Investigation of Mn2+-Doped Stearic-Acid Through XRD, Raman, and FT-IR, and Thermal Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth of Stearic Acid Crystals
2.2. XRD, Raman, FT-IR, and DSC Techniques
3. Results and Discussion
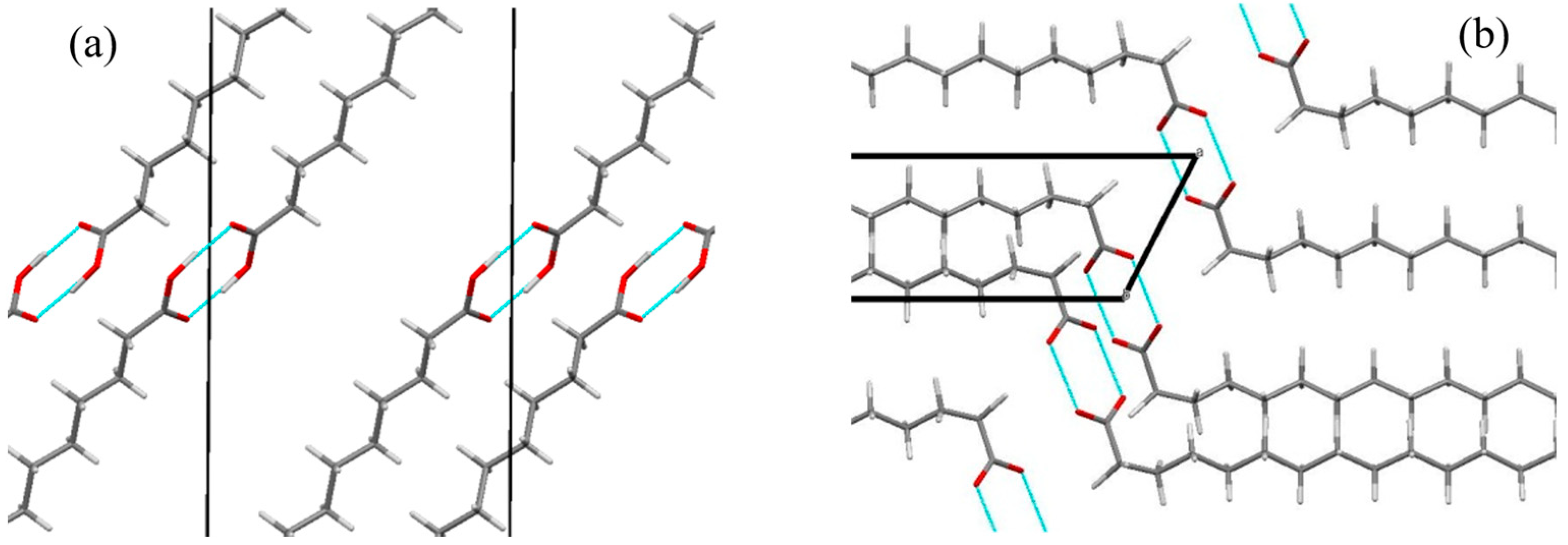
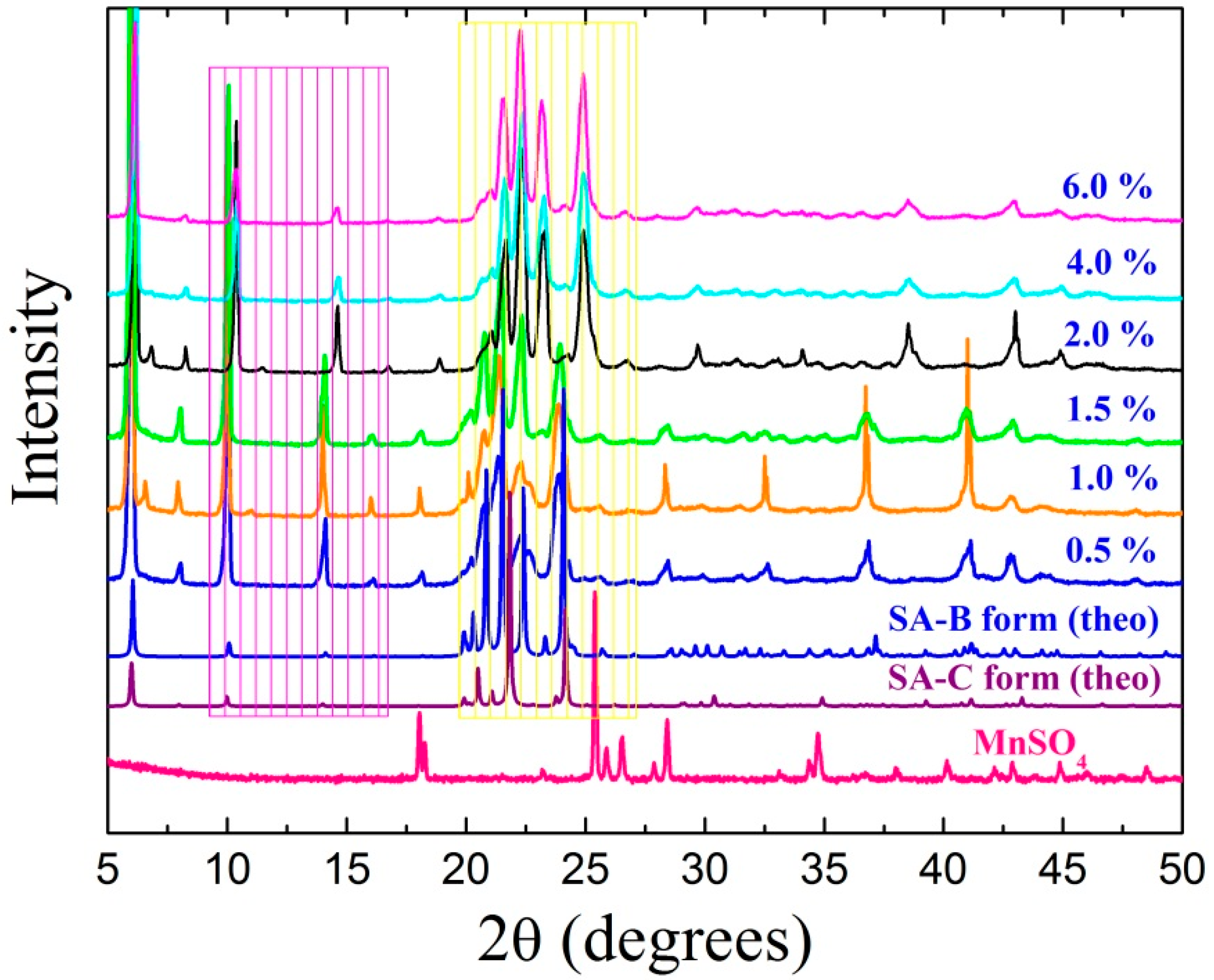
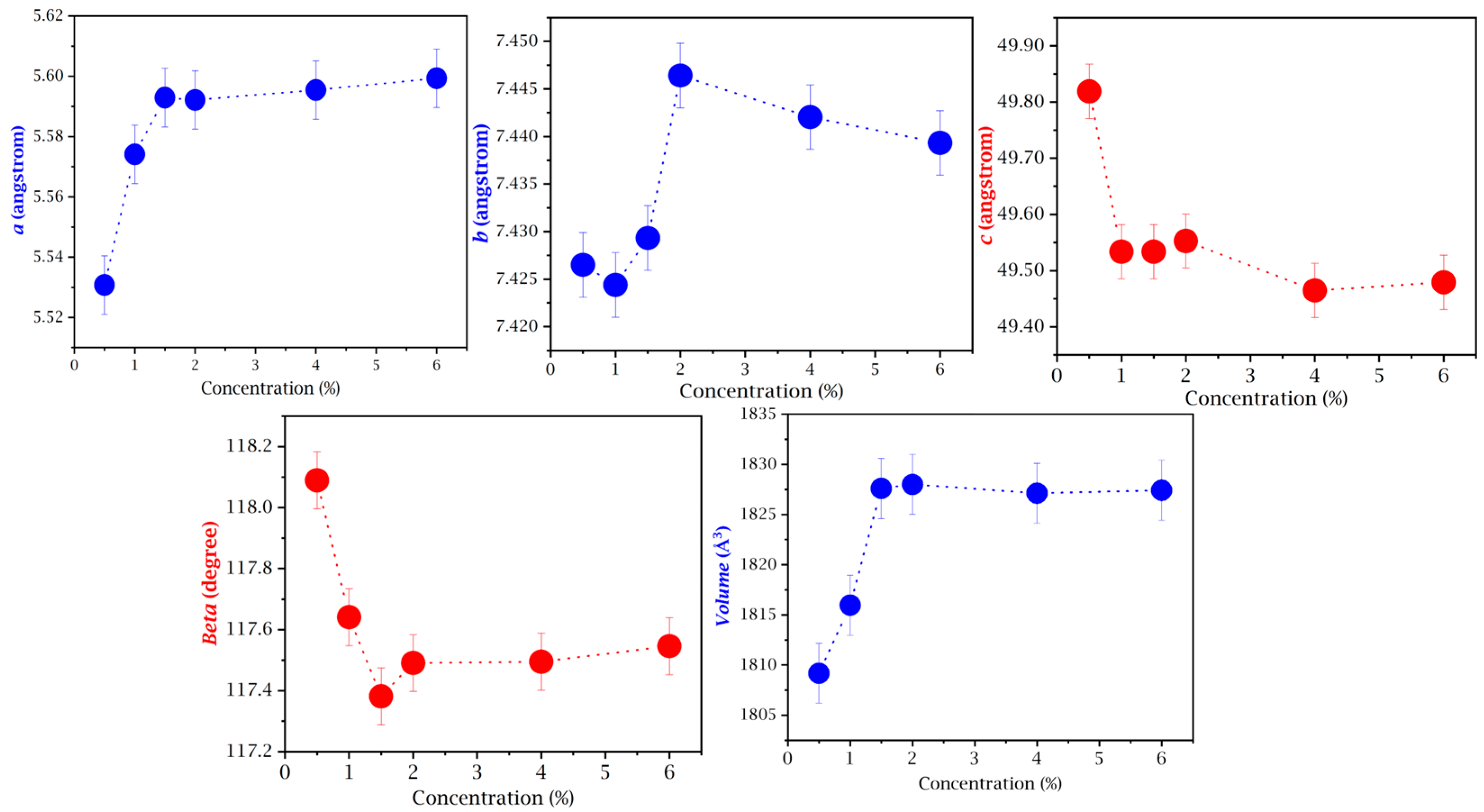
3.1. Growth, XRD, and Rietveld’s Refinement Studies
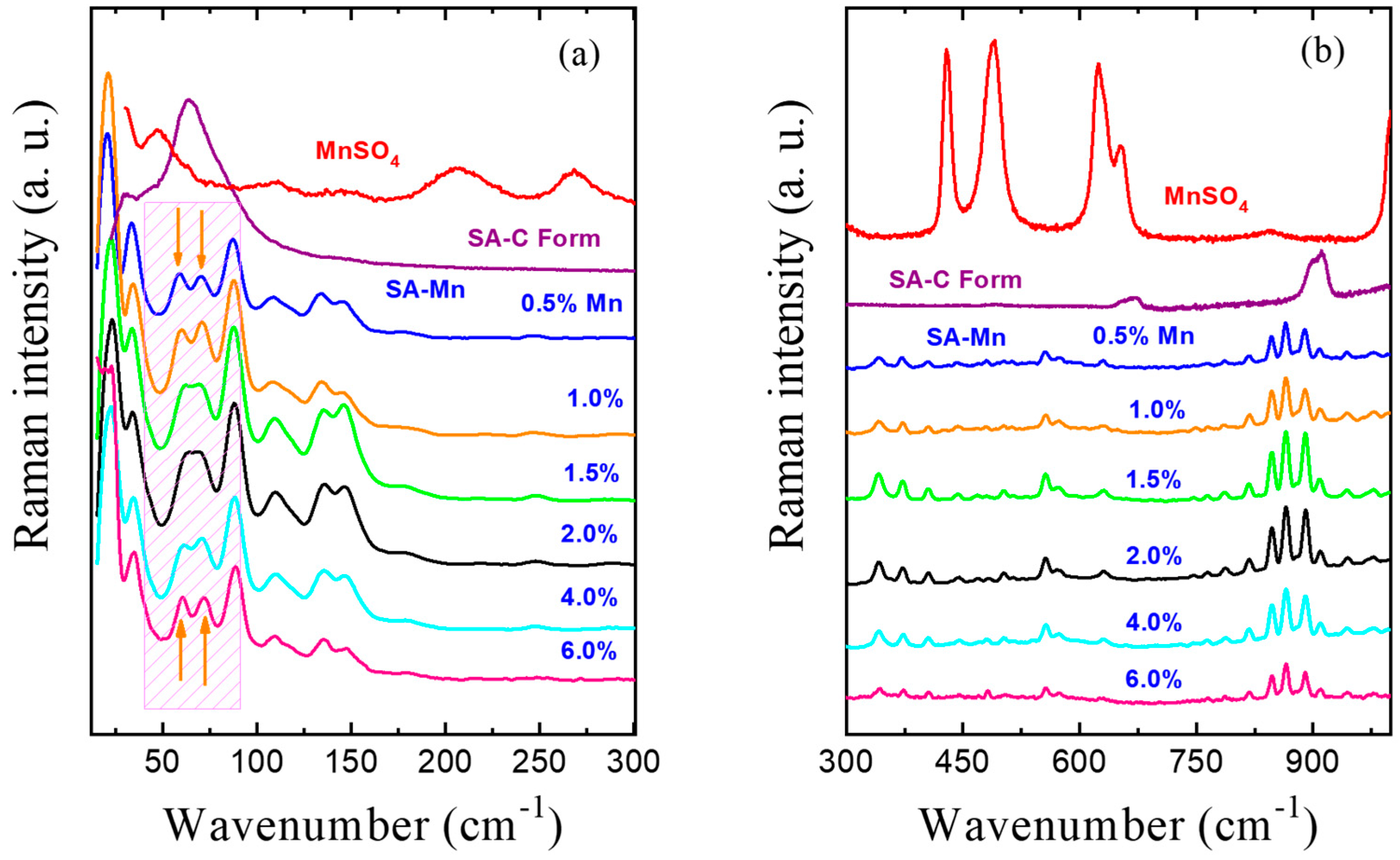
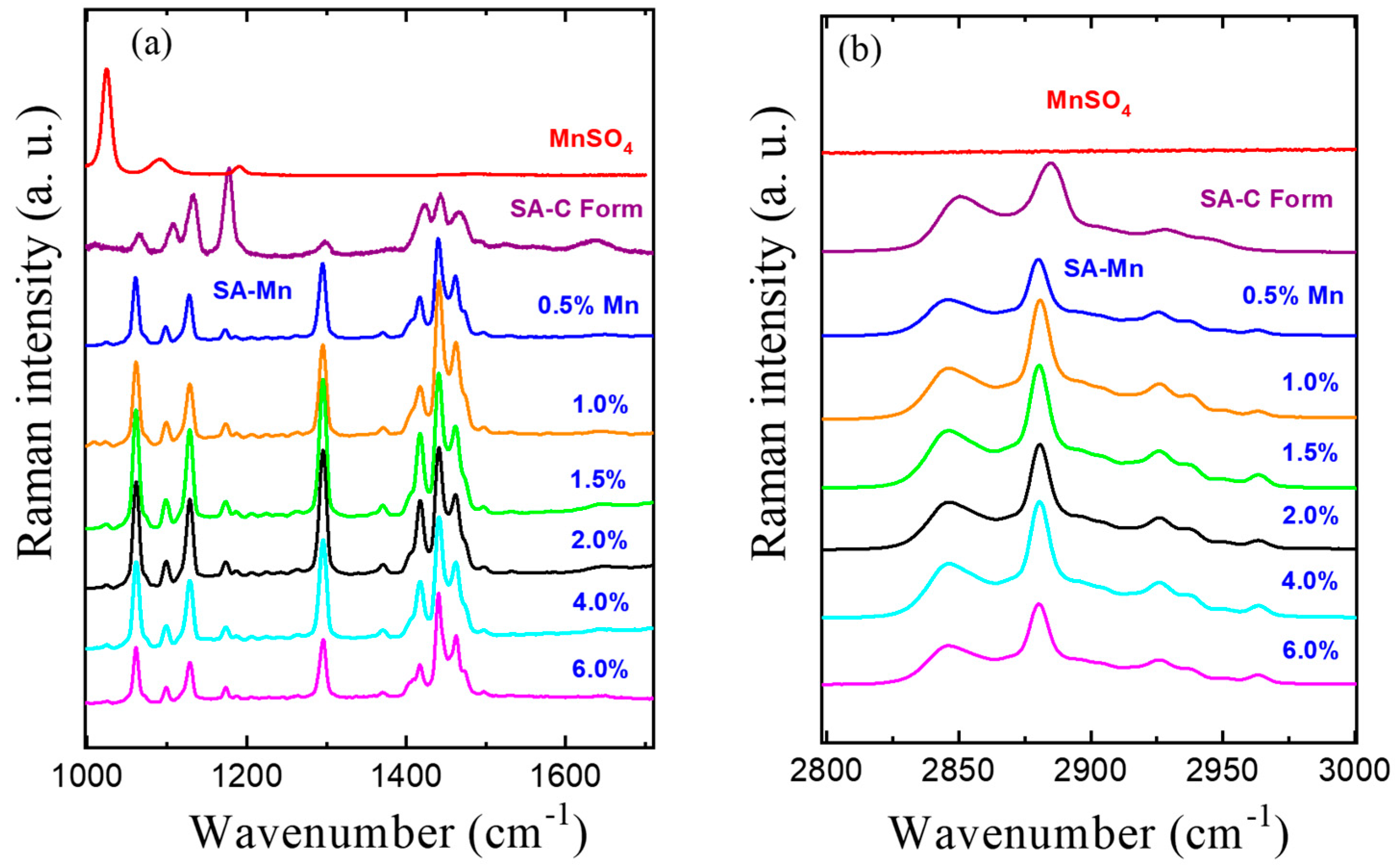
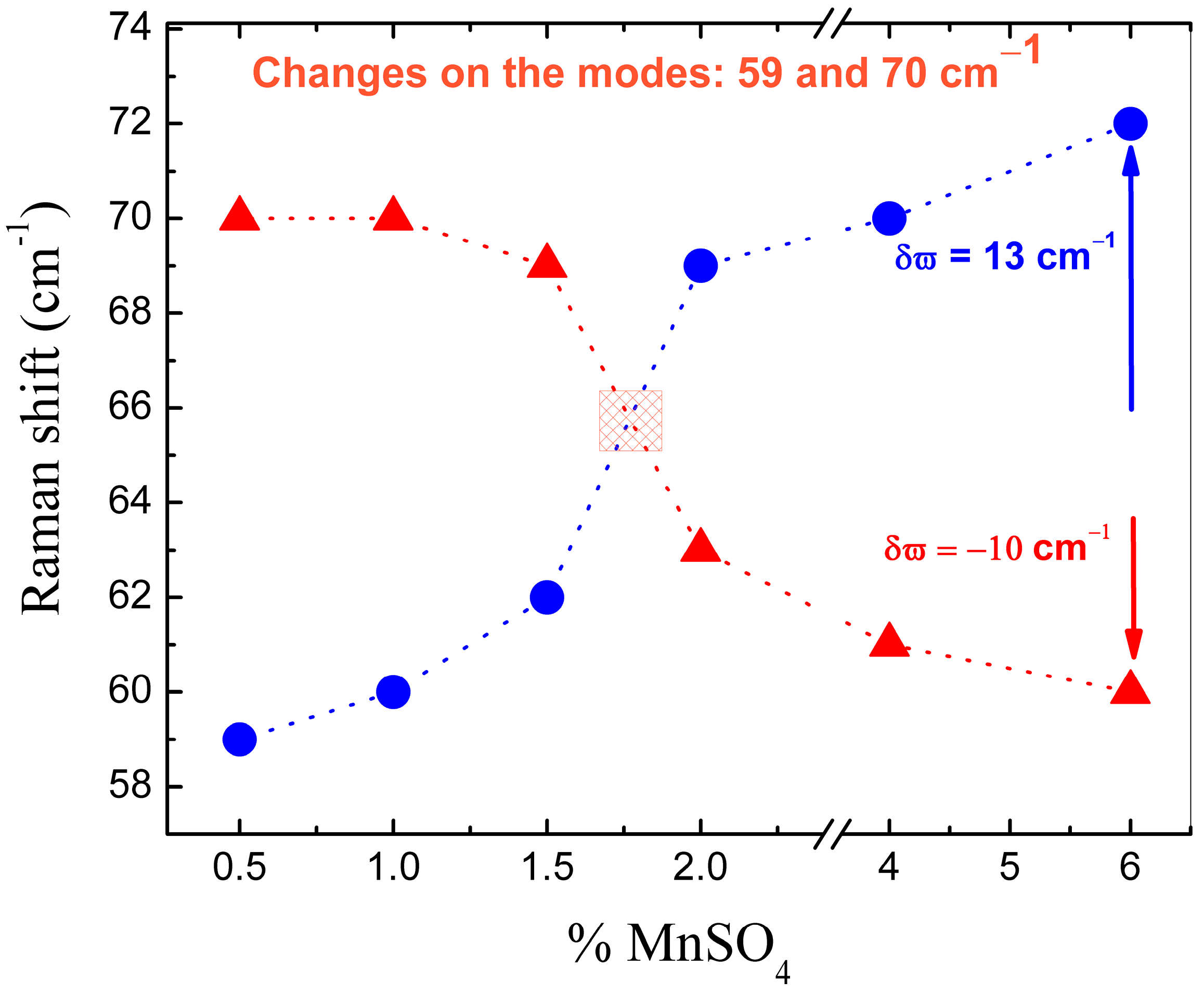
3.2. Raman Spectroscopy Studies
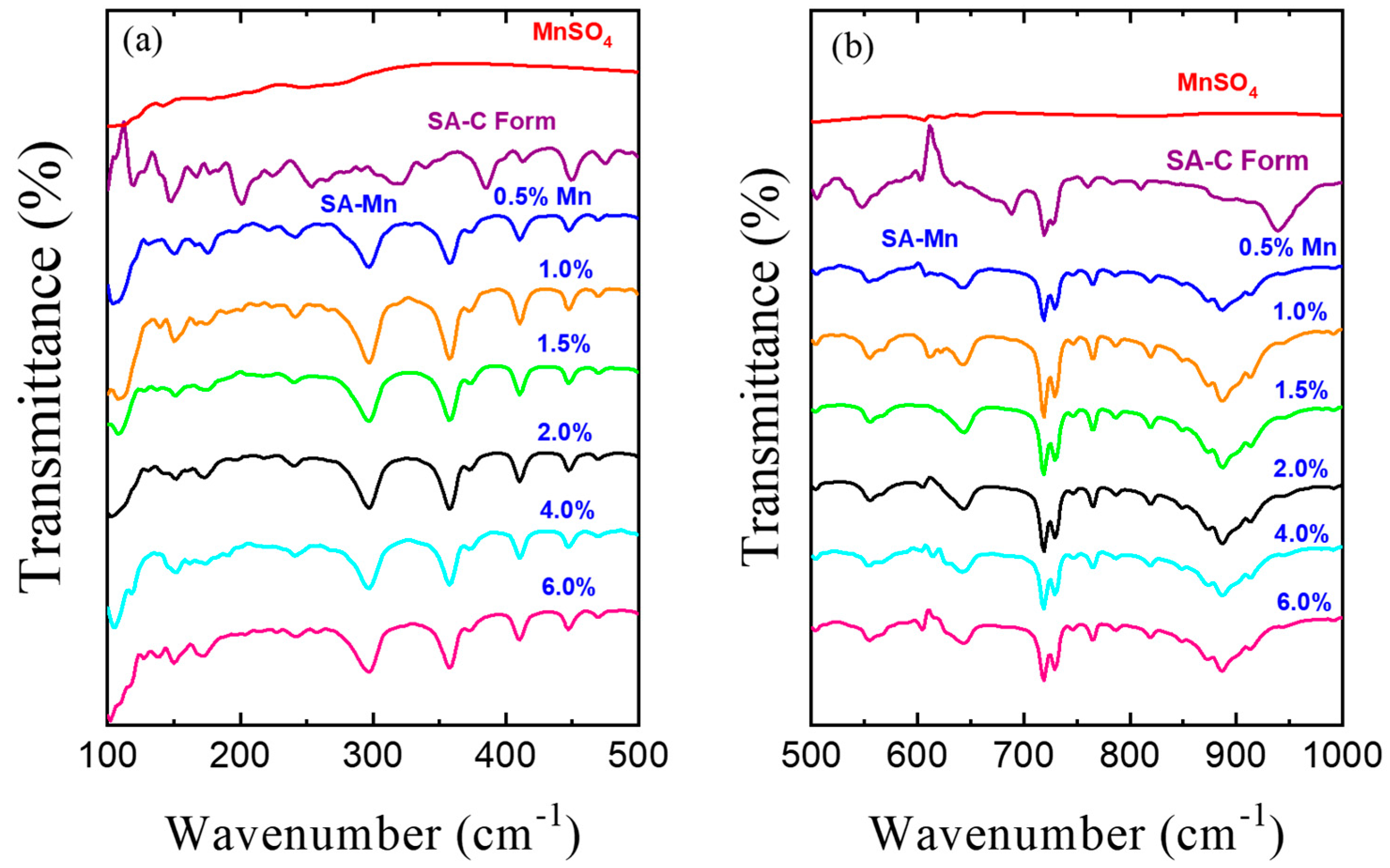
3.3. FT-IR Spectroscopy Studies
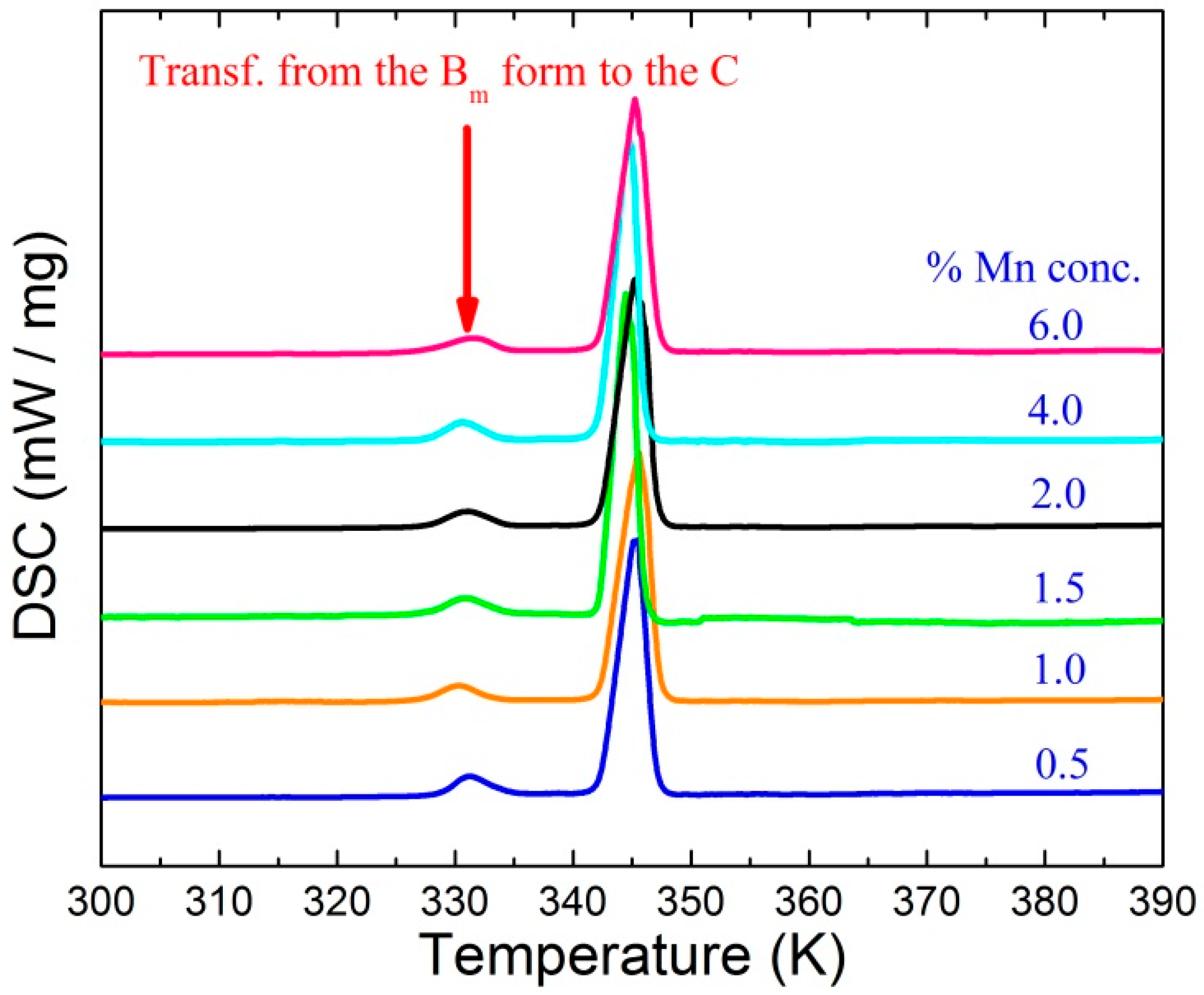
3.4. DSC Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, M.C.; Douglas, W.H.; Tanaka, J. Organic-Inorganic Interaction and the Growth Mechanism of Hydroxyapatite Crystals in Gelatin Matrices between 37 and 80 °C. J. Mater. Sci. Mater. Med. 2006, 17, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.R.; Volksen, W.; Huang, E.; Toney, M.; Frank, C.W.; Miller, R.D. Structure and Interaction of Organic/Inorganic Hybrid Nanocomposites for Microelectronic Applications. 1. MSSQ/P(MMA-Co-DMAEMA) Nanocomposites. Chem. Mater. 2002, 14, 3676–3685. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Yuan, Y.; Cao, X.; Yang, X. Effect of Carbon Nanotubes on the Thermal Behavior of Palmitic–Stearic Acid Eutectic Mixtures as Phase Change Materials for Energy Storage. Sol. Energy 2014, 110, 64–70. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Du, Y.; Cao, X.; Yuan, Y. Preparation and Properties of Palmitic-Stearic Acid Eutectic Mixture/Expanded Graphite Composite as Phase Change Material for Energy Storage. Energy 2014, 78, 950–956. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Schnütgen, F.; Scapin, G.; Börchers, T.; Xhong, N.; Lim, K.; Godbout, R.; Spener, F.; Sacchettini, J.C. Crystal Structure and Thermodynamic Analysis of Human Brain Fatty Acid-Binding Protein. J. Biol. Chem. 2000, 275, 27045–27054. [Google Scholar] [CrossRef]
- Akhiani, A.R.; Mehrali, M.; Tahan Latibari, S.; Mehrali, M.; Mahlia, T.M.I.; Sadeghinezhad, E.; Metselaar, H.S.C. One-Step Preparation of Form-Stable Phase Change Material through Self-Assembly of Fatty Acid and Graphene. J. Phys. Chem. C 2015, 119, 22787–22796. [Google Scholar] [CrossRef]
- Yabuuchi, Y.; Tani, M.; Matsushita, Y.; Otsuka, H.; Kobayashi, Y. Effects of Lauric Acid on Physical, Chemical and Microbial Characteristics in the Rumen of Steers on a High Grain Diet. Anim. Sci. J. 2007, 78, 387–394. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared With Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-Inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Yuan, Y.; Li, T.; Cao, X. Lauric–Palmitic–Stearic Acid/Expanded Perlite Composite as Form-Stable Phase Change Material: Preparation and Thermal Properties. Energy Build. 2014, 82, 505–511. [Google Scholar] [CrossRef]
- Yang, G.; Yim, Y.-J.; Lee, J.W.; Heo, Y.-J.; Park, S.-J. Carbon-Filled Organic Phase-Change Materials for Thermal Energy Storage: A Review. Molecules 2019, 24, 2055. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, R. Stearic Acid/Inorganic Porous Matrix Phase Change Composite for Hot Water Systems. Molecules 2019, 24, 1482. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Fuxreiter, M. Fuzzy Complexes: Polymorphism and Structural Disorder in Protein–Protein Interactions. Trends Biochem. Sci. 2008, 33, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, B.A.; Losev, E.A.; Boldyreva, E. V Polymorphism of “Glycine–Glutaric Acid” Co-Crystals: The Same Phase at Low Temperatures and High Pressures. CrystEngComm 2013, 15, 1693. [Google Scholar] [CrossRef]
- Moreno-Calvo, E.; Calvet, T.; Cuevas-Diarte, M.A.; Aquilano, D. Relationship between the Crystal Structure and Morphology of Carboxylic Acid Polymorphs. Predicted and Experimental Morphologies. Cryst. Growth Des. 2010, 10, 4262–4271. [Google Scholar] [CrossRef]
- Sato, K.; Kobayashi, M. Structure, Stability and Crystal Growth of Polymorphs and Polytypes of Long-Chain Aliphatic Compounds BT—Organic Crystals I: Characterization; Karl, N., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 978-3-642-76253-6. [Google Scholar]
- Kaneko, F.; Kobayashi, M.; Kitagawa, Y.; Matsuura, Y. Structure of Stearic Acid E Form. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 1490–1492. [Google Scholar] [CrossRef]
- Zerbi, G.; Conti, G.; Minoni, G.; Pison, S.; Bigotto, A. Premelting Phenomena in Fatty Acids: An Infrared and Raman Study. J. Phys. Chem. 1987, 91, 2386–2393. [Google Scholar] [CrossRef]
- Gbabode, G.; Negrier, P.; Mondieig, D.; Moreno Calvo, E.; Calvet, T.; Cuevas-Diarte, M.À. Structures of the High-Temperature Solid Phases of the Odd-Numbered Fatty Acids from Tridecanoic Acid to Tricosanoic Acid. Chem.–A Eur. J. 2007, 13, 3150–3159. [Google Scholar] [CrossRef]
- Moreno, E.; Cordobilla, R.; Calvet, T.; Cuevas-Diarte, M.A.; Gbabode, G.; Negrier, P.; Mondieig, D.; Oonk, H.A.J. Polymorphism of Even Saturated Carboxylic Acids from N-Decanoic to n-Eicosanoic Acid. New J. Chem. 2007, 31, 947. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chemie Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Lemmerer, A.; Esterhuysen, C.; Bernstein, J. Synthesis Characterization Molecular Modeling of a Pharmaceutical Co-Crystal: (2-Chloro-4-Nitrobenzoic Acid):(Nicotinamide). J. Pharm. Sci. 2010, 99, 4054–4071. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quiroz, A.; Moreira, S.G.C.; de Morais, A.V.; Silva, A.S.; da Rocha, G.N.; Alcantara, P. Physical and Chemical Analysis of Dielectric Properties and Differential Scanning Calorimetry Techniques on Buriti Oil. Instrum. Sci. Technol. 2003, 31, 93–101. [Google Scholar] [CrossRef]
- Albuquerque, M.L.S.; Guedes, I.; Alcantara, P.; Moreira, S.G.C. Infrared Absorption Spectra of Buriti (Mauritia Flexuosa L.) Oil. Vib. Spectrosc. 2003, 33, 127–131. [Google Scholar] [CrossRef]
- Hackett, M.J.; Zaro, J.L.; Shen, W.-C.; Guley, P.C.; Cho, M.J. Fatty Acids as Therapeutic Auxiliaries for Oral and Parenteral Formulations. Adv. Drug Deliv. Rev. 2013, 65, 1331–1339. [Google Scholar] [CrossRef]
- Nair, A.; Shah, J.; Al-Dhubiab, B.; Jacob, S.; Patel, S.; Venugopala, K.; Morsy, M.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin Solid Lipid Nanoparticles for Topical Ocular Therapy: Optimization, Evaluation and In Vivo Studies. Pharmaceutics 2021, 13, 523. [Google Scholar] [CrossRef]
- Jiang, M.; Fang, Q. Organic and Semiorganic Nonlinear Optical Materials. Adv. Mater. 1999, 11, 1147–1151. [Google Scholar] [CrossRef]
- Sagunthala, P.; Yasotha, P.; Vijaya, L. Growth and Characterization of Manganese (II) Sulphate and L-Lysine Doped Manganese (II) Sulphate (LMnSO4) Crystals. Int. J. Sci. Eng. Appl. 2013, 7560, 46–52. [Google Scholar] [CrossRef]
- Remédios, C.M.R.; dos Santos, A.O.; Lai, X.; Roberts, K.J.; Moreira, S.G.C.; Miranda, M.A.R.; de Menezes, A.S.; Rouxinol, F.P.; Cardoso, L.P. Experimental Evidence for the Influence of Mn3+ Concentration on the Impurity Incorporation and Habit Modification Mechanism of Potassium Dihydrogen Phosphate. Cryst. Growth Des. 2010, 10, 1053–1058. [Google Scholar] [CrossRef]
- Afanassyev, D.; Ubizskii, S.; Zhydachevskyy, Y.; Luchechko, A.; Popov, A.I.; Suchocki, A. Time-Resolved Pulsed OSL of Ceramic YAP:Mn Phosphors. Integr. Ferroelectr. 2019, 196, 24–31. [Google Scholar] [CrossRef]
- Shimotori, Y.; Yokoyama, M.; Harada, S.; Masugata, K.; Yatsui, K. Quick Deposition of ZuS:Mn Electroluminescent Thin Films by Intense, Pulsed, Ion Beam Evaporation. Jpn. J. Appl. Phys. 1989, 28, 468. [Google Scholar] [CrossRef]
- Adachi, S. Current Understanding of Anomalous Deep Red/NIR Emission Bands in Highly Mn4+, Mn2+, and Cr3+-Doped Phosphors: Exchange-Coupled Pair or Trap-Related Luminescence? Opt. Mater. X 2024, 22, 100312. [Google Scholar] [CrossRef]
- Vien, L.T.T.; Tu, N.; Viet, D.X.; Anh, D.D.; Nguyen, D.H.; Huy, P.T. Mn2+-Doped Zn2SnO4 Green Phosphor for WLED Applications. J. Lumin. 2020, 227, 117522. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Malta, V.; Celotti, G.; Zannetti, R.; Martelli, A.F. Crystal Structure of the C Form of Stearic Acid. J. Chem. Soc. B Phys. Org. 1971, 548–553. [Google Scholar] [CrossRef]
- Goto, M.; Asada, E. The Crystal Structure of the B-Form of Stearic Acid. Bull. Chem. Soc. Jpn. 1978, 51, 2456–2459. [Google Scholar] [CrossRef]
- Shimada, H.; Nibu, Y.; Shimada, R. Pressure Effects on Inter- and Intramolecular Vibrations in Hydrogen-bonded L-ascorbic Acid Crystal. J. Raman Spectrosc. 2008, 39, 32–39. [Google Scholar] [CrossRef]
- Luz-Lima, C.; Borges, J.A.; Moura, J.V.B.; Pinheiro, G.S.; Viana, B.C.; Mendes-Filho, J.; Freire, P.T.C. α-l-Glutamic Acid under High Pressure: Phase Transitions Studied by Raman Spectroscopy. Vib. Spectrosc. 2016, 86, 343–349. [Google Scholar] [CrossRef]
- L. da Silva, L.F.; Andrade-Filho, T.; Freire, P.T.C.; Filho, J.M.; da Silva Filho, J.G.; Saraiva, G.D.; Moreira, S.G.C.; de Sousa, F.F. Polarized Raman and Infrared Spectroscopy and Ab Initio Calculation of Palmitic and Stearic Acids in the B m and C Forms. J. Phys. Chem. A 2017, 121, 4830–4842. [Google Scholar] [CrossRef]
- de Sousa, F.F.; Saraiva, G.D.; Freire, P.T.C.; Lima, J.A.; Alcantara, P.; Melo, F.E.A.; Mendes Filho, J. Pressure-induced Phase Transitions in Palmitic Acid: C Form. J. Raman Spectrosc. 2012, 43, 146–152. [Google Scholar] [CrossRef]
- Hsu, M.F.; Dufresne, E.R.; Weitz, D.A. Charge Stabilization in Nonpolar Solvents. Langmuir 2005, 21, 4881–4887. [Google Scholar] [CrossRef]
- Tran, T.A.; Tran, H.C.; Nghiem, N.T.; Truong-Son, L.V.; Imanova, G.T.; Jabarov, S.H. Effect of Doping Mn Ion on the Crystal Structure and Cation Distribution in Co1-xMnxFe2O4 Compounds. J. Mater. Sci. Mater. Eng. 2025, 20, 5. [Google Scholar] [CrossRef]
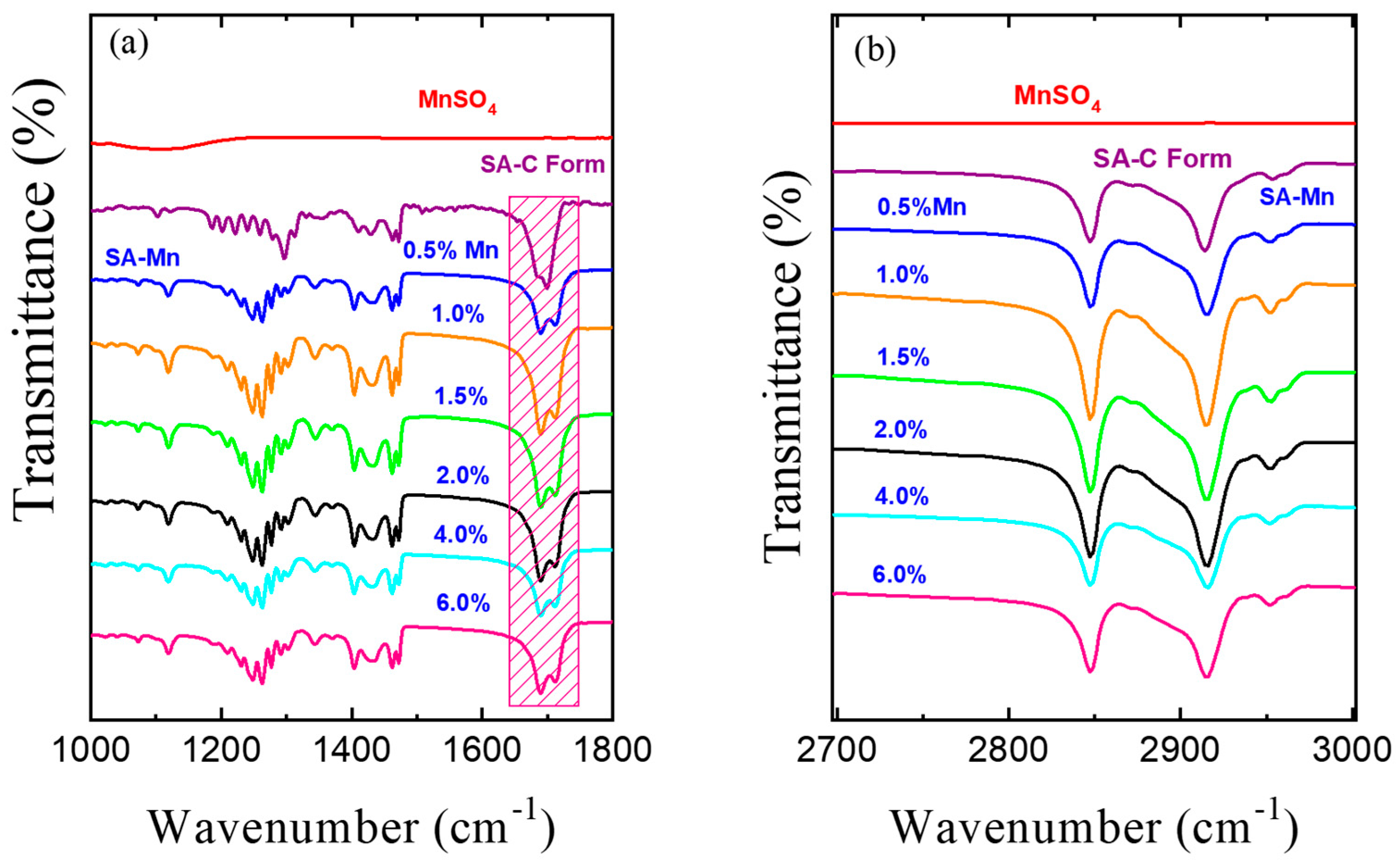
| Carbonyl IR-Active Modes | |
|---|---|
| Mn Concentration (%) | Wavenumber Range (cm−1) |
| C form | 1682–1700 |
| Bm form: 0.5 | 1690–1712 |
| Bm form: 1.0 | 1690–1712 |
| Bm form: 1.5 | 1690–1712 |
| Bm form: 2.0 | 1689–1712 |
| Bm form: 4.0 | 1690–1712 |
| Bm form: 6.0 | 1690–1713 |
| Melting of SA Crystals | ||||
| Mn Concentration (%) | Onset (K) | Peak (K) | Endset (K) | Width (K) |
| 0.5 | 342.3 | 345.5 | 347.1 | 3.2 |
| 1 | 342.3 | 345.6 | 347.2 | 3.4 |
| 1.5 | 342.2 | 344.5 | 346.0 | 2.6 |
| 2 | 342.3 | 345.3 | 347.0 | 3.3 |
| 4 | 342.0 | 344.7 | 346.1 | 2.7 |
| 6 | 342.1 | 345.2 | 347.2 | 3.3 |
| Transformation from the Bm form to the C | ||||
| Mn Concentration (%) | Onset (K) | Peak (K) | Endset (K) | Width (K) |
| 0.5 | 328.9 | 331.1 | 334.4 | 4.1 |
| 1 | 327.5 | 330.3 | 333.1 | 4.3 |
| 1.5 | 328.0 | 330.8 | 334.3 | 4.7 |
| 2 | 327.8 | 331.1 | 334.3 | 4.7 |
| 4 | 327.9 | 330.6 | 333.7 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, R.M.; de Souza Junior, M.V.; Silva, L.F.L.; Freire, P.T.C.; Pinheiro, G.S.; Paschoal, W., Jr.; de Sousa, F.F.; Moreira, S.G.C. Investigation of Mn2+-Doped Stearic-Acid Through XRD, Raman, and FT-IR, and Thermal Studies. Quantum Beam Sci. 2025, 9, 8. https://doi.org/10.3390/qubs9010008
Rocha RM, de Souza Junior MV, Silva LFL, Freire PTC, Pinheiro GS, Paschoal W Jr., de Sousa FF, Moreira SGC. Investigation of Mn2+-Doped Stearic-Acid Through XRD, Raman, and FT-IR, and Thermal Studies. Quantum Beam Science. 2025; 9(1):8. https://doi.org/10.3390/qubs9010008
Chicago/Turabian StyleRocha, Rodrigo M., Marinaldo V. de Souza Junior, Luiz F. L. Silva, Paulo T. C. Freire, Gardênia S. Pinheiro, Waldomiro Paschoal, Jr., Francisco F. de Sousa, and Sanclayton G. C. Moreira. 2025. "Investigation of Mn2+-Doped Stearic-Acid Through XRD, Raman, and FT-IR, and Thermal Studies" Quantum Beam Science 9, no. 1: 8. https://doi.org/10.3390/qubs9010008
APA StyleRocha, R. M., de Souza Junior, M. V., Silva, L. F. L., Freire, P. T. C., Pinheiro, G. S., Paschoal, W., Jr., de Sousa, F. F., & Moreira, S. G. C. (2025). Investigation of Mn2+-Doped Stearic-Acid Through XRD, Raman, and FT-IR, and Thermal Studies. Quantum Beam Science, 9(1), 8. https://doi.org/10.3390/qubs9010008








