.g., water, ethanol, plant extracts),
- Recovering and recycling ablation targets and solvents,
- Improving energy efficiency via high-repetition, low-energy laser systems or pulsed power sources,
- Developing life cycle assessments (LCA) for pulsed synthesis routes.
9.4. Trends in Industrial Adoption
9.5. Safety, Environmental, and Regulatory Considerations
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Trout, C.J.; Kumpf, P.; Sipps, K.; Griepenburg, J.C.; O’Malley, S.M. The Influence of Alkanethiols on the Production of Hydrophobic Gold Nanoparticles via Pulsed Laser Ablation in Liquids. Nanomanufacturing 2021, 1, 98–108. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Kylychbekov, S.; Majidov, I.; Khuzhakulov, Z.; Er, A.Y.; Kitchens, C.; Banga, S.; Er, A.O. The Role of Efflux Pump Inhibitor in Enhancing Antimicrobial Efficiency of Ag NPs and MB as an Effective Photodynamic Therapy Agent. Photodiagnosis Photodyn. Ther. 2024, 47, 104212. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, J.-P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.; Takeda, Y.; Kondow, T.; Sawabe, H. Formation and Size Control of Silver Nanoparticles by Laser Ablation in Aqueous Solution. J. Phys. Chem. B 2000, 104, 9111–9117. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Liang, C. Diverse Nanomaterials Synthesized by Laser Ablation of Pure Metals in Liquids. Sci. China Phys. Mech. Astron. 2022, 65, 274203. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Reich, S.; Schönfeld, P.; Letzel, A.; Kohsakowski, S.; Olbinado, M.; Gökce, B.; Barcikowski, S.; Plech, A. Fluence Threshold Behaviour on Ablation and Bubble Formation in Pulsed Laser Ablation in Liquids. ChemPhysChem 2017, 18, 1084–1090. [Google Scholar] [CrossRef]
- Khairani, I.Y.; Mínguez-Vega, G.; Doñate-Buendía, C.; Gökce, B. Green Nanoparticle Synthesis at Scale: A Perspective on Overcoming the Limits of Pulsed Laser Ablation in Liquids for High-Throughput Production. Phys. Chem. Chem. Phys. 2023, 25, 19380–19408. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of Surfactant-Free Colloidal Nanoparticles in Heterogeneous Catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Lee, S.J.; Shwetharani, R.; Kim, H.-S.; Pasha, S.K.K.; Ashokkumar, M.; Choi, M.Y. Fundamentals and Comprehensive Insights on Pulsed Laser Synthesis of Advanced Materials for Diverse Photo- and Electrocatalytic Applications. Light Sci. Appl. 2022, 11, 250. [Google Scholar] [CrossRef]
- Elahi, N.; Rizwan, M. Progress and Prospects of Magnetic Iron Oxide Nanoparticles in Biomedical Applications: A Review. Artif. Organs 2021, 45, 1272–1299. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Annamuradov, B.; Ateyeh, M.; Street, C.; Whipple, H.; Er, A.O. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem 2024, 4, 434–461. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Gaudiuso, R.; De Pascale, O.; De Giacomo, A. Mechanisms and Processes of Pulsed Laser Ablation in Liquids during Nanoparticle Production. Appl. Surf. Sci. 2015, 348, 4–9. [Google Scholar] [CrossRef]
- Gatsa, O.; Tahir, S.; Flimelová, M.; Riahi, F.; Doñate-Buendia, C.; Gökce, B.; Bulgakov, A.V. Unveiling Fundamentals of Multi-Beam Pulsed Laser Ablation in Liquids toward Scaling up Nanoparticle Production. Nanomaterials 2024, 14, 365. [Google Scholar] [CrossRef]
- Luo, J.; Niu, Z. Jet and Shock Wave from Collapse of Two Cavitation Bubbles. Sci. Rep. 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Subhan, A.; Mourad, A.-H.I.; Al-Douri, Y. Influence of Laser Process Parameters, Liquid Medium, and External Field on the Synthesis of Colloidal Metal Nanoparticles Using Pulsed Laser Ablation in Liquid: A Review. Nanomaterials 2022, 12, 2144. [Google Scholar] [CrossRef]
- Bulgakov, A.V.; Bulgakova, N.M. Recent Advances in Nanoparticle Generation in Liquids by Lasers: Revealing Formation Mechanisms and Tailoring Properties. Sci. China Phys. Mech. Astron. 2022, 65, 274207. [Google Scholar] [CrossRef]
- Alhajj, M.; Ghoshal, S.K. Sustainability, Safety, Biocompatibility and Benefits of Laser Ablated Gold, Silver and Copper Nanoparticles: A Comprehensive Review. J. Mol. Liq. 2024, 414, 126130. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Khuzhakulov, Z.; Kylychbekov, S.; Allamyradov, Y.; Majidov, I.; Ben Yosef, J.; Er, A.Y.; Kitchens, C.; Banga, S.; Badarudeen, S.; Er, A.O. Formation of Picosecond Laser-Induced Periodic Surface Structures on Steel for Knee Arthroplasty Prosthetics. Front. Met. Alloys 2023, 1, 1090104. [Google Scholar] [CrossRef]
- Majidov, I.; Allamyradov, Y.; Kylychbekov, S.; Khuzhakulov, Z.; Er, A.O. Phase Transition and Controlled Zirconia Implant Patterning Using Laser-Induced Shockwaves. Appl. Sci. 2025, 15, 362. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, Characteristics, and Applications in Analytical and Other Sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, T.-H.; Kim, H.-M.; Kim, H.-J.; Gwak, G.-H.; Paek, S.-M.; Oh, J.-M. Colloidal Behaviors of ZnO Nanoparticles in Various Aqueous Media. Toxicol. Environ. Health Sci. 2012, 4, 121–131. [Google Scholar] [CrossRef]
- Saimon, J.A.; Salim, E.T.; Amin, M.H.; Fakhri, M.A.; Azzahrani, A.S.; Ali, A.B.M.; Gopinath, S.C.B. Ag@WO3 Core–Shell Nanocomposite for Wide Range Photo Detection. Sci. Rep. 2024, 14, 28192. [Google Scholar] [CrossRef]
- Sahoo, A.; Dixit, T.; Kumari, A.; Gupta, S.; Kothandaraman, R.; Rajeev, P.P.; Rao, M.S.R.; Krishnan, S. Facile Control of Giant Green-Emission in Multifunctional ZnO Quantum Dots Produced in a Single-Step Process: Femtosecond Pulse Ablation. Nanoscale Adv. 2025, 7, 524–535. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef]
- Mehta, K.; Baruah, P.K. A Comprehensive Review and Outlook on the Experimental Techniques to Investigate the Complex Dynamics of Pulsed Laser Ablation in Liquid for Nanoparticle Synthesis. Rev. Sci. Instrum. 2022, 93, 091501. [Google Scholar] [CrossRef]
- Elsayed, K.A.; Imam, H.; Ahmed, M.A.; Ramadan, R. Effect of Focusing Conditions and Laser Parameters on the Fabrication of Gold Nanoparticles via Laser Ablation in Liquid. Opt. Laser Technol. 2013, 45, 495–502. [Google Scholar] [CrossRef]
- Blažeka, D.; Car, J.; Krstulović, N. Concentration Quantification of TiO2 Nanoparticles Synthesized by Laser Ablation of a Ti Target in Water. Materials 2022, 15, 3146. [Google Scholar] [CrossRef]
- Hwang, J.S.; Park, J.-E.; Kim, G.W.; Nam, H.; Yu, S.; Jeon, J.S.; Kim, S.; Lee, H.; Yang, M. Recycling Silver Nanoparticle Debris from Laser Ablation of Silver Nanowire in Liquid Media toward Minimum Material Waste. Sci. Rep. 2021, 11, 2262. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Leng, D.; Fang, S.; Yang, Y.; Du, Y. Machine Learning Applications in Nanomaterials: Recent Advances and Future Perspectives. Chem. Eng. J. 2024, 500, 156687. [Google Scholar] [CrossRef]
- De Bonis, A.; Sansone, M.; D’Alessio, L.; Galasso, A.; Santagata, A.; Teghil, R. Dynamics of Laser-Induced Bubble and Nanoparticles Generation during Ultra-Short Laser Ablation of Pd in Liquid. J. Phys. D Appl. Phys. 2013, 46, 445301. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-Mediated Cellular Response Is Size-Dependent. Nat. Nanotech 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-Dependent Internalization of Particles via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef]
- Al-Kinani, M.A.; Haider, A.J.; Al-Musawi, S. Design and Synthesis of Nanoencapsulation with a New Formulation of Fe@Au-CS-CU-FA NPs by Pulsed Laser Ablation in Liquid (PLAL) Method in Breast Cancer Therapy: In Vitro and In Vivo. Plasmonics 2021, 16, 1107–1117. [Google Scholar] [CrossRef]
- Talib, S.M.; Haider, A.J.; Al-Musawi, S.; Al-Joudi, F.S.; Ahmed, S.A. Laser-Fabricated Metal Oxide Core–Shell Nanoparticles for Biomedical Applications: A Mini Review. Plasmonics 2025, 20, 7509–7526. [Google Scholar] [CrossRef]
- Yu, Y.; Ni, M.; Zheng, Y.; Huang, Y. Airway Epithelial-Targeted Nanoparticle Reverses Asthma in Inhalation Therapy. J. Control. Release 2024, 367, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in Photodynamic Antimicrobial Chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Lavaee, F.; Motamedifar, M.; Rafiee, G. The Effect of Photodynamic Therapy by Gold Nanoparticles on Streptococcus Mutans and Biofilm Formation: An in Vitro Study. Lasers Med. Sci. 2022, 37, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Abdelgwad, M.; Sabry, D.; Mohamed Abdelgawad, L.; Mohamed Elroby Ali, D. In Vitro Differential Sensitivity of Head and Neck Squamous Cell Carcinoma to Cisplatin, Silver Nanoparticles, and Photodynamic Therapy. Rep. Biochem. Mol. Biol. 2022, 11, 224–237. [Google Scholar] [CrossRef]
- Liu, X.-L.; Dong, X.; Yang, S.-C.; Lai, X.; Liu, H.-J.; Gao, Y.; Feng, H.-Y.; Zhu, M.-H.; Yuan, Y.; Lu, Q.; et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy. Adv. Sci. 2021, 8, 2003679. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Mokeem, L.; Alsahafi, R.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Magnetic-Based Photosensitizer to Improve the Efficiency of Antimicrobial Photodynamic Therapy against Mature Dental Caries-Related Biofilms. MedComm–Biomater. Appl. 2023, 2, e67. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.; Cheon, J. Magnetic Nanoparticles for Multi-Imaging and Drug Delivery. Mol. Cells 2013, 35, 274–284. [Google Scholar] [CrossRef]
- Dessie, Y.; Tilahun, E.; Wondimu, T.H. Functionalized Carbon Electrocatalysts in Energy Conversion and Storage Applications: A Review. Heliyon 2024, 10, e39395. [Google Scholar] [CrossRef]
- Abdulwahid, F.S.; Haider, A.J.; Al-Musawi, S. Effect of Laser Parameter on Fe3O4 NPs Formation by Pulsed Laser Ablation in Liquid. AIP Conf. Proc. 2023, 2769, 020039. [Google Scholar] [CrossRef]
- Hesabizadeh, T.; Hicks, E.; Medina Cruz, D.; Bourdo, S.E.; Watanabe, F.; Bonney, M.; Nichols, J.; Webster, T.J.; Guisbiers, G. Synthesis of “Naked” TeO2 Nanoparticles for Biomedical Applications. ACS Omega 2022, 7, 23685–23694. [Google Scholar] [CrossRef]
- Mat Isa, S.Z.; Zainon, R.; Tamal, M. State of the Art in Gold Nanoparticle Synthesisation via Pulsed Laser Ablation in Liquid and Its Characterisation for Molecular Imaging: A Review. Materials 2022, 15, 875. [Google Scholar] [CrossRef] [PubMed]
- Altuwirqi, R.M. Graphene Nanostructures by Pulsed Laser Ablation in Liquids: A Review. Materials 2022, 15, 5925. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Panigrahi, D.; Patel, S.K.; Dhupal, D. Microchanneling on Bio-Inert Dental Ceramic Using Dry Pulsed Laser Ablation and Liquid Supported Pulsed Laser Ablation Approach. Opt. Lasers Eng. 2021, 144, 106654. [Google Scholar] [CrossRef]
- Al Baroot, A.; Elsayed, K.A.; Khan, F.A.; Haladu, S.A.; Ercan, F.; Çevik, E.; Drmosh, Q.A.; Almessiere, M.A. Anticancer Activity of Au/CNT Nanocomposite Fabricated by Nanosecond Pulsed Laser Ablation Method on Colon and Cervical Cancer. Micromachines 2023, 14, 1455. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Güner, S.; Slimani, Y.; Hassan, M.; Baykal, A.; Gondal, M.A.; Baig, U.; Trukhanov, S.V.; Trukhanov, A.V. Structural and Magnetic Properties of Co0.5Ni0.5Ga0.01Gd0.01Fe1.98O4/ZnFe2O4 Spinel Ferrite Nanocomposites: Comparative Study between Sol-Gel and Pulsed Laser Ablation in Liquid Approaches. Nanomaterials 2021, 11, 2461. [Google Scholar] [CrossRef]
- Popov, A.A.; Swiatkowska-Warkocka, Z.; Marszalek, M.; Tselikov, G.; Zelepukin, I.V.; Al-Kattan, A.; Deyev, S.M.; Klimentov, S.M.; Itina, T.E.; Kabashin, A.V. Laser-Ablative Synthesis of Ultrapure Magneto-Plasmonic Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials 2022, 12, 649. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Vilas, A.M.; Boutinguiza, M.; Rodríguez, D.; Arias-González, F.; Pou-Álvarez, P.; Riveiro, A.; Gil, J.; Pou, J. Palladium Nanoparticles Synthesized by Laser Ablation in Liquids for Antimicrobial Applications. Nanomaterials 2022, 12, 2621. [Google Scholar] [CrossRef]
- Hadi, A.J.; Nayef, U.M.; Jabir, M.S.; Mutlak, F.A.-H. Laser-Ablated Tin Dioxide Nanoparticle Synthesis for Enhanced Biomedical Applications. Plasmonics 2023, 18, 1667–1677. [Google Scholar] [CrossRef]
- Fakhri, M.A.; Salim, E.T.; Sulaiman, G.M.; Albukhaty, S.; Ali, H.S.; Salim, Z.T.; Gopinath, S.C.B.; Hashim, U.; Al-aqbi, Z.T. Gold Nanowires Based on Photonic Crystal Fiber by Laser Ablation in Liquid to Improve Colon Biosensor. Plasmonics 2023, 18, 2447–2463. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Karczewski, G. Laser Synthesis of Magnetic Nanoparticles in Liquids and Application in the Fabrication of Polymer–Nanoparticle Composites. ACS Appl. Nano Mater. 2021, 4, 6407–6440. [Google Scholar] [CrossRef]
- Pastukhov, A.I.; Belyaev, I.B.; Bulmahn, J.C.; Zelepukin, I.V.; Popov, A.A.; Zavestovskaya, I.N.; Klimentov, S.M.; Deyev, S.M.; Prasad, P.N.; Kabashin, A.V. Laser-Ablative Aqueous Synthesis and Characterization of Elemental Boron Nanoparticles for Biomedical Applications. Sci. Rep. 2022, 12, 9129. [Google Scholar] [CrossRef] [PubMed]
- Imran, H.J.; Hubeatir, K.A.; Aadim, K.A. A Novel Method for ZnO@NiO Core–Shell Nanoparticle Synthesis Using Pulse Laser Ablation in Liquid and Plasma Jet Techniques. Sci. Rep. 2023, 13, 5441. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Ali, A.; Abd, A. Bio-Logical Effect on Fungi, Bacteria and Cancer Cells with a Study of the Aging of Selenium Dioxide Particles Produced by Pulsed Laser. J. Optoelectron. Biomed. Mater. 2025, 17, 87–97. [Google Scholar] [CrossRef]
- Alheshibri, M.; Elsayed, K.A.; Khan, F.A.; Haladu, S.A.; Ercan, F.; Çevik, E.; Drmosh, Q.A.; Kayed, T.S.; Almessiere, M.A. Tuning the Morphology of Au/ZnO Nanocomposite Using Pulsed Laser Ablation for Anticancer Applications. Arab. J. Sci. Eng. 2024, 49, 1063–1074. [Google Scholar] [CrossRef]
- Khamees, E.J.; Rashid, E.Y.; Al-Kufaishi, Z.H.J.; Mohammed, K.A.; Al-Taee, R.A.M.; Sharma, S.; Kalyani, T.; Sharma, M.; Kulshreshta, A.; Kumar, A.; et al. Nanosecond-Pulsed Laser Ablation Synthesis of Gold Nanoparticles in DDDW, NaOH, and DMEM Liquid Media: Unveiling of Microstructural Morphological, Chemical, Optical, and Structural Characterizations and Cytotoxic Evaluation of Enhanced Anticancer Efficacy. Gold. Bull. 2025, 58, 10. [Google Scholar] [CrossRef]
- Costa, D.C.; Fernandes, M.; Moura, C.; Miranda, G.; Silva, F.; Carvalho, Ó.; Madeira, S. Laser Ablation in Liquid-Assisted Synthesis of Three Types of Nanoparticles for Enhanced Antibacterial Applications. Int. J. Precis. Eng. Manuf. Green Technol. 2025, 12, 1699–1717. [Google Scholar] [CrossRef]
- Hesabizadeh, T. Selenium Nanoparticles Synthesized by Pulsed Laser Ablation in Liquids for Antimicrobial Applications. Ph.D. Thesis, University of Arkansas at Little Rock, Little Rock, AR, USA, 2025. [Google Scholar]
- Cortes, F.R.U.; Falomir, E.; Doñate-Buendía, C.; Mínguez-Vega, G. A Review on Pulsed Laser-Based Synthesis of Carbon and Graphene Quantum Dots in Liquids: From Fundamentals, Chemistry to Bio Applications and Beyond. J. Phys. Chem. C 2025, 129, 10378–10414. [Google Scholar] [CrossRef]
- Al-Helaly, M.T.; Hussain, S.A.; Shaheed, M.A. Investigation of Structural and Optical Properties of Palladium–Copper Nanoparticles Synthesized by Pulsed Laser Ablation Method for Biomedical Applications. J. Appl. Bioanal. 2025, 11, 521–531. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying–Realloying Enabled High Durability for Pt–Pd-3d-Transition Metal Nanoparticle Fuel Cell Catalysts. Nat. Commun. 2021, 12, 859. [Google Scholar] [CrossRef]
- Lasemi, N.; Wicht, T.; Bernardi, J.; Liedl, G.; Rupprechter, G. Defect-Rich CuZn Nanoparticles for Model Catalysis Produced by Femtosecond Laser Ablation. ACS Appl. Mater. Interfaces 2024, 16, 38163–38176. [Google Scholar] [CrossRef]
- Pandith, A.; Jayaprakash, G.K.; ALOthman, Z.A. Surface-Modified CuO Nanoparticles for Photocatalysis and Highly Efficient Energy Storage Devices. Environ. Sci. Pollut. Res. 2023, 30, 43320–43330. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Raza, M.A.; Chishti, U.N.; Hussnain, A.; Maqsood, M.F.; Iqbal, M.Z.; Iqbal, M.J.; Latif, U. Role of Carbon Nanomaterials on Enhancing the Supercapacitive Performance of Manganese Oxide-Based Composite Electrodes. Arab. J. Sci. Eng. 2023, 48, 8371–8386. [Google Scholar] [CrossRef]
- Patil, P.H.; Kulkarni, V.V.; Jadhav, S.A. An Overview of Recent Advancements in Conducting Polymer–Metal Oxide Nanocomposites for Supercapacitor Application. J. Compos. Sci. 2022, 6, 363. [Google Scholar] [CrossRef]
- Alshammari, T.K.; Ghoshal, S.K.; Bakhtiar, H.; Salim, A.A.; Alias, S.S. Elucidating the Structural and Spectroscopic Attributes of Titania-Iron Oxide (TiO2-(α-Fe2O3)) Nanocomposites: Showcasing a Pulsed Laser Ablation in Liquid Approach. Mater. Chem. Phys. 2024, 318, 129235. [Google Scholar] [CrossRef]
- Siddiq, M.; Ur Rehman, Z.; Asim Rasheed, M.; Ul Hassan, S.M.; Qayyum, H.; Mehmood, S.; Qayyum, A. Synthesis of Bimetallic Core/Shell Nanoparticles via Pulse Laser Ablation and Their Catalytic Effectiveness in Dye Degradation. J. Laser Appl. 2024, 36, 032008. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A.; Awwad, N.S.; Ibrahium, H.A. Synthesis of Multi-Walled Carbon Nanotubes Decorated with Silver Metallic Nanoparticles as a Catalytic Degradable Material via Pulsed Laser Ablation in Liquid Media. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 126992. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A.; Awwad, N.S.; Ibrahium, H.A. Catalytic Activity of Ag Nanoparticles and Au/Ag Nanocomposite Prepared by Pulsed Laser Ablation Technique against 4-Nitrophenol for Environmental Applications. J. Mater. Sci. Mater. Electron. 2021, 32, 11978–11988. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Zheng, Z.; Yan, J.; Sun, L.; Huang, Z.; Li, X. A Review on Pulsed Laser Fabrication of Nanomaterials in Liquids for (Photo)Catalytic Degradation of Organic Pollutants in the Water System. Nanomaterials 2023, 13, 2628. [Google Scholar] [CrossRef]
- Lin, Z.; Shen, S.; Wang, Z.; Zhong, W. Laser Ablation in Air and Its Application in Catalytic Water Splitting and Li-Ion Battery. iScience 2021, 24, 102469. [Google Scholar] [CrossRef]
- Lo Pò, C.; Boscarino, S.; Scalese, S.; Boninelli, S.; Grimaldi, M.G.; Ruffino, F. Pulsed Laser Ablation of Recycled Copper in Methanol: A New Route toward Sustainable Plasmonic and Catalytic Nanostructures. Appl. Surf. Sci. Adv. 2025, 26, 100712. [Google Scholar] [CrossRef]
- Aziz, S.M.A.; Nayef, U.M.; Rasheed, M. Synthesis of CuO@ZnO Nanoparticle Core–Shell Formed via Laser Ablation in Liquid for Photocatalytic Applications. Plasmonics 2025, 20, 2595–2605. [Google Scholar] [CrossRef]
- Attallah, A.H.; Abdulwahid, F.S.; Ali, Y.A.; Haider, A.J. Enhanced Characteristics of Iron Oxide Nanoparticles for Efficient Pollutant Degradation via Pulsed Laser Ablation in Liquid. Plasmonics 2024, 19, 2581–2594. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Svetlichnyi, V.A.; Kulinich, S.A. Green Laser Ablation-Based Synthesis of Functional Nanomaterials for Generation, Storage, and Detection of Hydrogen. Curr. Opin. Green Sustain. Chem. 2022, 33, 100566. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shukla, S.; Sastikumar, D.; Koinkar, P. Progress in Pulsed Laser Ablation in Liquid (PLAL) Technique for the Synthesis of Carbon Nanomaterials: A Review. Appl. Phys. A 2021, 127, 810. [Google Scholar] [CrossRef]
- Chen, C.; Zhigilei, L.V. Atomistic Modeling of Pulsed Laser Ablation in Liquid: Spatially and Time-Resolved Maps of Transient Nonequilibrium States and Channels of Nanoparticle Formation. Appl. Phys. A 2023, 129, 288. [Google Scholar] [CrossRef]
- Oh, Y.; Vamsi Krishna, B.N.; Jung, H.J.; Toor, A.; Lee, S.J. Rhodium Nanoparticle-Supported Graphitic Carbon-Encapsulated Nickel Metal Core Electrocatalyst via Pulsed Laser Ablation for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2025, 17, 44402–44410. [Google Scholar] [CrossRef]
- Li, S.; Du, B.; Cui, Q.; Ye, J.; Cui, H.; Gao, H.; Wang, Y.; Zheng, Y.; Han, J. A Review on Liquid Pulsed Laser Propulsion. Aerospace 2025, 12, 604. [Google Scholar] [CrossRef]
- Xue, S.; Cao, S.; Huang, Z.; Yang, D.; Zhang, G. Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials 2021, 14, 4263. [Google Scholar] [CrossRef]
- Feng, Z.; Gao, C.; Ma, X.; Zhan, J. Well-Dispersed Pd Nanoparticles on Porous ZnO Nanoplates via Surface Ion Exchange for Chlorobenzene-Selective Sensor. RSC Adv. 2019, 9, 42351–42359. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Ankitha, M.; Pillai, V.K.; Alwarappan, S. Graphene Quantum Dots for Biosensing and Bioimaging. RSC Adv. 2024, 14, 16001–16023. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Huang, Y.; Chen, Z.; Pan, Y.; Zhang, X.; Long, Q.; Yang, Q.; Wu, T.; Xie, T.-Z.; Wang, M.; et al. Terpyridine-Based Metallo-Cuboctahedron Nanomaterials for Efficient Photocatalytic Degradation of Persistent Organic Pollutants. Nano Res. 2024, 17, 6833–6840. [Google Scholar] [CrossRef]
- Gera, T.; Kondász, B.; Smausz, T.; Kopniczky, J.; Hodovány, S.; Ajtai, T.; Szabó-Révész, P.; Ambrus, R.; Csóka, I.; Hopp, B. Pulsed Laser Ablation of Polymer-Based Magnetic Nanocomposites for Oil Spill Remediation. Clean. Mater. 2024, 11, 100235. [Google Scholar] [CrossRef]
- Hassan, N.; Fakhri, M.; Salim, E. Physical Properties of Pure Gold Nanoparticles and Gold Doped ZnO Nanoparticles Using Laser Ablation in Liquid For Sensor Applications. Eng. Technol. J. 2022, 40, 422–427. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.; Jung, S.; Lee, J.; Shin, B.S. Surface Morphological Growth Characteristics of Laser-Induced Graphene with UV Pulsed Laser and Sensor Applications. ACS Mater. Lett. 2023, 5, 1261–1270. [Google Scholar] [CrossRef]
- Kalai Priya, A.; Yogesh, G.K.; Subha, K.; Kalyanavalli, V.; Sastikumar, D. Synthesis of Silver Nano-Butterfly Park by Using Laser Ablation of Aqueous Salt for Gas Sensing Application. Appl. Phys. A 2021, 127, 292. [Google Scholar] [CrossRef]
- Lanza, G.; Betancourth, D.; Avila, A.; Riascos, H.; Perez-Taborda, J.A. Control of the Size Distribution of AuNPs for Colorimetric Sensing by Pulsed Laser Ablation in Liquids. Kuwait J. Sci. 2025, 52, 100294. [Google Scholar] [CrossRef]
- Choi, J.-G.; Baek, S.; Lee, J.; Park, S. Scalable Metal-Based Nanoparticle Synthesis via Laser Ablation in Liquids for Transformative Sensory and Synaptic Devices. Int. J. Extrem. Manuf. 2025, 7, 062001. [Google Scholar] [CrossRef]
- Nancy, P.; Arya Nair, J.S.; Thomas, S.; Sandhya, K.Y.; Kalarikkal, N. Laser Driven Exfoliation and in Situ Engineering of MoS2/WS2–Ag Nanocomposites for High-Performance Electrochemical Sensing and Photonic Applications. New J. Chem. 2025, 49, 15023–15037. [Google Scholar] [CrossRef]
- Puliyasseri, R.; Anbalagan, K.P.; Sastikumar, D. Single-Step Synthesis of N, B Co-Doped Graphene Oxide by Nanosecond Pulsed Laser Ablation in Liquid for Clad-Modified Fiber Optic Gas Sensing Application. Appl. Phys. A 2025, 131, 596. [Google Scholar] [CrossRef]
- Sabbar, G.; Mohammed, W.M.; Al-Nafiey, A. Temperature-Dependent Gas Sensing Properties of Cobalt Oxide, Reduced Graphene Oxide, and Composite Thin Films Synthesized by Pulsed Laser Ablation. Arab. J. Sci. Eng. 2025, 50, 1–15. [Google Scholar] [CrossRef]
- Byram, C.; Moram, S.S.B.; Banerjee, D.; Beeram, R.; Rathod, J.; Soma, V.R. Review of Ultrafast Laser Ablation for Sensing and Photonic Applications. J. Opt. 2023, 25, 043001. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Kozak, K.; Duraczyńska, D.; Wiertel-Pochopień, A.; Zawała, J.; Szczepanowicz, K. Silver Shell Thickness-Dependent Conductivity of Coatings Based on Ni@Ag Core@shell Nanoparticles. Nanotechnol. Sci. Appl. 2023, 16, 73–84. [Google Scholar] [CrossRef]
- Didde, S.; Dubey, R.S. Sol–Gel Derived Ceramic Nanoparticles as an Alternative Material for Microstrip Patch Antenna in WLAN Applications. Sci. Rep. 2024, 14, 13684. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Vázquez, M.; Brabazon, D. Post-Fabrication Adjustment of Metalloid Mg–C-Graphene Nanoparticles via Pulsed Laser Ablation for Paper Electronics and Process Optimisation. Mater. Des. 2024, 240, 112869. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Magnesium Nanoparticle Synthesis from Powders via Pulsed Laser Ablation in Liquid for Nanocolloid Production. Appl. Sci. 2021, 11, 10974. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Fitzpatrick, B.; Brabazon, D. Effect of Liquid Medium and Laser Processing Parameters on the Fabrication of Carbon Nanoparticles via Pulsed Laser Ablation in Liquid towards Paper Electronics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128151. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Fu, Y.; Yao, Q.; Li, Z.; Sugioka, K. Liquid Vortexes and Flows Induced by Femtosecond Laser Ablation in Liquid Governing Formation of Circular and Crisscross LIPSS. Opto-Electron. Adv. 2022, 5, 210066. [Google Scholar] [CrossRef]
- Marabotti, P.; Peggiani, S.; Vidale, A.; Spartaco Casari, C. Pulsed Laser Ablation in Liquid of Sp-Carbon Chains: Status and Recent Advances. Chin. Phys. B 2022, 31, 125202. [Google Scholar] [CrossRef]
- Parameswaran Sreekala, A.; Raveendran Nair, P.; Kundalam Kadavath, J.; Krishnan, B.; Avellaneda, D.A.; Anantharaman, M.R.; Shaji, S. Laser Processing in Liquids: Insights into Nanocolloid Generation and Thin Film Integration for Energy, Photonic, and Sensing Applications. Beilstein J. Nanotechnol. 2025, 16, 1428–1498. [Google Scholar] [CrossRef]
- Abdul Amir, H.A.A.; Fakhri, M.A.; Alwahib, A.A. Synthesized of GaN Nanostructure Using 1064 Nm Laser Wavelength by Pulsed Laser Ablation in Liquid. Eng. Technol. J. 2022, 40, 404–411. [Google Scholar] [CrossRef]
- Ismail, R.A.; Erten-Ela, S.; Ali, A.K.; Yavuz, C.; Hassoon, K.I. Pulsed Laser Ablation of Tin Oxide Nanoparticles in Liquid for Optoelectronic Devices. Silicon 2021, 13, 3229–3237. [Google Scholar] [CrossRef]
- Altowyan, A.S.; Mostafa, A.M.; Ahmed, H.A. Effect of Liquid Media and Laser Energy on the Preparation of Ag Nanoparticles and Their Nanocomposites with Au Nanoparticles via Laser Ablation for Optoelectronic Applications. Optik 2021, 241, 167217. [Google Scholar] [CrossRef]
- Ye, F.; Musselman, K.P. Synthesis of Low Dimensional Nanomaterials by Pulsed Laser Ablation in Liquid. APL Mater. 2024, 12, 050602. [Google Scholar] [CrossRef]
- Tsuta, M.; Nakamura, S.; Kato, A. Micronization of KSrPO4:Eu and KBaPO4:Eu Phosphor Particles for White Light-Emitting Diodes by Pulsed Laser Ablation in Liquid. Opt. Laser Technol. 2021, 135, 106725. [Google Scholar] [CrossRef]
- Sulaiman, E.M.; Mutlak, F.A.-H.; Nayef, U.M. High-Performance Photodetector of Au–MgO/PS Nanostructure Manufactured via Pulsed Laser Ablation Technique. Opt. Quantum Electron. 2022, 54, 744. [Google Scholar] [CrossRef]
- Rasheed, S.A.; Nayef, U.M.; Muayad, M.W. Synthesis of Al2O3 Nanoparticles via Laser Ablation for Photodetectors Application. Plasmonics 2025, 20, 6611–6621. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Tsuji, T.; Sakaki, S.; Koshizaki, N. Pulsed Laser Melting in Liquid for Crystalline Spherical Submicrometer Particle Fabrication–Mechanism, Process Control, and Applications. Prog. Mater. Sci. 2023, 131, 101004. [Google Scholar] [CrossRef]
- Ding, J.; Yuan, Q.; Xin, B.; Li, J.; Liu, S.; Wang, Y.; Deng, H.; Chen, Q. Atomic-Scale Defect and Surface Chemistry Control via Pulsed Laser Ablation to Boost Ethanol Response of MXene Sensors. Surf. Interfaces 2025, 73, 107469. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vazquez, M.; Brabazon, D. A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals 2023, 13, 253. [Google Scholar] [CrossRef]
- Altakroury, A.R.; Gatsa, O.; Riahi, F.; Fu, Z.; Flimelová, M.; Samokhvalov, A.; Barcikowski, S.; Doñate-Buendía, C.; Bulgakov, A.V.; Gökce, B. Size Control of Nanoparticles Synthesized by Pulsed Laser Ablation in Liquids Using Donut-Shaped Beams. Beilstein J. Nanotechnol. 2025, 16, 407–417. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef]
- Kuzmin, P.G.; Shafeev, G.A.; Bukin, V.V.; Garnov, S.V.; Farcau, C.; Carles, R.; Warot-Fontrose, B.; Guieu, V.; Viau, G. Silicon Nanoparticles Produced by Femtosecond Laser Ablation in Ethanol: Size Control, Structural Characterization, and Optical Properties. J. Phys. Chem. C 2010, 114, 15266–15273. [Google Scholar] [CrossRef]
- Spellauge, M.; Doñate-Buendía, C.; Barcikowski, S.; Gökce, B.; Huber, H.P. Comparison of Ultrashort Pulse Ablation of Gold in Air and Water by Time-Resolved Experiments. Light Sci. Appl. 2022, 11, 68. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; von Alvensleben, F.; Tünnermann, A. Femtosecond, Picosecond and Nanosecond Laser Ablation of Solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Pou-Álvarez, P.; Riveiro, A.; Nóvoa, X.R.; Fernández-Arias, M.; del Val, J.; Comesaña, R.; Boutinguiza, M.; Lusquiños, F.; Pou, J. Nanosecond, Picosecond and Femtosecond Laser Surface Treatment of Magnesium Alloy: Role of Pulse Length. Surf. Coat. Technol. 2021, 427, 127802. [Google Scholar] [CrossRef]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem.–A Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef] [PubMed]
- Mirghassemzadeh, N.; Ghamkhari, M.; Dorranian, D. Dependence of Laser Ablation Produced Gold Nanoparticles Characteristics on the Fluence of Laser Pulse. Soft Nanosci. Lett. 2013, 3, 121–125. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, A.; Chattopadhyaya, S.; Ding, C.-F. A Review on Critical Challenges in Additive Manufacturing via Laser-Induced Forward Transfer. Opt. Laser Technol. 2024, 168, 109893. [Google Scholar] [CrossRef]
- Zhang, H.; van Oosten, D.; Krol, D.M.; Dijkhuis, J.I. Saturation Effects in Femtosecond Laser Ablation of Silicon-on-Insulator. Appl. Phys. Lett. 2011, 99, 231108. [Google Scholar] [CrossRef]
- Yang, F.; Kang, R.; Ma, H.; Ma, G.; Wu, D.; Dong, Z. Effect of Femtosecond Laser Processing Parameters on the Ablation Microgrooves of RB-SiC Composites. Materials 2023, 16, 2536. [Google Scholar] [CrossRef]
- Mustafa, H.; Matthews, D.T.A.; Römer, G.R.B.E. The Role of Pulse Repetition Rate on Picosecond Pulsed Laser Processing of Zn and Zn-Coated Steel. Opt. Laser Technol. 2020, 131, 106408. [Google Scholar] [CrossRef]
- Ryabchikov, Y.V.; Kana, A.; Mirza, I. Performance of Pico-Second Laser-Designed Silicon/Gold Composite Nanoparticles Affected by Precision of Focus Position. Crystals 2025, 15, 132. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Makhesana, M.; Heidarinassab, S.; Plouze, A.; Vazquez, M.; Brabazon, D. A Review of Physical, Chemical and Biological Synthesis Methods of Bimetallic Nanoparticles and Applications in Sensing, Water Treatment, Biomedicine, Catalysis and Hydrogen Storage. Adv. Colloid Interface Sci. 2023, 321, 103010. [Google Scholar] [CrossRef]
- Schoppink, J.J.; Krizek, J.; Moser, C.; Fernandez Rivas, D. Cavitation Induced by Pulsed and Continuous-Wave Fiber Lasers in Confinement. Exp. Therm. Fluid Sci. 2023, 146, 110926. [Google Scholar] [CrossRef]
- Fazio, E.; Saija, R.; Santoro, M.; Abir, S.; Neri, F.; Tommasini, M.; Ossi, P.M. On the Optical Properties of Ag–Au Colloidal Alloys Pulsed Laser Ablated in Liquid: Experiments and Theory. J. Phys. Chem. C 2020, 124, 24930–24939. [Google Scholar] [CrossRef]
- Forte, G.; D’Urso, L.; Fazio, E.; Patanè, S.; Neri, F.; Puglisi, O.; Compagnini, G. The Effects of Liquid Environments on the Optical Properties of Linear Carbon Chains Prepared by Laser Ablation Generated Plasmas. Appl. Surf. Sci. 2013, 272, 76–81. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, P.; Zhang, R. The Effect of Different Solvents on the Morphology and Gas-Sensitive Properties of Tungsten Oxide Nanoparticles. Appl. Phys. A 2025, 131, 104. [Google Scholar] [CrossRef]
- Gholami, T.; Seifi, H.; Dawi, E.A.; Pirsaheb, M.; Seifi, S.; Aljeboree, A.M.; Hamoody, A.-H.M.; Altimari, U.S.; Ahmed Abass, M.; Salavati-Niasari, M. A Review on Investigating the Effect of Solvent on the Synthesis, Morphology, Shape and Size of Nanostructures. Mater. Sci. Eng. B 2024, 304, 117370. [Google Scholar] [CrossRef]
- Hettiarachchi, B.S.; Takaoka, Y.; Uetake, Y.; Yakiyama, Y.; Yoshikawa, H.Y.; Maruyama, M.; Sakurai, H. Mechanistic Study in Gold Nanoparticle Synthesis through Microchip Laser Ablation in Organic Solvents. Metals 2024, 14, 155. [Google Scholar] [CrossRef]
- Hettiarachchi, B.S.; Takaoka, Y.; Uetake, Y.; Yakiyama, Y.; Lim, H.H.; Taira, T.; Maruyama, M.; Mori, Y.; Yoshikawa, H.Y.; Sakurai, H. Uncovering Gold Nanoparticle Synthesis Using a Microchip Laser System through Pulsed Laser Ablation in Aqueous Solution. Ind. Chem. Mater. 2024, 2, 340–347. [Google Scholar] [CrossRef]
- Leekumjorn, S.; Gullapalli, S.; Wong, M.S. Understanding the Solvent Polarity Effects on Surfactant-Capped Nanoparticles. J. Phys. Chem. B 2012, 116, 13063–13070. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Gupta, S.K.; Manohar, R.; Varia, M.C.; Kumar, S.; Kumar, A. Effect of Cadmium Selenide Quantum Dots on the Dielectric and Physical Parameters of Ferroelectric Liquid Crystal. J. Appl. Phys. 2014, 116, 034106. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Tai, Y.-C. Effects of Alcohol Solvents on Anatase TiO2 Nanocrystals Prepared by Microwave-Assisted Solvothermal Method. J. Nanoparticle Res. 2013, 15, 1686. [Google Scholar] [CrossRef]
- Chemin, A.; Lam, J.; Laurens, G.; Trichard, F.; Motto-Ros, V.; Ledoux, G.; Jarý, V.; Laguta, V.; Nikl, M.; Dujardin, C.; et al. Doping Nanoparticles Using Pulsed Laser Ablation in a Liquid Containing the Doping Agent. Nanoscale Adv. 2019, 1, 3963–3972. [Google Scholar] [CrossRef]
- Kanitz, A.; Kalus, M.-R.; Gurevich, E.L.; Ostendorf, A.; Barcikowski, S.; Amans, D. Review on Experimental and Theoretical Investigations of the Early Stage, Femtoseconds to Microseconds Processes during Laser Ablation in Liquid-Phase for the Synthesis of Colloidal Nanoparticles. Plasma Sources Sci. Technol. 2019, 28, 103001. [Google Scholar] [CrossRef]
- Itina, T.E. On Nanoparticle Formation by Laser Ablation in Liquids. J. Phys. Chem. C 2011, 115, 5044–5048. [Google Scholar] [CrossRef]
- Motto-Ros, V.; Pelascini, F.; Dell’Aglio, M.; De Giacomo, A. Fate of Laser-Induced Plasma Material: Particle Formation and Re-Deposition Effects in Micro-LIBS Scanning Applications. Spectrochim. Acta Part B At. Spectrosc. 2025, 230, 107240. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Li, P.; Tian, Z.; Liang, C. Recent Advances in Surfactant-Free, Surface-Charged, and Defect-Rich Catalysts Developed by Laser Ablation and Processing in Liquids. ChemNanoMat 2017, 3, 512–533. [Google Scholar] [CrossRef]
- Ye, F.; Ayub, A.; Karimi, R.; Wettig, S.; Sanderson, J.; Musselman, K.P. Defect-Rich MoSe2 2H/1T Hybrid Nanoparticles Prepared from Femtosecond Laser Ablation in Liquid and Their Enhanced Photothermal Conversion Efficiencies. Adv. Mater. 2023, 35, 2301129. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.; Reichenberger, S.; Haxhiaj, I.; Barcikowski, S.; Müller, A.M. Mechanism of Laser-Induced Bulk and Surface Defect Generation in ZnO and TiO2 Nanoparticles: Effect on Photoelectrochemical Performance. ACS Appl. Energy Mater. 2018, 1, 5366–5385. [Google Scholar] [CrossRef]
- Liu, P.; Cai, W.; Zeng, H. Fabrication and Size-Dependent Optical Properties of FeO Nanoparticles Induced by Laser Ablation in a Liquid Medium. J. Phys. Chem. C 2008, 112, 3261–3266. [Google Scholar] [CrossRef]
- Švrček, V.; Kondo, M. Blue Luminescent Silicon Nanocrystals Prepared by Short Pulsed Laser Ablation in Liquid Media. Appl. Surf. Sci. 2009, 255, 9643–9646. [Google Scholar] [CrossRef]
- Tarasenka, N.; Butsen, A.; Pankov, V.; Tarasenko, N. Structural Defects and Magnetic Properties of Gadolinium Silicide Nanoparticles Synthesized by Laser Ablation Technique in Liquid. Phys. Status Solidi (b) 2013, 250, 809–814. [Google Scholar] [CrossRef]
- Singh, S.C.; Kotnala, R.K.; Gopal, R. Room Temperature Ferromagnetism in Liquid-Phase Pulsed Laser Ablation Synthesized Nanoparticles of Nonmagnetic Oxides. J. Appl. Phys. 2015, 118, 064305. [Google Scholar] [CrossRef]
- Honda, M.; Goto, T.; Owashi, T.; Rozhin, A.G.; Yamaguchi, S.; Ito, T.; Kulinich, S.A. ZnO Nanorods Prepared via Ablation of Zn with Millisecond Laser in Liquid Media. Phys. Chem. Chem. Phys. 2016, 18, 23628–23637. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Ye, Y.; Tian, Z.; Zhu, X.; Liang, C. Oxygen Defects Induce Strongly Coupled Pt/Metal Oxides/rGO Nanocomposites for Methanol Oxidation Reaction. ACS Appl. Energy Mater. 2019, 2, 5577–5583. [Google Scholar] [CrossRef]
- Yu, M.; Waag, F.; Chan, C.K.; Weidenthaler, C.; Barcikowski, S.; Tüysüz, H. Laser Fragmentation-Induced Defect-Rich Cobalt Oxide Nanoparticles for Electrochemical Oxygen Evolution Reaction. ChemSusChem 2020, 13, 520–528. [Google Scholar] [CrossRef]
- Ou, G.; Fan, P.; Ke, X.; Xu, Y.; Huang, K.; Wei, H.; Yu, W.; Zhang, H.; Zhong, M.; Wu, H.; et al. Defective Molybdenum Sulfide Quantum Dots as Highly Active Hydrogen Evolution Electrocatalysts. Nano Res. 2018, 11, 751–761. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Liu, J.; Chen, Q.; Zhu, X.; Liang, C. Carbon-Encapsulated Metal/Metal Carbide/Metal Oxide Core–Shell Nanostructures Generated by Laser Ablation of Metals in Organic Solvents. ACS Appl. Nano Mater. 2019, 2, 28–39. [Google Scholar] [CrossRef]
- Lv, J.; Tian, Z.; Dai, K.; Ye, Y.; Liang, C. Interface and Defect Engineer of Titanium Dioxide Supported Palladium or Platinum for Tuning the Activity and Selectivity of Electrocatalytic Nitrogen Reduction Reaction. J. Colloid Interface Sci. 2019, 553, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Two Mechanisms of Nanoparticle Generation in Picosecond Laser Ablation in Liquids: The Origin of the Bimodal Size Distribution—Nanoscale (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2018/nr/c7nr08614h (accessed on 7 September 2025).
- Ko, B.; Lu, W.; Sokolov, A.V.; Lee, H.W.H.; Scully, M.O.; Zhang, Z. Multi-Pulse Laser-Induced Bubble Formation and Nanoparticle Aggregation Using MoS2 Nanoparticles. Sci. Rep. 2020, 10, 15753. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Schönfeld, P.; Wagener, P.; Letzel, A.; Ibrahimkutty, S.; Gökce, B.; Barcikowski, S.; Menzel, A.; dos Santos Rolo, T.; Plech, A. Pulsed Laser Ablation in Liquids: Impact of the Bubble Dynamics on Particle Formation. J. Colloid Interface Sci. 2017, 489, 106–113. [Google Scholar] [CrossRef]
- Riahi, F.; Bußmann, A.; Doñate-Buendia, C.; Adami, S.; Adams, N.A.; Barcikowski, S.; Gökce, B. Characterizing Bubble Interaction Effects in Synchronous-Double-Pulse Laser Ablation for Enhanced Nanoparticle Synthesis. Photon. Res. PRJ 2023, 11, 2054–2071. [Google Scholar] [CrossRef]
- Baig, U.; Khan, A.; Gondal, M.A.; Dastageer, M.A.; Akhtar, S. Single-Step Synthesis of Silicon Carbide Anchored Graphitic Carbon Nitride Nanocomposite Photo-Catalyst for Efficient Photoelectrochemical Water Splitting under Visible-Light Irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125886. [Google Scholar] [CrossRef]
- Miskin, C.K.; Deshmukh, S.D.; Vasiraju, V.; Bock, K.; Mittal, G.; Dubois-Camacho, A.; Vaddiraju, S.; Agrawal, R. Lead Chalcogenide Nanoparticles and Their Size-Controlled Self-Assemblies for Thermoelectric and Photovoltaic Applications. ACS Appl. Nano Mater. 2019, 2, 1242–1252. [Google Scholar] [CrossRef]
- Bonatti, L.; Gil, G.; Giovannini, T.; Corni, S.; Cappelli, C. Plasmonic Resonances of Metal Nanoparticles: Atomistic vs. Continuum Approaches. Front. Chem. 2020, 8, 340. [Google Scholar] [CrossRef]
- Hakimov, S.; Kylychbekov, S.; Harness, B.; Neupane, S.; Hurley, J.; Brooks, A.; Banga, S.; Er, A.O. Evaluation of Silver Nanoparticles Attached to Methylene Blue as an Antimicrobial Agent and Its Cytotoxicity. Photodiagnosis Photodyn. Ther. 2022, 39, 102904. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Choe, K.; Zheng, F.; Wang, H.; Yuan, Y.; Zhao, W.; Xue, G.; Qiu, X.; Ri, M.; Shi, X.; Wang, Y.; et al. Fast and Selective Semihydrogenation of Alkynes by Palladium Nanoparticles Sandwiched in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2020, 59, 3650–3657. [Google Scholar] [CrossRef]
- Mastalir, Á.; Molnár, Á. Palladium Nanoparticles Supported on Porous Silica Materials as Heterogeneous Catalysts of C−C Coupling and Cross-Coupling Reactions. ChemCatChem 2023, 15, e202300643. [Google Scholar] [CrossRef]
- Díaz-Vázquez, E.D.; Cuellar, M.A.; Heredia, M.D.; Barolo, S.M.; González-Bakker, A.; Padrón, J.M.; Budén, M.E.; Martín, S.E.; Uberman, P.M. Palladium Nanoparticles for the Synthesis of Phenanthridinones and Benzo[c]Chromenes via C–H Activation Reaction. RSC Adv. 2024, 14, 18703–18715. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Molahalli, V.; Sharma, A.; Bijapur, K.; Soman, G.; Shetty, A.; Sirichandana, B.; Patel, B.G.M.; Chattham, N.; Hegde, G. Nanomaterials. In Copper-Based Nanomaterials in Organic Transformations; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2024; Volume 1466, pp. 1–33. [Google Scholar]
- Koga, K.; Hirasawa, M. Anisotropic Growth of NiO Nanorods from Ni Nanoparticles by Rapid Thermal Oxidation. Nanotechnology 2013, 24, 375602. [Google Scholar] [CrossRef]
- Wang, P.; Shi, T.; Mehta, N.; Yang, S.; Wang, H.; Liu, D.; Zhu, Z. Changes in Magnetic Properties of Magnetite Nanoparticles Upon Microbial Iron Reduction. Geochem. Geophys. Geosyst. 2022, 23, e2021GC010212. [Google Scholar] [CrossRef]
- Puscasu, E.; Sacarescu, L.; Lupu, N.; Grigoras, M.; Oanca, G.; Balasoiu, M.; Creanga, D. Iron Oxide-Silica Nanocomposites Yielded by Chemical Route and Sol–Gel Method. J. Sol-Gel Sci. Technol. 2016, 79, 457–465. [Google Scholar] [CrossRef]
- Yasmin, H.; Giwa, S.O.; Noor, S.; Sharifpur, M. Thermal Conductivity Enhancement of Metal Oxide Nanofluids: A Critical Review. Nanomaterials 2023, 13, 597. [Google Scholar] [CrossRef] [PubMed]
- Sutunkova, M.P.; Klinova, S.V.; Ryabova, Y.V.; Tazhigulova, A.V.; Minigalieva, I.A.; Shabardina, L.V.; Solovyeva, S.N.; Bushueva, T.V.; Privalova, L.I. Comparative Evaluation of the Cytotoxic Effects of Metal Oxide and Metalloid Oxide Nanoparticles: An Experimental Study. Int. J. Mol. Sci. 2023, 24, 8383. [Google Scholar] [CrossRef]
- Mbewana-Ntshanka, N.G.; Moloto, M.J.; Mubiayi, P.K. Antimicrobial Activity of the Synthesized of Copper Chalcogenide Nanoparticles. J. Nanotechnol. 2021, 2021, 6675145. [Google Scholar] [CrossRef]
- Khongiang, L.; Deb, S.; Kalita, P.K. Influence of Capping Agents for Controlling Structural and Optical Properties of Copper Chalcogenide (CuS) Nanoparticles. J. Phys. Conf. Ser. 2024, 2919, 012008. [Google Scholar] [CrossRef]
- Ramirez, O.; Ramasamy, P.; Chan Choi, Y.; Lee, J.-S. Morphology Transformation of Chalcogenide Nanoparticles Triggered by Cation Exchange Reactions. Chem. Mater. 2019, 31, 268–276. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ma, Z.; Wu, M.; Wang, L. Preparation of MXene with High Conductivity and Its Application on Conductive Fabrics. Appl. Nanosci. 2022, 12, 2317–2329. [Google Scholar] [CrossRef]
- Park, C.E.; Jeong, G.H.; Theerthagiri, J.; Lee, H.; Choi, M.Y. Moving beyond Ti2C3Tx MXene to Pt-Decorated TiO2@TiC Core–Shell via Pulsed Laser in Reshaping Modification for Accelerating Hydrogen Evolution Kinetics. ACS Nano 2023, 17, 7539–7549. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Gogotsi, O.; Baginskiy, I.; Balitskyi, V.; Zahorodna, V.; Husak, Y.; Yanko, I.; Pernakov, M.; Roshchupkin, A.; Lyndin, M.; et al. MXene-Assisted Ablation of Cells with a Pulsed Near-Infrared Laser. ACS Appl. Mater. Interfaces 2022, 14, 28683–28696. [Google Scholar] [CrossRef]
- Yang, H.-L.; Bai, L.-F.; Geng, Z.-R.; Chen, H.; Xu, L.-T.; Xie, Y.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon Quantum Dots: Preparation, Optical Properties, and Biomedical Applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Okafor, O.; Kim, K. Cytotoxicity of Quantum Dots in Receptor-Mediated Endocytic and Pinocytic Pathways in Yeast. Int. J. Mol. Sci. 2024, 25, 4714. [Google Scholar] [CrossRef]
- Alheshibri, M. Fabrication of Au–Ag Bimetallic Nanoparticles Using Pulsed Laser Ablation for Medical Applications: A Review. Nanomaterials 2023, 13, 2940. [Google Scholar] [CrossRef] [PubMed]
- De Anda Villa, M.; Gaudin, J.; Amans, D.; Boudjada, F.; Bozek, J.; Evaristo Grisenti, R.; Lamour, E.; Laurens, G.; Macé, S.; Nicolas, C.; et al. Assessing the Surface Oxidation State of Free-Standing Gold Nanoparticles Produced by Laser Ablation. Langmuir 2019, 35, 11859–11871. [Google Scholar] [CrossRef] [PubMed]
- Agati, M.; Hamdan, A.; Boninelli, S. Formation of Sn/Zn Alloy or Core-Shell Nanoparticles via Pulsed Nanosecond Discharges in Liquid Toluene. Mater. Chem. Phys. 2022, 292, 126858. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Tseng, K.-H.; Chang, J.-T.; Chung, M.-Y.; Lin, Z.-Y. A Study of Nano-Tungsten Colloid Preparing by the Electrical Spark Discharge Method. Micromachines 2022, 13, 2009. [Google Scholar] [CrossRef]
- Kohut, A.; Kéri, A.; Horváth, V.; Kopniczky, J.; Ajtai, T.; Hopp, B.; Galbács, G.; Geretovszky, Z. Facile and Versatile Substrate Fabrication for Surface Enhanced Raman Spectroscopy Using Spark Discharge Generation of Au/Ag Nanoparticles. Appl. Surf. Sci. 2020, 531, 147268. [Google Scholar] [CrossRef]
- Titov, E.; Bodrikov, I.; Titov, D. Control of the Energy Impact of Electric Discharges in a Liquid Phase. Energies 2023, 16, 1683. [Google Scholar] [CrossRef]
- Casian-Plaza, F.A.; Janovszky, P.M.; Palásti, D.J.; Kohut, A.; Geretovszky, Z.; Kopniczky, J.; Schubert, F.; Živković, S.; Galbács, Z.; Galbács, G. Comparison of Three Nanoparticle Deposition Techniques Potentially Applicable to Elemental Mapping by Nanoparticle-Enhanced Laser-Induced Breakdown Spectroscopy. Appl. Surf. Sci. 2024, 657, 159844. [Google Scholar] [CrossRef]
- Asyunin, V.I.; Bushin, S.A.; Davydov, S.G.; Dolgov, A.N.; Pilyushenko, A.V.; Pshenichnyi, A.A.; Revazov, V.O.; Yakubov, R.K. The Effect of Pulsed Vacuum-Arc Discharge on the Surface of Elements of a Discharger. Tech. Phys. Lett. 2016, 42, 368–371. [Google Scholar] [CrossRef]
- Shao, T.; Wang, R.; Zhang, C.; Yan, P. Atmospheric-Pressure Pulsed Discharges and Plasmas: Mechanism, Characteristics and Applications. High Volt. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Dobrynin, D.; Rakhmanov, R.; Fridman, A. Nanosecond-Pulsed Spark Discharge Plasma in Liquid Nitrogen: Synthesis of Polynitrogen from NaN3. J. Phys. D Appl. Phys. 2019, 52, 455502. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, H.; Wang, H.; Liu, Z.; Zhang, L.; Li, Y.; Zou, X.; Wang, X. Simultaneous Hydrodynamic Cavitation and Nanosecond Pulse Discharge Plasma Enhanced by Oxygen Injection. Ultrason. Sonochem. 2023, 99, 106552. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Yokochi, A.; Jovanovic, G.; Zhang, S.; von Jouanne, A. Application-Oriented Non-Thermal Plasma in Chemical Reaction Engineering: A Review. Green Energy Resour. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Anwar, M.; Saraswati, T.E.; Anjarwati, L.; Moraru, D.; Udhiarto, A.; Adriyanto, F.; Maghfiroh, H.; Nuryadi, R. Probing Ionization Characteristics of Under-Water Plasma Arc Discharge Using Simultaneous Current and Voltage versus Time Measurement in Carbon Nanoparticle Synthesis. Micro Nano Eng. 2022, 14, 100099. [Google Scholar] [CrossRef]
- Hayashi, Y.; Diono, W.; Takada, N.; Kanda, H.; Goto, M. Glycine Oligomerization by Pulsed Discharge Plasma over Aqueous Solution under Atmospheric Pressure. ChemEngineering 2018, 2, 17. [Google Scholar] [CrossRef]
- Ramezani, S. NANOPARTICLES, from Theory to Application (Gunter Schmid). Available online: https://www.academia.edu/5517804/NANOPARTICLES_from_theory_to_application_gunter_schmid_ (accessed on 6 April 2025).
- Duma, Z.-S.; Sihvonen, T.; Havukainen, J.; Reinikainen, V.; Reinikainen, S.-P. Optimizing Energy Dispersive X-Ray Spectroscopy (EDS) Image Fusion to Scanning Electron Microscopy (SEM) Images. Micron 2022, 163, 103361. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Dover Publications: Garden City, NY, USA, 2000; ISBN 978-0-486-41155-2. [Google Scholar]
- DosRamos, J.G. Acoustic Attenuation Spectroscopy for Process Control of Dispersed Systems. IOP Conf. Ser. Mater. Sci. Eng. 2012, 42, 012023. [Google Scholar] [CrossRef]
- Bellotti, R.; Picotto, G.B.; Ribotta, L. AFM Measurements and Tip Characterization of Nanoparticles with Different Shapes. Nanomanuf. Metrol. 2022, 5, 127–138. [Google Scholar] [CrossRef]
- Majeed, M.S.; Hassan, S.M.; Fadhil, S.A. AgO Nanoparticles Synthesis by Different Nd:YAG Laser Pulse Energies. Lasers Manuf. Mater. Process. 2022, 9, 228–240. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Addison-Wesley Publishing Company: Boston, MA, USA, 1956; ISBN 978-0-201-01230-9. [Google Scholar]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution Monochromated X-Ray Photoelectron Spectroscopy of Organic Polymers: A Comparison between Solid State Data for Organic Polymers and Gas Phase Data for Small Molecules. Mol. Phys. 1992, 76, 919–936. [Google Scholar] [CrossRef]
- Griffiths, P.R. Fourier Transform Infrared Spectrometry. Science 1983, 222, 297–302. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Camarda, P.; Messina, F.; Vaccaro, L.; Agnello, S.; Buscarino, G.; Schneider, R.; Popescu, R.; Gerthsen, D.; Lorenzi, R.; Gelardi, F.M.; et al. Luminescence Mechanisms of Defective ZnO Nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 16237–16244. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, J.; Qiu, M. Metallic Photoluminescence of Plasmonic Nanoparticles in Both Weak and Strong Excitation Regimes. Nanophotonics 2024, 13, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lai, J.; Lu, J.; Li, Z. Pulsed Laser Ablation of Bulk Target and Particle Products in Liquid for Nanomaterial Fabrication. AIP Adv. 2019, 9, 015307. [Google Scholar] [CrossRef]
- Tsuji, T.; Thang, D.H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of Silver Nanoparticles by Laser Ablation in Polyvinylpyrrolidone Solutions. Appl. Surf. Sci. 2008, 254, 5224–5230. [Google Scholar] [CrossRef]
- Ibrahimkutty, S.; Wagener, P.; Rolo, T.D.S.; Karpov, D.; Menzel, A.; Baumbach, T.; Barcikowski, S.; Plech, A. A Hierarchical View on Material Formation during Pulsed-Laser Synthesis of Nanoparticles in Liquid. Sci. Rep. 2015, 5, 16313. [Google Scholar] [CrossRef]
- Reich, S.; Göttlicher, J.; Letzel, A.; Gökce, B.; Barcikowski, S.; dos Santos Rolo, T.; Baumbach, T.; Plech, A. X-Ray Spectroscopic and Stroboscopic Analysis of Pulsed-Laser Ablation of Zn and Its Oxidation. Appl. Phys. A 2017, 124, 71. [Google Scholar] [CrossRef]
- Reich, S.; Göttlicher, J.; Ziefuss, A.; Streubel, R.; Letzel, A.; Menzel, A.; Mathon, O.; Pascarelli, S.; Baumbach, T.; Zuber, M.; et al. In Situ Speciation and Spatial Mapping of Zn Products during Pulsed Laser Ablation in Liquids (PLAL) by Combined Synchrotron Methods. Nanoscale 2020, 12, 14011–14020. [Google Scholar] [CrossRef]
- Sánchez Aké, C.; Sobral, H.; Villagrán Muniz, M.; Escobar-Alarcón, L.; Camps, E. Characterization of Laser Ablation Plasmas by Laser Beam Deflection. Opt. Lasers Eng. 2003, 39, 581–588. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Gaudiuso, R.; ElRashedy, R.; De Pascale, O.; Palazzo, G.; De Giacomo, A. Collinear Double Pulse Laser Ablation in Water for the Production of Silver Nanoparticles. Phys. Chem. Chem. Phys. 2013, 15, 20868–20875. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Alva, M.A.; García-Fernández, T.; Villagrán-Muniz, M.; Sánchez-Aké, C.; Castañeda-Guzmán, R.; Esparza-Alegría, E.; Sánchez-Valdés, C.F.; Llamazares, J.L.S.; Herrera, C.E.M. Synthesis of Silver Nanoparticles by Laser Ablation in Ethanol: A Pulsed Photoacoustic Study. Appl. Surf. Sci. 2015, 355, 341–349. [Google Scholar] [CrossRef]
- Matsukura, M.; Ito, Y. Time-Resolved Photoelasticity Imaging of Transient Stress Fields in Solids Induced by Intense Laser Pulses. J. Phys. Conf. Ser. 2007, 59, 749. [Google Scholar] [CrossRef]
- Choudhury, K.; Singh, R.K.; Narayan, S.; Srivastava, A.; Kumar, A. Time Resolved Interferometric Study of the Plasma Plume Induced Shock Wave in Confined Geometry: Two-Dimensional Mapping of the Ambient and Plasma Density. Phys. Plasmas 2016, 23, 042108. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Gu, Y.; Wang, H.; Song, X.; Zeng, H. Probing Mesoscopic Process of Laser Ablation in Liquid by Integrated Method of Optical Beam Deflection and Time-Resolved Shadowgraphy. J. Colloid Interface Sci. 2017, 489, 38–46. [Google Scholar] [CrossRef]
- Soliman, W.; Takada, N.; Sasaki, K. Growth Processes of Nanoparticles in Liquid-Phase Laser Ablation Studied by Laser-Light Scattering. Appl. Phys. Express 2010, 3, 035201. [Google Scholar] [CrossRef]
- Xu, R. Light Scattering: A Review of Particle Characterization Applications. Particuology 2015, 18, 11–21. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Mechanisms of Pulsed Laser Ablation of Biological Tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef]
- Fankuchen, I. Small-Angle Scattering of X-Rays. André Guinier and Gérard Fournet. Translated by Christopher B. Walker. Wiley, New York; Chapman & Hall, London, 1955. Xi + 268 Pp. Illus. $7.50. Science 1956, 123, 591–592. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, A.; Yang, Z.; Gilbert, E.P.; Knott, R.; de Campo, L.; Storer, B.; Hemar, Y.; Singh, H. Small-Angle X-Ray Scattering (SAXS) and Small-Angle Neutron Scattering (SANS) Study on the Structure of Sodium Caseinate in Dispersions and at the Oil-Water Interface: Effect of Calcium Ions. Food Struct. 2022, 32, 100276. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Spellauge, M.; Redka, D.; Mo, M.; Song, C.; Huber, H.P.; Plech, A. Time-Resolved Probing of Laser-Induced Nanostructuring Processes in Liquids. Beilstein J. Nanotechnol. 2025, 16, 968–1002. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Spellauge, M.; Redka, D.; Auer, R.; Doñate-Buendia, C.; Barcikowski, S.; Gökce, B.; Huber, H.P.; Zhigilei, L.V. Time-Resolved Probing and Modeling of Optical Signatures of Ultrashort-Pulse Laser Spallation and Phase Explosion in Iron-Nickel Targets. Phys. Rev. B 2025, 111, 174301. [Google Scholar] [CrossRef]
- Chen, C.; Zhigilei, L.V. Ultrashort Pulse Laser Ablation in Liquids: Probing the First Nanoseconds of Underwater Phase Explosion. Light Sci. Appl. 2022, 11, 111. [Google Scholar] [CrossRef]
- Ando, K.; Nakajima, T. Clear Observation of the Formation of Nanoparticles inside the Ablation Bubble through a Laser-Induced Flat Transparent Window by Laser Scattering. Nanoscale 2020, 12, 9640–9646. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, X.; Yang, J.; Wang, X.; Wang, M. Time-Resolved Shadowgraphs of Material Ejection in Intense Femtosecond Laser Ablation of Aluminum. Phys. Rev. Lett. 2007, 99, 167602. [Google Scholar] [CrossRef]
- Coviello, V.; Reffatto, C.; Fawaz, M.W.; Mahler, B.; Sollier, A.; Lukic, B.; Rack, A.; Amans, D.; Amendola, V. Time-Resolved Dynamics of Laser Ablation in Liquid with Gas-Evolving Additives: Toward Molding the Atomic Structure of Nonequilibrium Nanoalloys. Adv. Sci. 2025, 12, 2416035. [Google Scholar] [CrossRef]
- Cheng, N.; Sun, H.; Pivak, Y.; Liebscher, C.H. Direct Visualization and Quantitative Insights into the Formation and Phase Evolution of Cu Nanoparticles via In Situ Liquid Phase 4D-STEM. Adv. Sci. 2025, 12, 2500706. [Google Scholar] [CrossRef]
- Basagni, A.; Torresan, V.; Marzola, P.; van Raap, M.B.F.; Nodari, L.; Amendola, V. Structural Evolution under Physical and Chemical Stimuli of Metastable Au–Fe Nanoalloys Obtained by Laser Ablation in Liquid. Faraday Discuss. 2023, 242, 286–300. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Albert, T.J.; Li, H.; Arefev, M.I.; Chen, Y.; Dunne, M.; Glownia, J.M.; Jerman, M.; Hoffmann, M.; et al. Dynamics of Nanoscale Phase Decomposition in Laser Ablation. Commun. Mater. 2025, 6, 69. [Google Scholar] [CrossRef]
- Wu, C.; Zhigilei, L.V. Microscopic Mechanisms of Laser Spallation and Ablation of Metal Targets from Large-Scale Molecular Dynamics Simulations. Appl. Phys. A 2014, 114, 11–32. [Google Scholar] [CrossRef]
- Plettenberg, P.; Bauerhenne, B.; Garcia, M.E. Neural Network Interatomic Potential for Laser-Excited Materials. Commun. Mater. 2023, 4, 63. [Google Scholar] [CrossRef]
- Nyabadza, A.; Brabazon, D. Machine Learning-Based Recommender System for Pulsed Laser Ablation in Liquid: Recommendation of Optimal Processing Parameters for Targeted Nanoparticle Size and Concentration Using Cosine Similarity and KNN Models. Crystals 2025, 15, 662. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Yiu, T.-H. Investigation of Laser Ablation Quality Based on Data Science and Machine Learning XGBoost Classifier. Appl. Sci. 2024, 14, 326. [Google Scholar] [CrossRef]
- Kawaoto, R.; Namba, T.; Ohtsuki, Y.; Yan, F.; Nakajima, T. Predicting Ablation-Assisted Nanosecond Laser Fabrication of Glass Optical Diffusers by Machine Learning. Results Opt. 2025, 21, 100865. [Google Scholar] [CrossRef]
- Guo, Y.; Kalinin, S.V.; Cai, H.; Xiao, K.; Krylyuk, S.; Davydov, A.V.; Guo, Q.; Lupini, A.R. Defect Detection in Atomic-Resolution Images via Unsupervised Learning with Translational Invariance. Npj Comput. Mater. 2021, 7, 180. [Google Scholar] [CrossRef]
- Groschner, C.K.; Choi, C.; Scott, M.C. Machine Learning Pipeline for Segmentation and Defect Identification from High-Resolution Transmission Electron Microscopy Data. Microsc. Microanal. 2021, 27, 549–556. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, J.; Wang, J.; Jiang, M.; Wang, Z.; Bai, X.; Wang, X. A Deep Learning-Based Framework for Automatic Analysis of the Nanoparticle Morphology in SEM/TEM Images. Nanoscale 2022, 14, 10761–10772. [Google Scholar] [CrossRef]
- Novotný, K.; Krempl, I.; Afonin, I.; Prokeš, L.; Vaňhara, P.; Havel, J. Laser-Induced Plasma Spectroscopy in Pulsed Laser Ablation Synthesis of Nanoparticles in Liquid. Talanta 2025, 290, 127767. [Google Scholar] [CrossRef]
- Belekov, E. Improved Antimicrobial Properties of Methylene Blue Attached to Silver Nanoparticles. Photodiagnosis Photodyn. Ther. 2020, 32, 102012. [Google Scholar] [CrossRef]
- Kholikov, K. Improved Singlet Oxygen Generation and Antimicrobial Activity of Sulphur-Doped Graphene Quantum Dots Coupled with Methylene Blue for Photodynamic Therapy Applications. Photodiagn. Photodyn. Ther. 2018, 24, 7–14. [Google Scholar] [CrossRef]
- Mekki-Berrada, F.; Ren, Z.; Huang, T.; Wong, W.K.; Zheng, F.; Xie, J.; Tian, I.P.S.; Jayavelu, S.; Mahfoud, Z.; Bash, D.; et al. Two-Step Machine Learning Enables Optimized Nanoparticle Synthesis. Npj Comput. Mater. 2021, 7, 55. [Google Scholar] [CrossRef]
- Ortiz-Perez, A.; van Tilborg, D.; van der Meel, R.; Grisoni, F.; Albertazzi, L. Machine Learning-Guided High Throughput Nanoparticle Design. Digit. Discov. 2024, 3, 1280–1291. [Google Scholar] [CrossRef]
- Lv, H.; Chen, X. Intelligent Control of Nanoparticle Synthesis through Machine Learning. Nanoscale 2022, 14, 6688–6708. [Google Scholar] [CrossRef] [PubMed]
- Kusne, A.G.; Yu, H.; Wu, C.; Zhang, H.; Hattrick-Simpers, J.; DeCost, B.; Sarker, S.; Oses, C.; Toher, C.; Curtarolo, S.; et al. On-the-Fly Closed-Loop Materials Discovery via Bayesian Active Learning. Nat. Commun. 2020, 11, 5966. [Google Scholar] [CrossRef]
- Johnson, J.E.; Jamil, I.R.; Pan, L.; Lin, G.; Xu, X. Bayesian Optimization with Gaussian-Process-Based Active Machine Learning for Improvement of Geometric Accuracy in Projection Multi-Photon 3D Printing. Light Sci. Appl. 2025, 14, 56. [Google Scholar] [CrossRef]
- Chitturi, S.R.; Ramdas, A.; Wu, Y.; Rohr, B.; Ermon, S.; Dionne, J.; da Jornada, F.H.; Dunne, M.; Tassone, C.; Neiswanger, W.; et al. Targeted Materials Discovery Using Bayesian Algorithm Execution. Npj Comput. Mater. 2024, 10, 156. [Google Scholar] [CrossRef]
- Di Fiore, F.; Nardelli, M.; Mainini, L. Active Learning and Bayesian Optimization: A Unified Perspective to Learn with a Goal. Arch. Comput. Methods Eng. 2024, 31, 2985–3013. [Google Scholar] [CrossRef]
- Jiang, Y.; Salley, D.; Sharma, A.; Keenan, G.; Mullin, M.; Cronin, L. An Artificial Intelligence Enabled Chemical Synthesis Robot for Exploration and Optimization of Nanomaterials. Sci. Adv. 2022, 8, eabo2626. [Google Scholar] [CrossRef]
- Harris, S.B.; Biswas, A.; Yun, S.J.; Roccapriore, K.M.; Rouleau, C.M.; Puretzky, A.A.; Vasudevan, R.K.; Geohegan, D.B.; Xiao, K. Autonomous Synthesis of Thin Film Materials with Pulsed Laser Deposition Enabled by In Situ Spectroscopy and Automation. Small Methods 2024, 8, 2301763. [Google Scholar] [CrossRef]
- Harris, S.B.; López Fajardo, R.Y.; Puretzky, A.A.; Xiao, K.; Bao, F.; Vasudevan, R.K. Online Bayesian State Estimation for Real-Time Monitoring of Growth Kinetics in Thin Film Synthesis. Nano Lett. 2025, 25, 2444–2451. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.M.; Hong, S.; Han, B.; Nam, K.T.; Jung, Y. Closed-Loop Optimization of Nanoparticle Synthesis Enabled by Robotics and Machine Learning. Matter 2023, 6, 677–690. [Google Scholar] [CrossRef]
- Citrine Informatics. AI-Guided Closed-Loop Synthesis of Nanoparticles for Catalysis. Case Study. 2021. Available online: https://citrine.io/Ai-Guided-Closed-Loop-Synthesis-of-Nanoparticles-for-Catalysis/ (accessed on 26 May 2025).
- Harris, S.B.; Biswas, A.; Yun, S.J.; Rouleau, C.M.; Puretzky, A.A.; Vasudevan, R.K.; Geohegan, D.B.; Xiao, K. Autonomous Synthesis of Thin Film Materials with Pulsed Laser Deposition Enabled by In Situ Spectroscopy and Automation. arXiv 2023, arXiv:2308.08700. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.B.; Fajardo, R.; Puretzky, A.A.; Xiao, K.; Bao, F.; Vasudevan, R.K. Bayesian State Estimation Unlocks Real-Time Control in Thin Film Synthesis. arXiv 2024, arXiv:2410.23895. [Google Scholar] [CrossRef]
- Balachandran, A.; Sreenilayam, S.P.; Madanan, K.; Thomas, S.; Brabazon, D. Nanoparticle Production via Laser Ablation Synthesis in Solution Method and Printed Electronic Application—A Brief Review. Results Eng. 2022, 16, 100646. [Google Scholar] [CrossRef]
- Wu, R.; Du, Q.; Zhang, H.; Zhang, P.; Lei, X.; Zhang, F. A Comprehensive Review: The Approach for Fabrication of Core/Shell Au Nanocomposite and Modification, Properties, Applications of Au NPs. J. Saudi Chem. Soc. 2024, 28, 101824. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef]
- Hamad, A.H. The Effect of Microwave Irradiation on the Laser-Generated Ag-TiO2 Compound Nanoparticles. ARO-Sci. J. KOYA Univ. 2025, 13, 111–116. [Google Scholar] [CrossRef]
- Zito, C.A.; Orlandi, M.O.; Volanti, D.P. Accelerated Microwave-Assisted Hydrothermal/Solvothermal Processing: Fundamentals, Morphologies, and Applications. J. Electroceram 2018, 40, 271–292. [Google Scholar] [CrossRef]
- Paul, N.; Sawate, A.; Sugano, S.; Katayama, T.; Furube, A.; Koinkar, P. Formation of WO3/MoS2/rGO Composite Prepared by Integration of Pulse Laser Ablation and Hydrothermal Method to Enhance Optical and Photocatalytic Activity. Surf. Rev. Lett. 2025, 32, 2540005. [Google Scholar] [CrossRef]
- Towards Scalable Large-Area Pulsed Laser Deposition. Available online: https://www.mdpi.com/1996-1944/14/17/4854 (accessed on 10 September 2025).
- Khairani, I.Y.; Spellauge, M.; Riahi, F.; Huber, H.P.; Gökce, B.; Doñate-Buendía, C. Parallel Diffractive Multi-Beam Pulsed-Laser Ablation in Liquids Toward Cost-Effective Gram Per Hour Nanoparticle Productivity. Adv. Photonics Res. 2024, 5, 2300290. [Google Scholar] [CrossRef]
- Barcikowski, S.; Menéndez-Manjón, A.; Chichkov, B.; Brikas, M.; Račiukaitis, G. Generation of Nanoparticle Colloids by Picosecond and Femtosecond Laser Ablations in Liquid Flow. Appl. Phys. Lett. 2007, 91, 083113. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano–Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global Life Cycle Releases of Engineered Nanomaterials. J. Nanopart Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef]
- Petersen, E.J.; Ceger, P.; Allen, D.G.; Coyle, J.; Derk, R.; Garcia-Reyero, N.; Gordon, J.; Kleinstreuer, N.C.; Matheson, J.; McShan, D.; et al. U.S. Federal Agency Interests and Key Considerations for New Approach Methodologies for Nanomaterials. ALTEX-Altern. Anim. Exp. 2022, 39, 183–206. [Google Scholar] [CrossRef]
- Hunt, N.; Kestens, V.; Rasmussen, K.; Badetti, E.; Soeteman-Hernández, L.G.; Oomen, A.G.; Peijnenburg, W.; Hristozov, D.; Rauscher, H. Regulatory Preparedness for Multicomponent Nanomaterials: Current State, Gaps and Challenges of REACH. NanoImpact 2025, 37, 100538. [Google Scholar] [CrossRef]
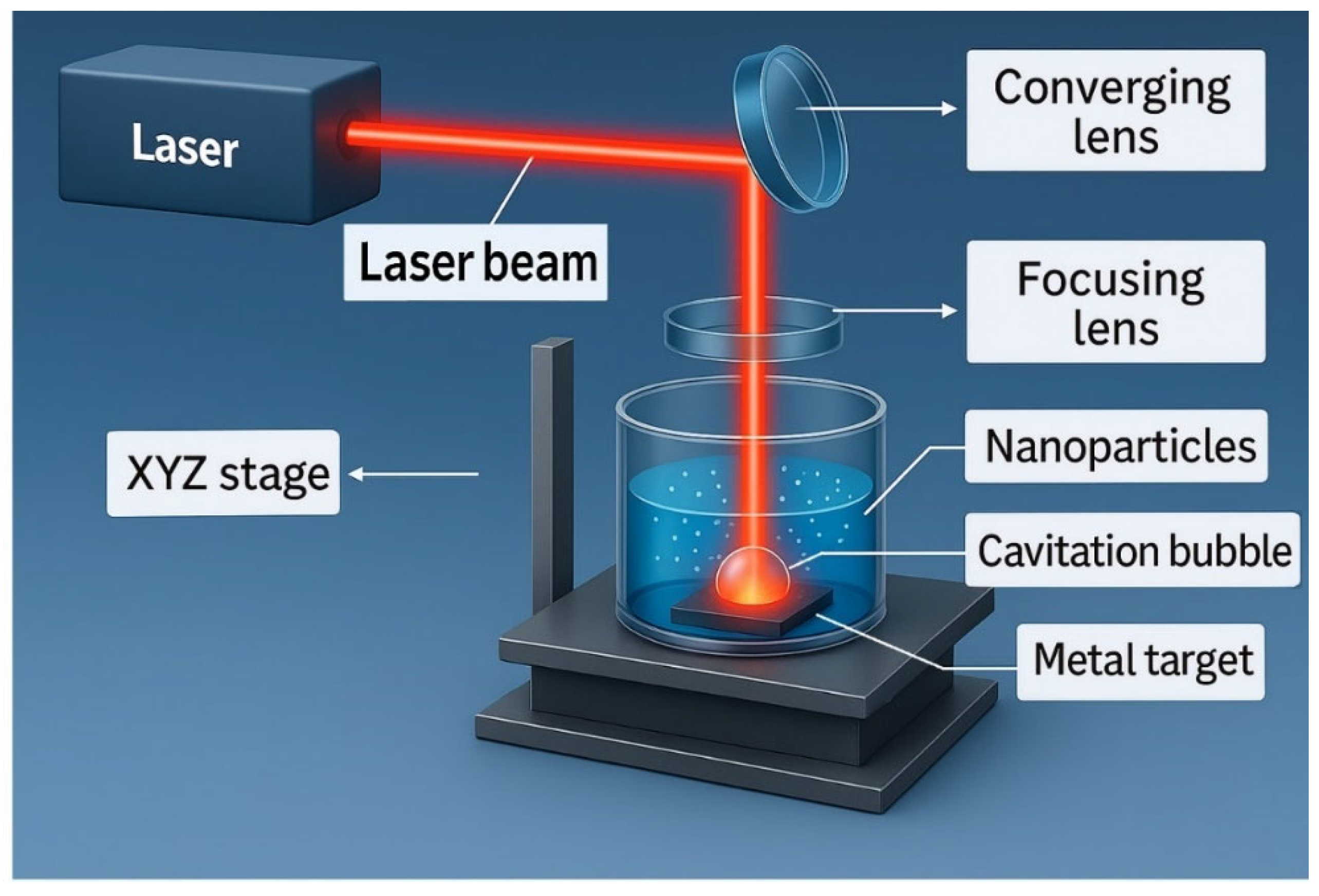


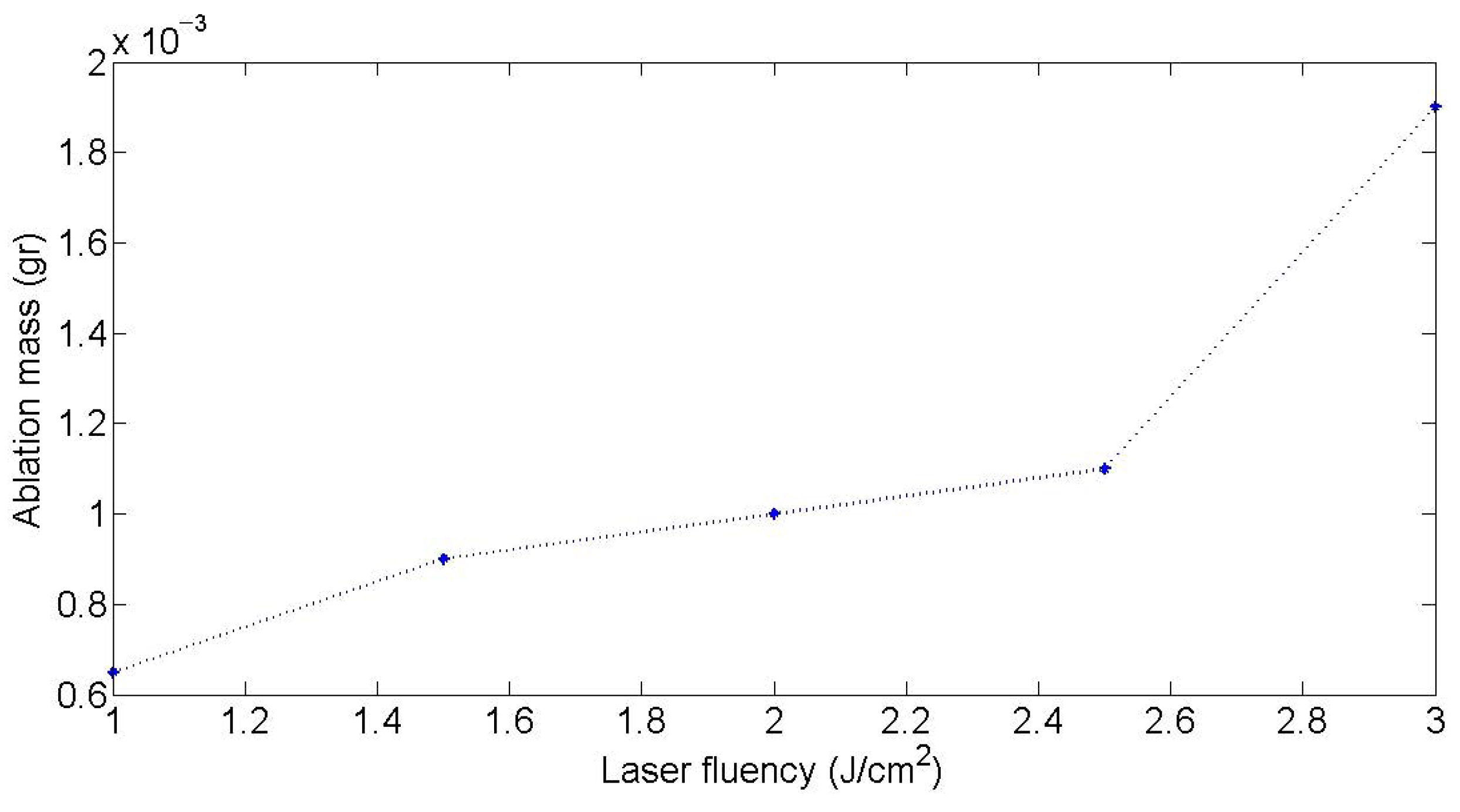
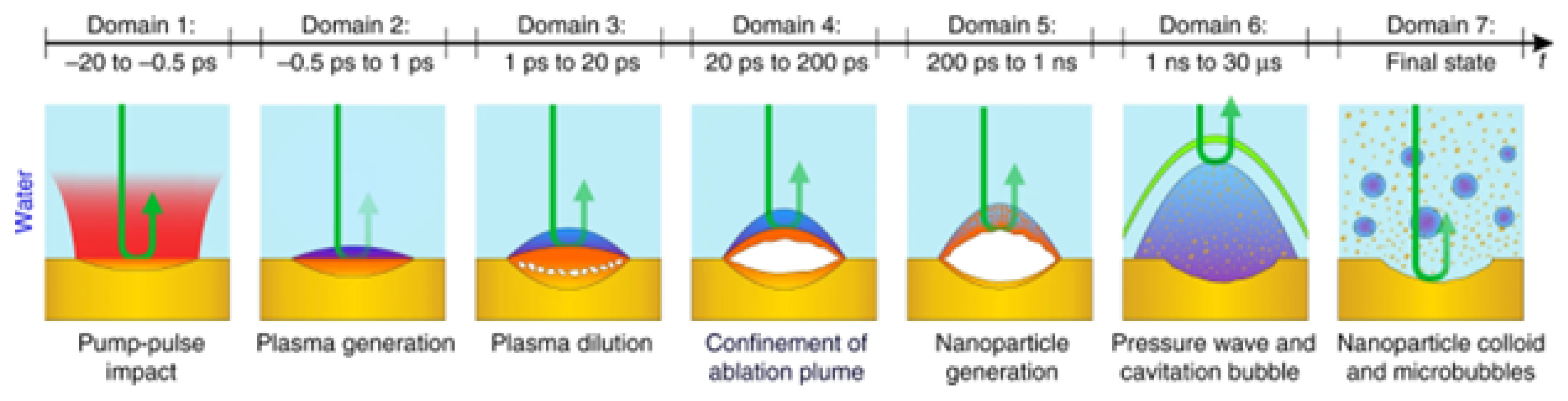
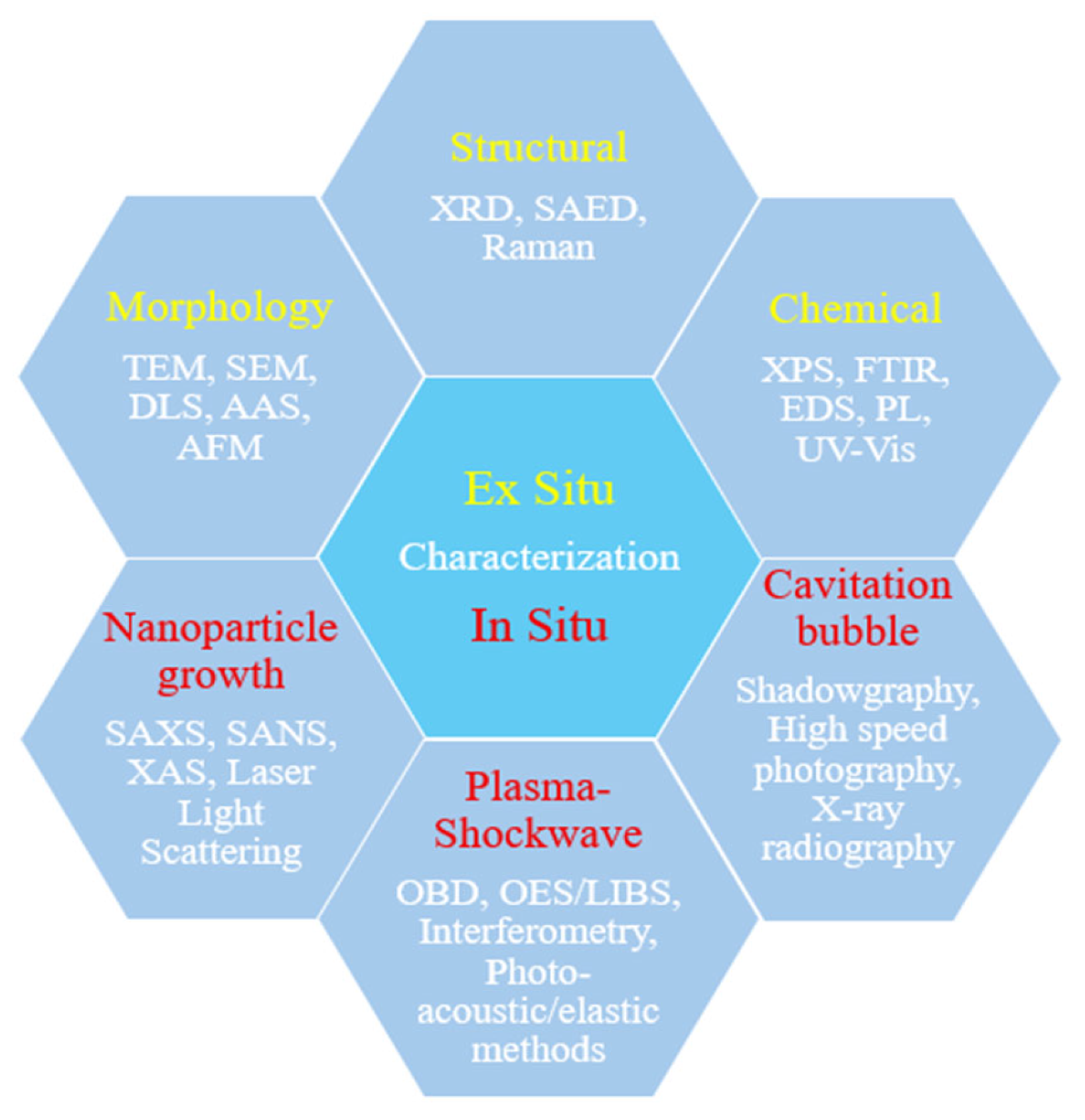
| Material (Nanomaterial) | Laser Type | Wavelength | Pulse | Fluence/Energy | Rep. Rate | Pulse Time | Medium | Size Range | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Mg–C–Graphene nanoparticles | Nd:YAG (WEDGE HF 1064) | 1064 nm | 0.2–0.9 ns | 1–2 J/cm2 | 10–20 kHz | 85 min | IPA/IPA–HCl/IPA–NaOH | 60–300 nm → <90 nm | Paper electronics, printed inks | [107] |
| Magnesium (Mg) nanoparticles from powders | Nd:YAG (WEDGE HF) | 1064 nm | 600 ps | 1.83–1.91 J/cm2 | 10 kHz | 2, 5, 25 min | IPA | 53–239 nm | Flexible electronics, conductive inks | [108] |
| Carbon nanoparticles (CNPs) | Nd:YAG (WEDGE HF) | 1064 nm | 600 ps | 1.63–1.91 J/cm2 | 10 kHz | 8 min | Water/Ethanol/Mg-solution | 10–1389 nm (by medium) | Paper/flexible electronics | [109] |
| LIPSS on Si and W (fs structuring) | Femtosecond (FCPA μJewel D-1000-UG3) | 1045 nm | 457 fs | 5.5–77 J/cm2 | 100 kHz | scanning | Water | ~100–200 nm periodicity | Photonics: antireflection, colorization | [110] |
| sp-carbon chains (polyynes/cumulenes) | Nd:YAG, fs Ti:Sapph, excimer | 193–1064 nm | fs/ns | 0.3–5 J/cm2 | 10 Hz–1 kHz | 5–180 min | Water & organics | CnH2 (n = 6–30) | NLO, optoelectronics, sensing | [111] |
| Ag nanoparticles via donut beams | Nd:YAG + DOE | 532 nm | 7 ns | 3.2 vs. 1.2 J/cm2 | 10 Hz | 15 min | Water | 20–30 nm (narrower with donut) | Plasmonics, SERS | [112] |
| GaN nanostructures (on porous Si) | Q-sw Nd:YAG | 1064 nm | ns | 1600 mJ/pulse | 4 Hz | 500 pulses | Ethanol (drop-cast) | Grain ~132 nm | UV detectors, LEDs | [113] |
| SnO2 nanoparticles (device) | Q-sw Nd:YAG (SHG) | 532 nm | 7 ns | ~12 J/cm2 | 10 Hz | — | Methanol/3 mM NaCl | 25–65 nm | n-SnO2/p-Si photodetectors | [114] |
| PLAL for photonics/opto/quantum (review) | Ti:Sapph, Nd:YAG, fiber, excimer | 193–1064 nm | fs/ps/ns | 0.01–10 J/cm2 | Hz–MHz | min–h | Water, ethanol, acetone | 2–200 nm | Photonics, quantum emitters, metamaterials | [12] |
| Ag NPs; Au@Ag core–shell (NLO) | Nd:YAG (fund.) | 1064 nm | 7 ns | 50–250 mJ/pulse | 10 Hz | 10 min | Water/HAuCl4 | 9–40 nm | Optical limiting/switching | [115] |
| Low-dimensional nanomaterials (review) | Nd:YAG, Ti:Sapph, excimer, fiber | 193–1064 nm | fs/ps/ns | 0.01–10 J/cm2 | Hz–kHz | min–h | Water/ethanol/DMF/PEG/IPA | QDs 1–200 nm; 2D; 1D | PL devices, UV PDs, bioimaging | [116] |
| Phosphor micronization (KSrPO4:Eu, KBaPO4:Eu) | Nd:YAG/Nd:YVO4 harmonics | 532/355/266 nm | 4–10 ns | ~1–8 J/cm2 (est.) | 10–20 Hz | 15–30 min | Water | ~2.0 µm → ~1.0 µm | W-LED phosphors | [117] |
| Au:MgO on porous Si (photodetector) | Nd:YAG | 1064 nm | ns | 600–1000 mJ/pulse | 10 Hz | 100 pulses | CTAB aq.; Au → Mg colloid | —(size ↑ with energy) | UV–Vis–NIR photodetection | [118] |
| Al2O3 nanoparticles (device) | Q-sw Nd:YAG | 532 nm | 10 ns | 400–1000 mJ/pulse | 1 Hz | — | DI water (3 mL) | 30–100 nm; XRD ~53 nm | Photodetectors on porous Si | [119] |
| PLM crystalline spheres (review) | Nd:YAG, Ti:Sapph, excimer | 193–1064 nm | fs/ps/ns | 0.1–10 J/cm2 | Hz–kHz | min–h | Water/ethanol/organics | 10–200 nm (uniform spheres) | Optical materials, photonics (primary), catalysis, biomedicine | [120] |
| Method | Laser/Energy Source | Material State | Mechanism | Particle Type | Liquid Role |
|---|---|---|---|---|---|
| PLAL (Pulsed Laser Ablation in Liquid) | Pulsed Laser | Solid Target | Ablation → Plasma → Bubble Collapse | Freshly generated nanoparticles | Medium for plasma formation, cooling, and confinement |
| LFL (Laser Fragmentation in Liquid) | Pulsed/Continuous Laser | Colloidal Suspension | Laser-induced photofragmentation | Smaller or uniform nanoparticles | Energy transfer and cooling |
| LML (Laser Melting in Liquid) | Pulsed/Continuous Laser | Colloidal Suspension | Particle melting → Reshaping | Spherical nanoparticles | Shape control and thermal sink |
| EDM (Electrical Discharge Machining in Liquid) | Electrical Discharge | Solid Electrodes | Micro-explosions, vaporization, quenching | Metal or metal oxide nanoparticles | Dielectric medium enables discharge and cooling |
| Technique | Key Parameters | Typical Defects Generated | Properties Affected | Representative Applications |
|---|---|---|---|---|
| PLAL (Pulsed Laser Ablation in Liquid) | Pulse width, fluence, repetition rate, wavelength, liquid chemistry | Oxygen vacancies, surface terminations, lattice strain, dislocations | Bandgap narrowing, enhanced catalytic site density, increased photothermal conversion | Photocatalysis (H2 evolution, CO2 reduction), photothermal therapy, sensors |
| PLFL (Pulsed Laser Fragmentation in Liquid) | High fluence, short pulses, multipulse irradiation | Vacancies (O, S, N), high surface disorder, amorphous shells | Optical emission tuning, increased carrier recombination/trap density, conductivity modulation | Quantum dots for LEDs, bioimaging, defect-rich catalysts for OER/HER |
| PLML (Pulsed Laser Melting in Liquid) | Nanosecond–millisecond pulses, lower fluence, controlled heating | Grain boundaries, twin defects, recrystallization-induced strain | Particle size/morphology uniformity, magnetic domain behavior | Magnetic nanomaterials, plasmonic tuning, shape-controlled catalysts |
| PLPP (Pulsed Laser Post-Processing in Liquid) | Secondary irradiation of preformed colloids, wavelength-specific targeting | Controlled surface vacancies, interface modifications, cation exchange | Surface chemistry tailoring, charge transfer kinetics | Electrocatalysis (methanol oxidation, N2 reduction), hybrid composites with tailored interfaces |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurbandurdyyev, B.; Annamuradov, B.; Er, S.B.; Gross, B.; Er, A.O. Advances in Pulsed Liquid-Based Nanoparticles: From Synthesis Mechanism to Application and Machine Learning Integration. Quantum Beam Sci. 2025, 9, 32. https://doi.org/10.3390/qubs9040032
Gurbandurdyyev B, Annamuradov B, Er SB, Gross B, Er AO. Advances in Pulsed Liquid-Based Nanoparticles: From Synthesis Mechanism to Application and Machine Learning Integration. Quantum Beam Science. 2025; 9(4):32. https://doi.org/10.3390/qubs9040032
Chicago/Turabian StyleGurbandurdyyev, Begench, Berdimyrat Annamuradov, Sena B. Er, Brayden Gross, and Ali Oguz Er. 2025. "Advances in Pulsed Liquid-Based Nanoparticles: From Synthesis Mechanism to Application and Machine Learning Integration" Quantum Beam Science 9, no. 4: 32. https://doi.org/10.3390/qubs9040032
APA StyleGurbandurdyyev, B., Annamuradov, B., Er, S. B., Gross, B., & Er, A. O. (2025). Advances in Pulsed Liquid-Based Nanoparticles: From Synthesis Mechanism to Application and Machine Learning Integration. Quantum Beam Science, 9(4), 32. https://doi.org/10.3390/qubs9040032





