Time-Resolved Radioluminescence Dosimetry Applications and the Influence of Ge Dopants In Silica Optical Fiber Scintillators
Abstract
:1. Introduction
2. Materials & Methods
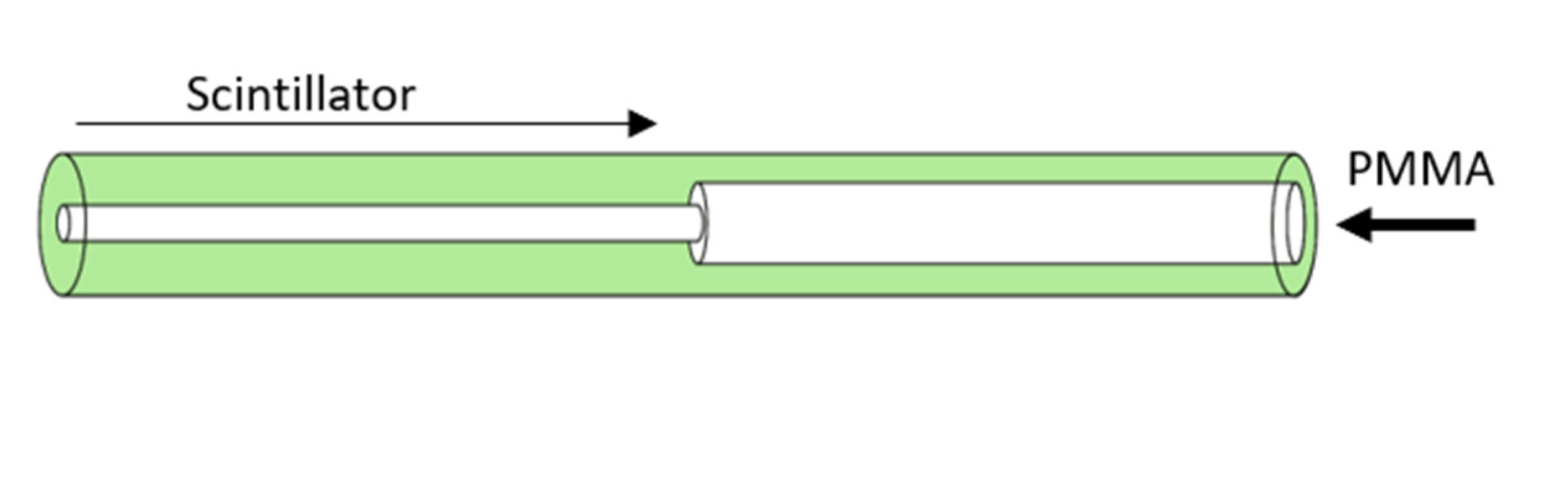
2.1. Scintillator Design and Fabrication
2.2. Scintillator Probe
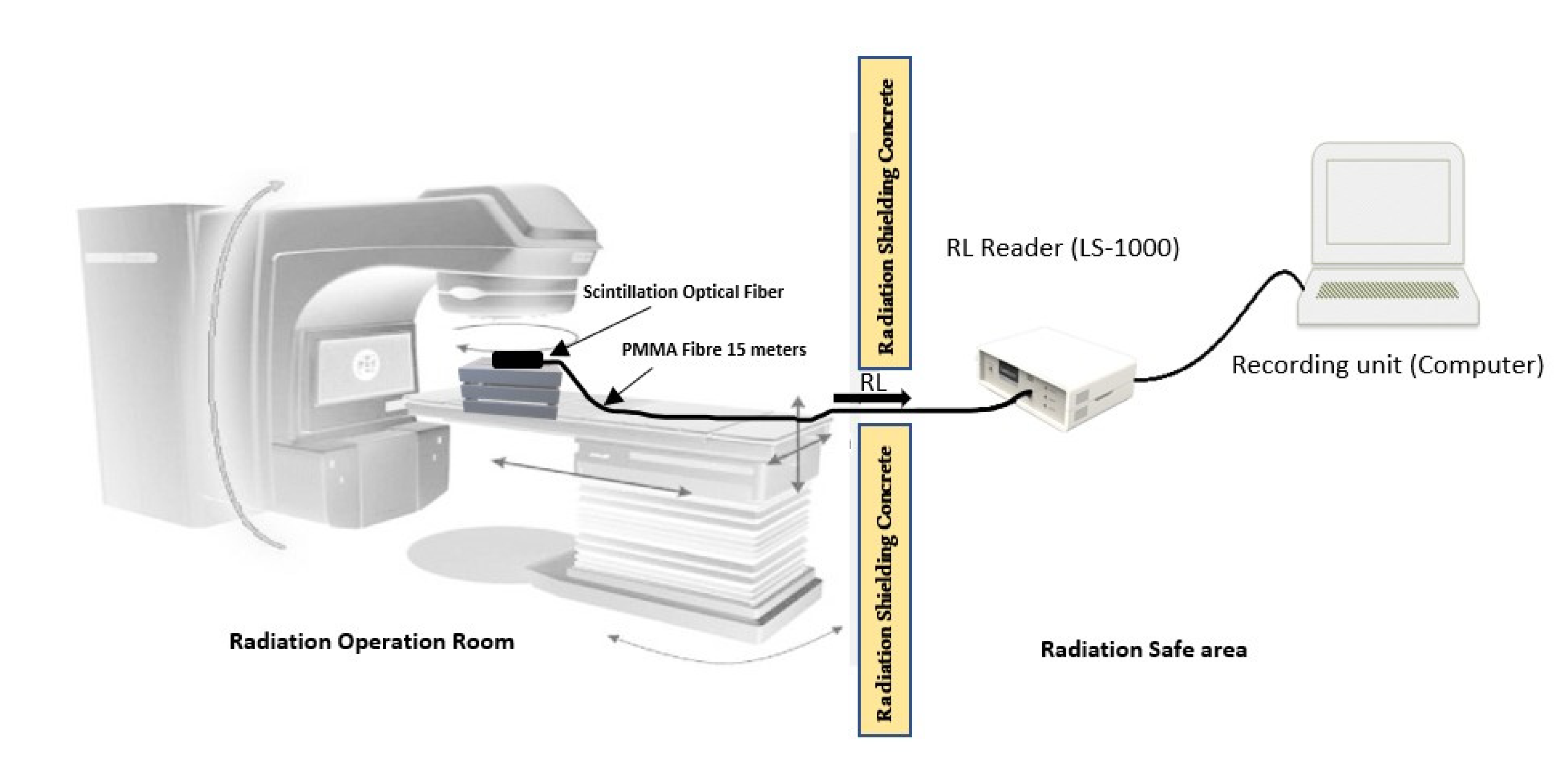
2.3. Experimental Setup
2.4. Characterization
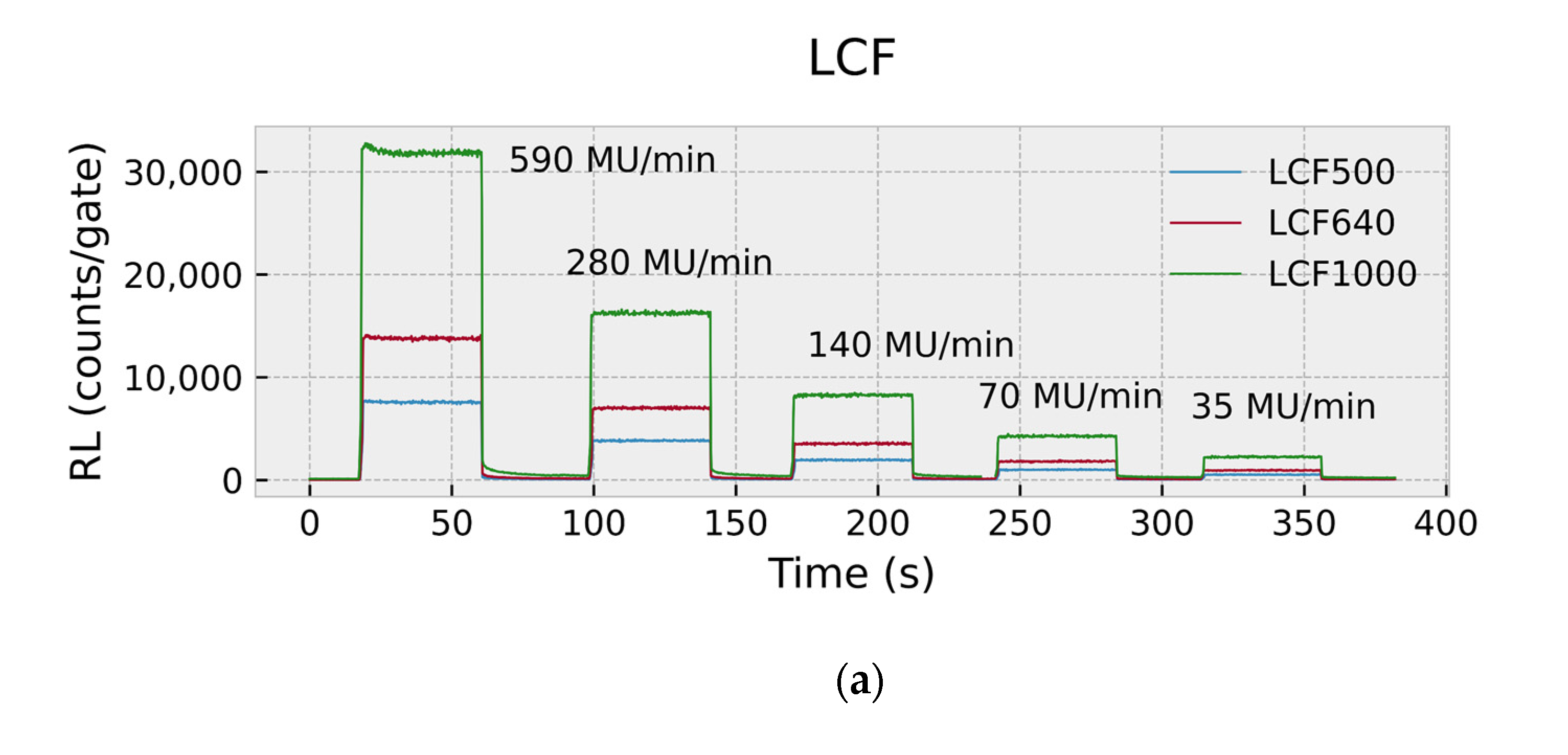
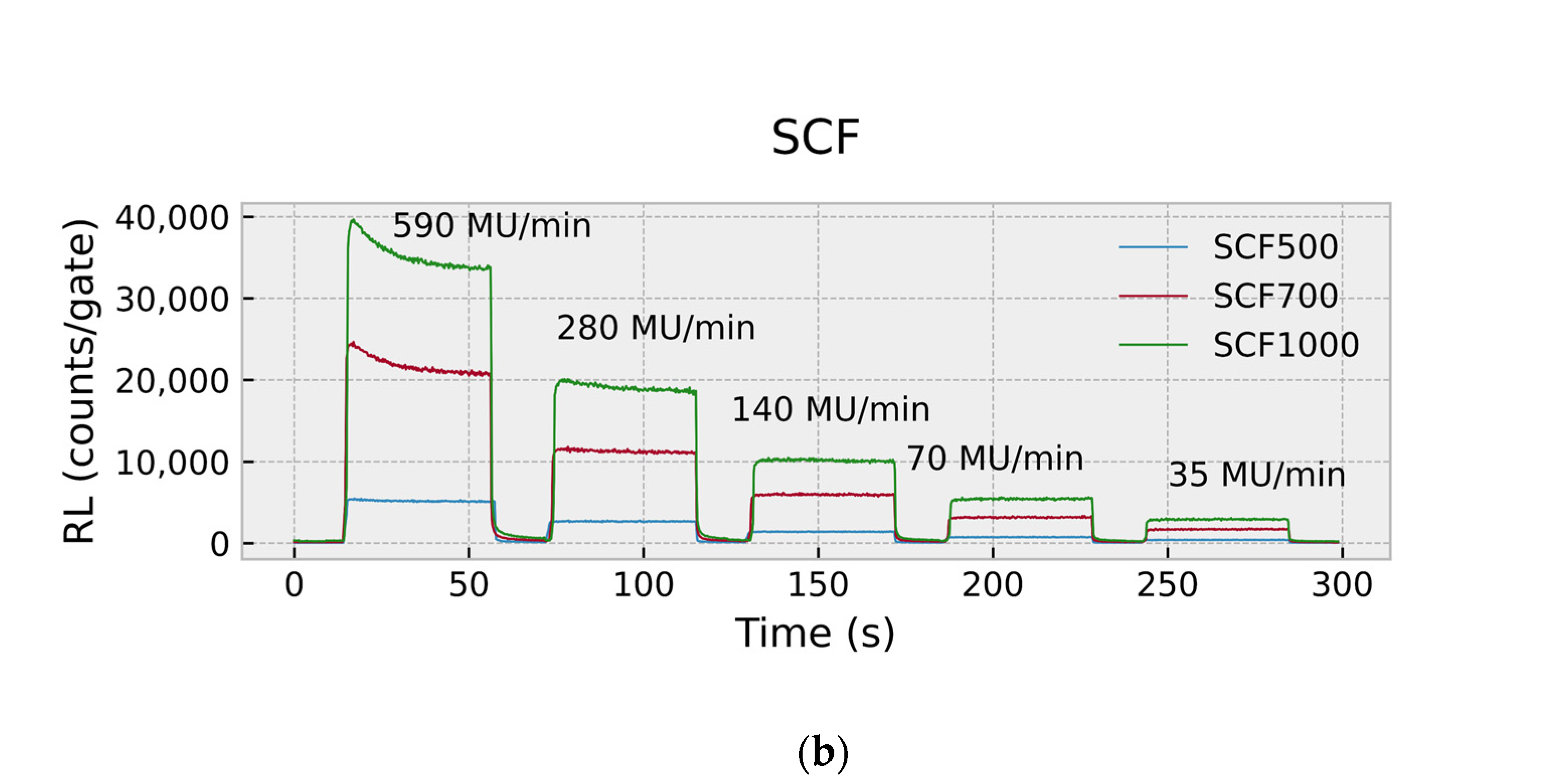
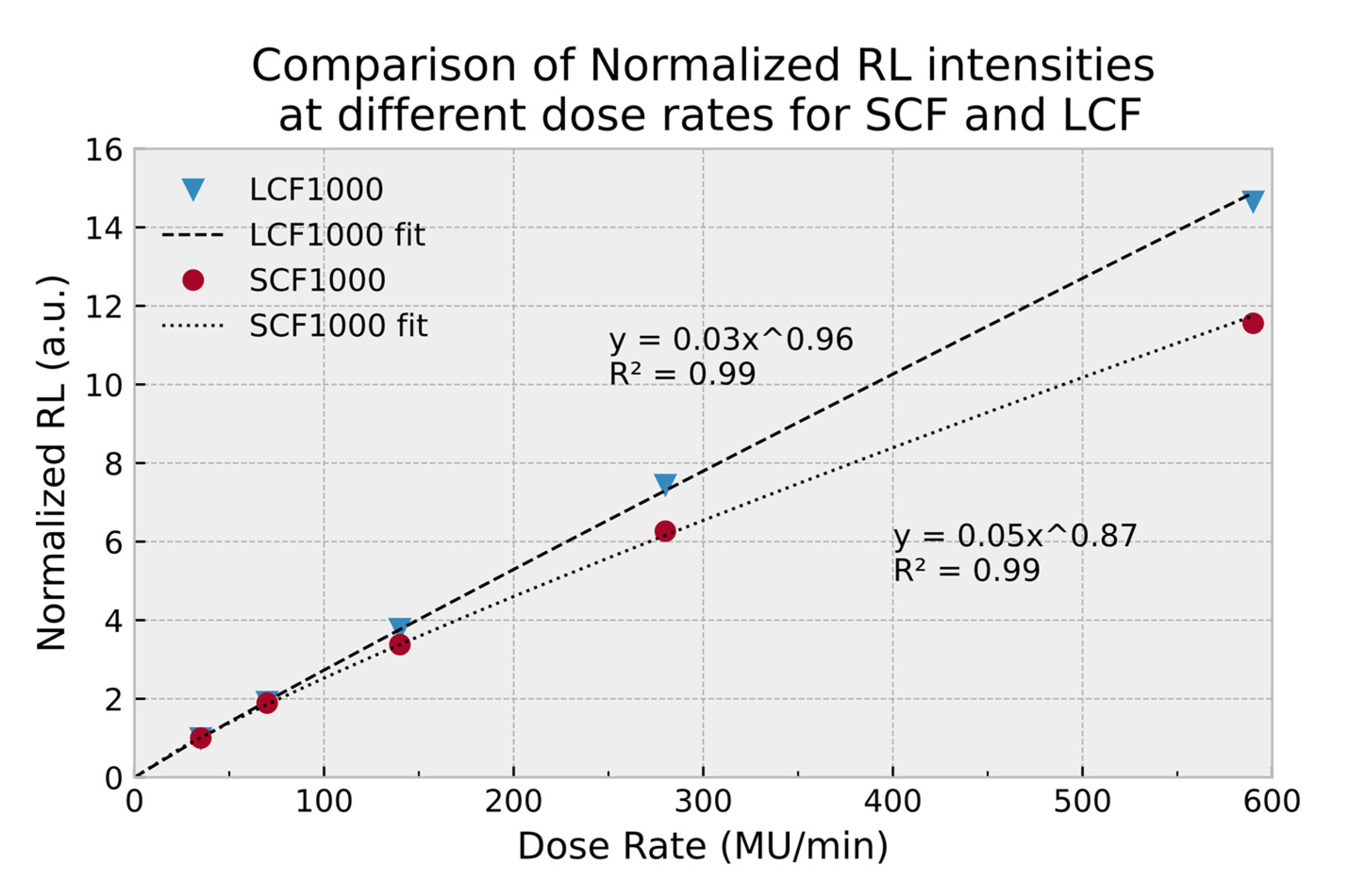
2.4.1. RL Response and Dose-Rate Dependence
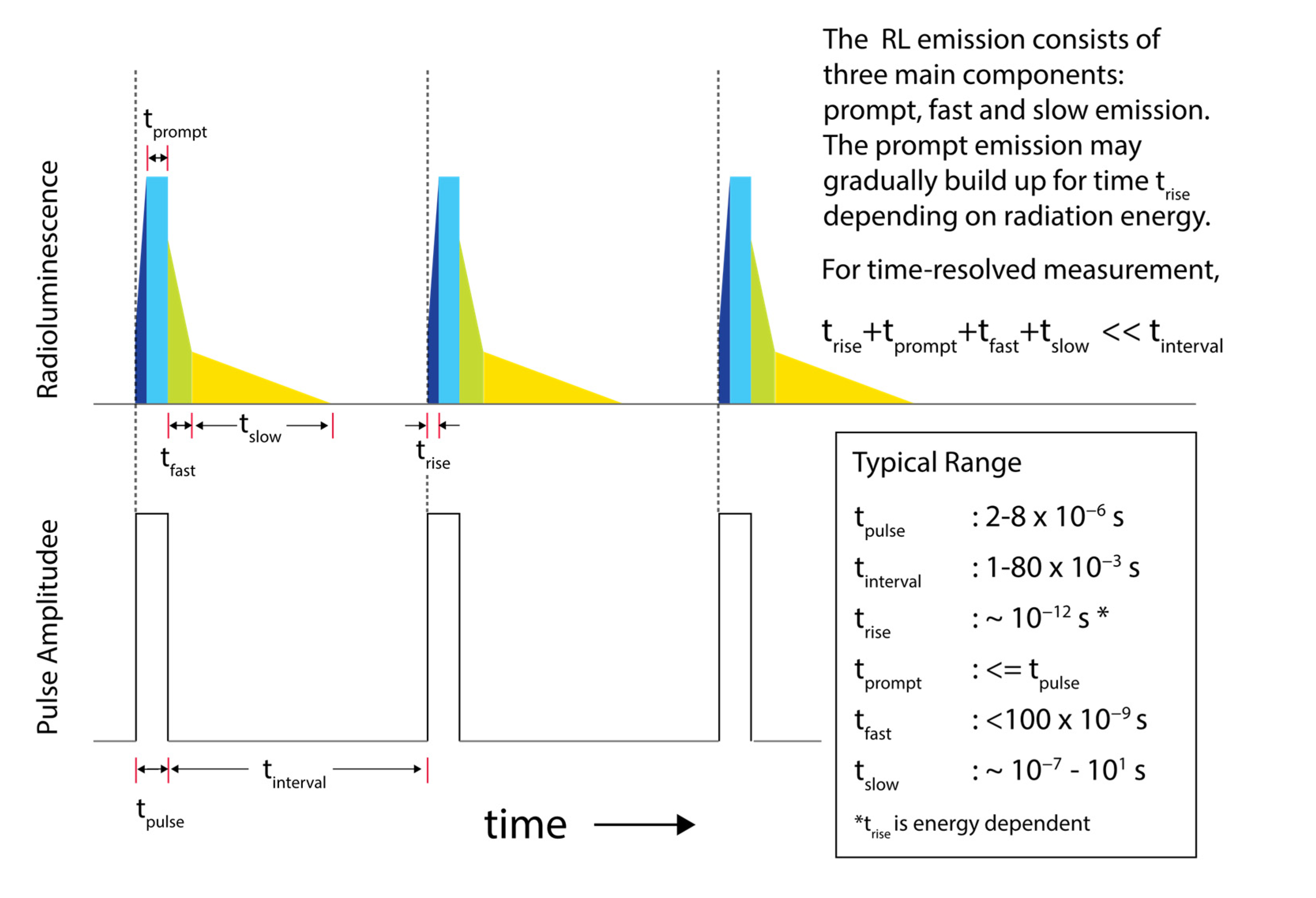
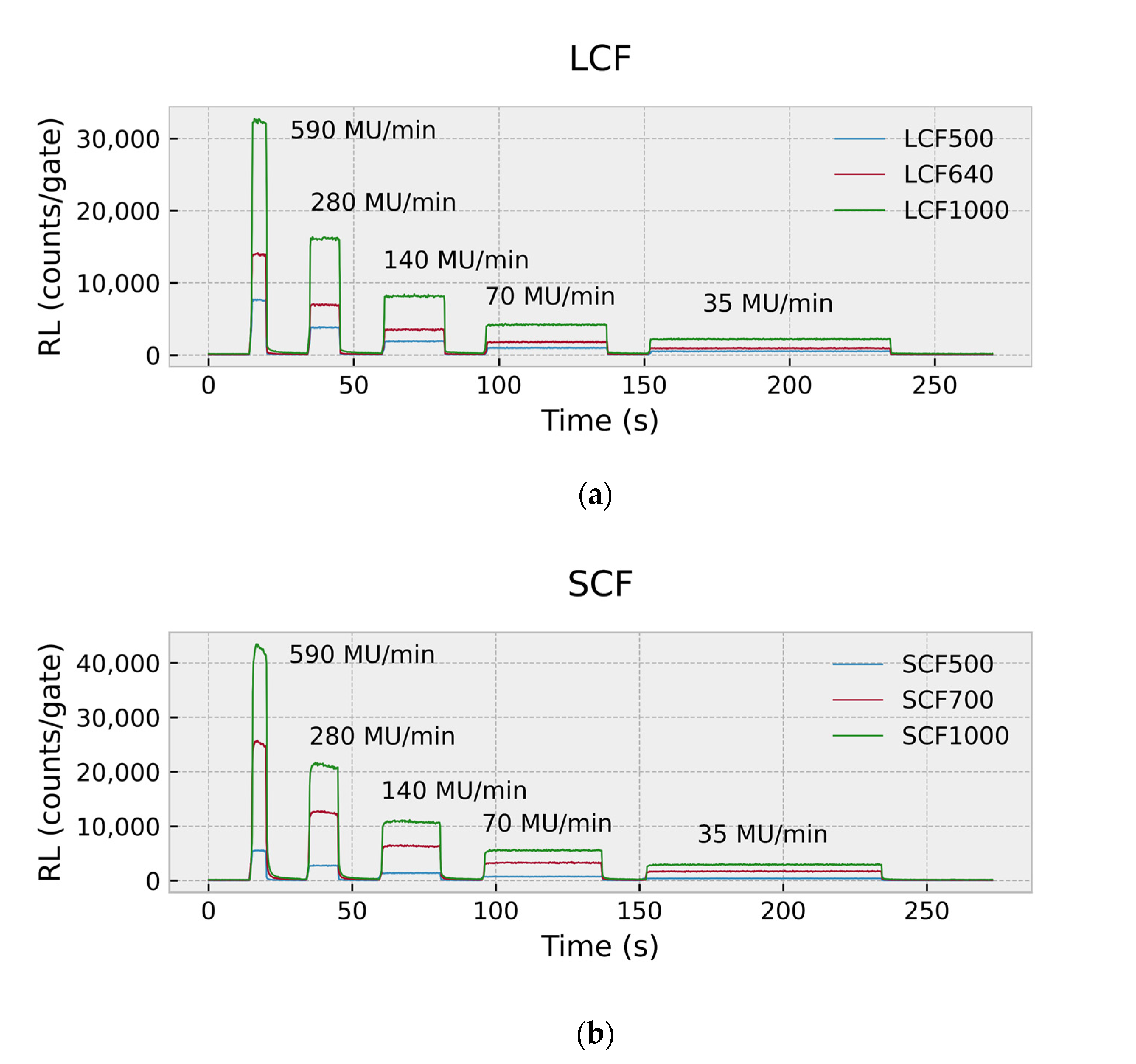
2.4.2. Time-Resolved Assessment
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goulet, M.; Archambault, L.; Beaulieu, L.; Gingras, L. 3D Tomodosimetry Using Long Scintillating Fibers: A Feasibility Study. Med. Phys. 2013, 40, 101703. [Google Scholar] [CrossRef] [PubMed]
- Elsey, J.; McKenzie, D.R.; Lambert, J.; Suchowerska, N.; Law, S.L.; Fleming, S.C. Optimal Coupling of Light from a Cylindrical Scintillator into an Optical Fiber. Appl. Opt. 2007, 46, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.V. Radiotherapy Near Misses, Incidents and Errors: Radiotherapy Incident at Glasgow. Clin. Oncol. 2007, 19, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, F.; Barca, P.; Barone, S.; Bortoli, E.; Borgheresi, R.; De Stefano, S.; Di Francesco, M.; Faillace, L.; Giuliano, L.; Grasso, L.; et al. FLASH Radiotherapy With Electrons: Issues Related to the Production, Monitoring, and Dosimetric Characterization of the Beam. Front. Phys. 2020, 8, 481. [Google Scholar] [CrossRef]
- Beddar, A.S.; Kinsella, K.J.; Ikhlef, A.; Sibata, C.H. A Miniature “Scintillator-Fiberoptic-PMT” Detector System for the Dosimetry of Small Fields in Stereotactic Radiosurgery. IEEE Trans. Nucl. Sci. 2001, 48, 924–928. [Google Scholar] [CrossRef]
- Cho, J.D.; Son, J.; Sung, J.; Choi, C.H.; Kim, J.S.; Wu, H.; Park, J.M.; Kim, J. Flexible Film Dosimeter for in Vivo Dosimetry. Med. Phys. 2020, 47, 3204–3213. [Google Scholar] [CrossRef]
- Boadu, M.; Rehani, M.M. Unintended Exposure in Radiotherapy: Identification of Prominent Causes. Radiother. Oncol. 2009, 93, 609–617. [Google Scholar] [CrossRef]
- Mahdiraji, G.A.; Adikan, F.R.M.; Bradley, D.A. Collapsed Optical Fiber: A Novel Method for Improving Thermoluminescence Response of Optical Fiber. J. Lumin. 2015, 161, 442–447. [Google Scholar] [CrossRef]
- Correia, A.; Pirraco, R.; Rosa, C.C.; Chiquita, S.; Hussain, N.S. A Multi-Sensor Dosimeter for Brachytherapy Based on Radioluminescent Fiber Sensors. Fifth Eur. Workshop Opt. Fibre Sens. 2013, 8794, 87941S. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.; McKenzie, D.R.; Law, S.; Elsey, J.; Suchowerska, N. A Plastic Scintillation Dosimeter for High Dose Rate Brachytherapy. Phys. Med. Biol. 2006, 51, 5505–5516. [Google Scholar] [CrossRef]
- Therriault-Proulx, F.; Briere, T.M.; Mourtada, F.; Aubin, S.; Beddar, S.; Beaulieu, L. A Phantom Study of an in Vivo Dosimetry System Using Plastic Scintillation Detectors for Real-Time Verification of 192Ir HDR Brachytherapy. Med. Phys. 2011, 38, 2542–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.K.M.M.; Begum, M.; Begum, M.; Zubair, H.T.; Abdul-Rashid, H.A.; Yusoff, Z.; Bradley, D.A. Radioluminescence of Ge-Doped Silica Optical Fibre and Al2O3:C Dosimeters. Sens. Actuators A Phys. 2018, 270, 72–78. [Google Scholar] [CrossRef]
- Archer, J.; Li, E.; Petasecca, M.; Dipuglia, A.; Cameron, M.; Stevenson, A.; Hall, C.; Hausermann, D.; Rosenfeld, A.; Lerch, M. X-Ray Microbeam Measurements with a High Resolution Scintillator Fibre-Optic Dosimeter. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Laissue, J.A.; Lyubimova, N.; Wagner, H.-P.; Archer, D.W.; Slatkin, D.N.; Di Michiel, M.; Nemoz, C.; Renier, M.; Brauer, E.; Spanne, P.O.; et al. Microbeam Radiation Therapy. In Medical Applications of Penetrating Radiation; Barber, H.B., Roehrig, H., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 1999; p. 38. [Google Scholar]
- Velthuis, J.J.; Page, R.F.; Purves, T.M.; Beck, L.; Hanifa, M.A.M.; Hugtenburg, R.P. Toward Pulse by Pulse Dosimetry Using an SC CVD Diamond Detector. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Van Eijk, C.W.E. Inorganic Scintillators in Medical Imaging. Phys. Med. Biol. 2002, 47, R85–R106. [Google Scholar] [CrossRef]
- Basaif, A.; Oresegun, A.; Tarif, Z.H.; Zin, H.; Choo, K.Y.; Ibrahim, S.A.; Abdul-Rashid, H.A.; Bradley, D.A. Ge-Doped Silica Optical Fibre for Time Resolved Radiation Dosimetry. Radiat. Phys. Chem. 2021, 189, 109669. [Google Scholar] [CrossRef]
- Mizanur Rahman, A.K.M.; Zubair, H.T.; Begum, M.; Abdul-Rashid, H.A.; Yusoff, Z.; Ung, N.M.; Mat-Sharif, K.A.; Wan Abdullah, W.S.; Amouzad Mahdiraji, G.; Amin, Y.; et al. Germanium-Doped Optical Fiber for Real-Time Radiation Dosimetry. Radiat. Phys. Chem. 2015, 116, 170–175. [Google Scholar] [CrossRef]
- Justus, B.L.; Falkenstein, P.; Huston, A.L.; Plazas, M.C.; Ning, H.; Miller, R.W. Gated Fiber-Optic-Coupled Detector for In Vivo Real-Time Radiation Dosimetry. Appl. Opt. 2004, 43, 1663. [Google Scholar] [CrossRef]
- Teichmann, T.; Sponner, J.; Radtke, J.; Henniger, J. Gated Discrimination of the Stem Signal in Pulsed Radiation Fields for a Fiber Optic Dosimetry System Based on the Radioluminescence of Beryllium Oxide. Radiat. Meas. 2017, 106, 552–555. [Google Scholar] [CrossRef]
- Lecoq, P.; Annenkov, A.; Gektin, A.; Korzhik, M.; Pedrini, C. Inorganic Scintillators for Detector Systems. In Particle Acceleration and Detection; Springer: Berlin/Heidelberg, Germany, 2006; pp. 81–122. ISBN 3-540-27766-8. [Google Scholar]
- Lam, S.E.; Bradley, D.A.; Mahmud, R.; Pawanchek, M.; Abdul Rashid, H.A.; Mohd Noor, N. Dosimetric Characteristics of Fabricated Ge-Doped Silica Optical Fibre for Small-Field Dosimetry. Results Phys. 2019, 12, 816–826. [Google Scholar] [CrossRef]
- Butson, M.J.; Rozenfeld, A.; Mathur, J.N.; Carolan, M.; Wong, T.P.Y.; Metcalfe, P.E. A New Radiotherapy Surface Dose Detector: The MOSFET. Med. Phys. 1996, 23, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Entezam, A.; Khandaker, M.U.; Amin, Y.M.; Ung, N.M.; Bradley, D.A.; Maah, J.; Safari, M.J.; Moradi, F. Thermoluminescence Response of Ge-Doped Cylindrical-, Flat- And Photonic Crystal Silica-Fibres to Electron and Photon Radiation. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pain, F.; Laniece, P.; Mastrippolito, R.; Charon, Y.; Comar, D.; Leviel, V.; Pujol, J.F.; Valentin, L. SIC, an Intracerebral Radiosensitive Probe for In Vivo Neuropharmacology Investigations in Small Laboratory Animals: Theoretical Considerations and Practical Characteristics. IEEE Trans. Nucl. Sci. 2000, 47, 25–32. [Google Scholar] [CrossRef]
- Mones, E.; Veronese, I.; Vedda, A.; Loi, G.; Fasoli, M.; Moretti, F.; Chiodini, N.; Cannillo, B.; Brambilla, M. Ce-Doped Optical Fibre as Radioluminescent Dosimeter in Radiotherapy. Radiat. Meas. 2008, 43, 888–892. [Google Scholar] [CrossRef]
- Hugtenburg, R.P.; Johnston, K.; Chalmers, G.J.; Beddoe, A.H. Application of Diamond Detectors to the Dosimetry of 45 and 100 KVp Therapy Beams: Comparison with a Parallel-Plate Ionization Chamber and Monte Carlo. Phys. Med. Biol. 2001, 46, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Balter, P.; Duhon, J.; White, G.A.; Vassy, D.L.; Miller, R.A.; Serago, C.F.; Fairobent, L.A. AAPM Medical Physics Practice Guideline 8.a.: Linear Accelerator Performance Tests. J. Appl. Clin. Med. Phys. 2017, 18, 23–39. [Google Scholar] [CrossRef]
- Leybovich, L.B.; Sethi, A.; Dogan, N. Comparison of Ionization Chambers of Various Volumes for IMRT Absolute Dose Verification. Med. Phys. 2003, 30, 119–123. [Google Scholar] [CrossRef]
- Low, D.A.; Parikh, P.; Dempsey, J.F.; Wahab, S.; Huq, S. Ionization Chamber Volume Averaging Effects in Dynamic Intensity Modulated Radiation Therapy Beams. Med. Phys. 2003, 30, 1706–1711. [Google Scholar] [CrossRef]
- Bradley, D.A.; Khandaker, M.U.; Alanazi, A. Irradiated Glass and Thermoluminescence Yield: Dosimetric Utility Reviewed. Radiat. Phys. Chem. 2020, 170, 108680. [Google Scholar] [CrossRef]
- Almond, P.R.; Biggs, P.J.; Coursey, B.M.; Hanson, W.F.; Huq, M.S.; Nath, R.; Rogers, D.W.O. AAPM’s TG-51 Protocol for Clinical Reference Dosimetry of High-Energy Photon and Electron Beams. Med. Phys. 1999, 26, 1847–1870. [Google Scholar] [CrossRef] [Green Version]
- Tanyi, J.A.; Krafft, S.P.; Ushino, T.; Huston, A.L.; Justus, B.L. Performance Characteristics of a Gated Fiber-Optic-Coupled Dosimeter in High-Energy Pulsed Photon Radiation Dosimetry. Appl. Radiat. Isot. 2010, 68, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.T.; Oresegun, A.; Rahman, A.K.M.M.; Ung, N.M.; Mat Sharif, K.A.; Zulkifli, M.I.; Yassin, S.Z.M.; Maah, M.J.; Yusoff, Z.; Abdul-Rashid, H.A.; et al. Real-Time Radiation Dosimetry Using P-Doped Silica Optical Fiber. Measurement 2019, 146, 119–124. [Google Scholar] [CrossRef]
- Bradley, D.A.; Zubair, H.T.; Oresegun, A.; Louay, G.T.; Abdul-Rashid, H.A.; Ung, N.M.; Alzimami, K.S. Towards the Development of Doped Silica Radioluminescence Dosimetry. Radiat. Phys. Chem. 2019, 154, 46–52. [Google Scholar] [CrossRef]
- Bradley, D.A.; Zubair, H.T.; Oresegun, A.; Louay, G.T.; Zin, H.M.; Ung, N.M.; Abdul-Rashid, H.A. Time-Resolved Dose Measurements of Linear Accelerator Pulses Using a Fibre Optic Sensor: Applications and Challenges. Radiat. Phys. Chem. 2020, 167, 108212. [Google Scholar] [CrossRef]
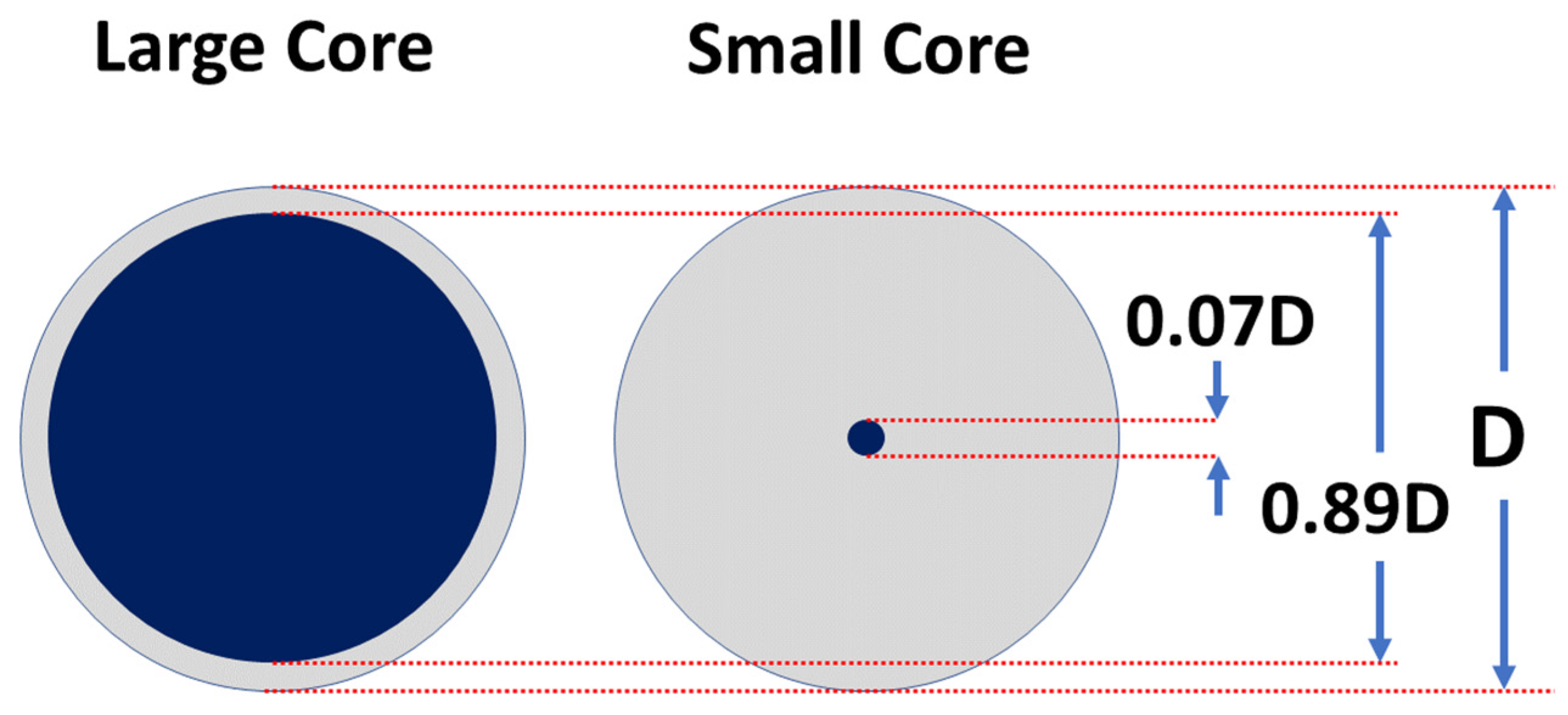
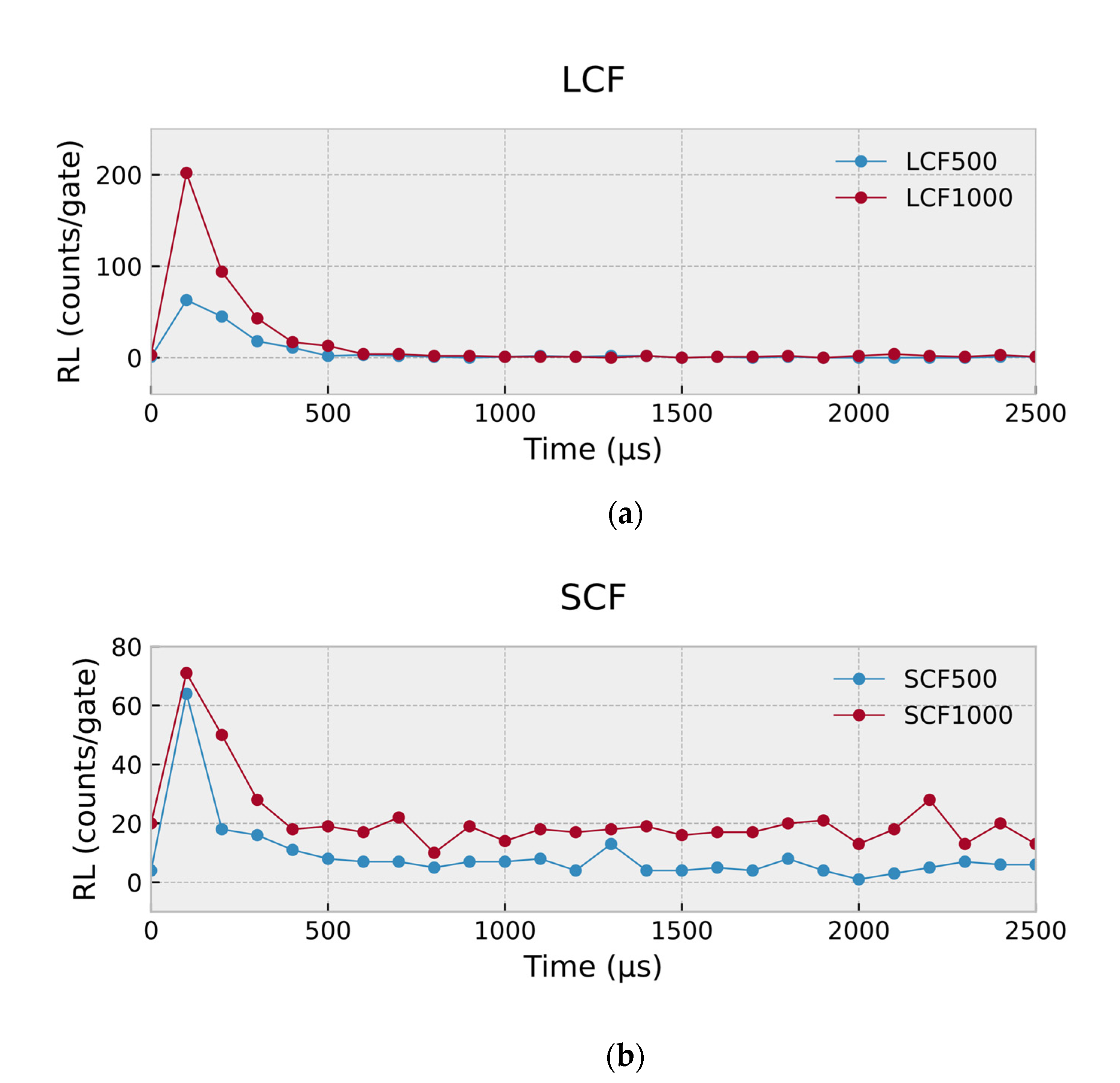
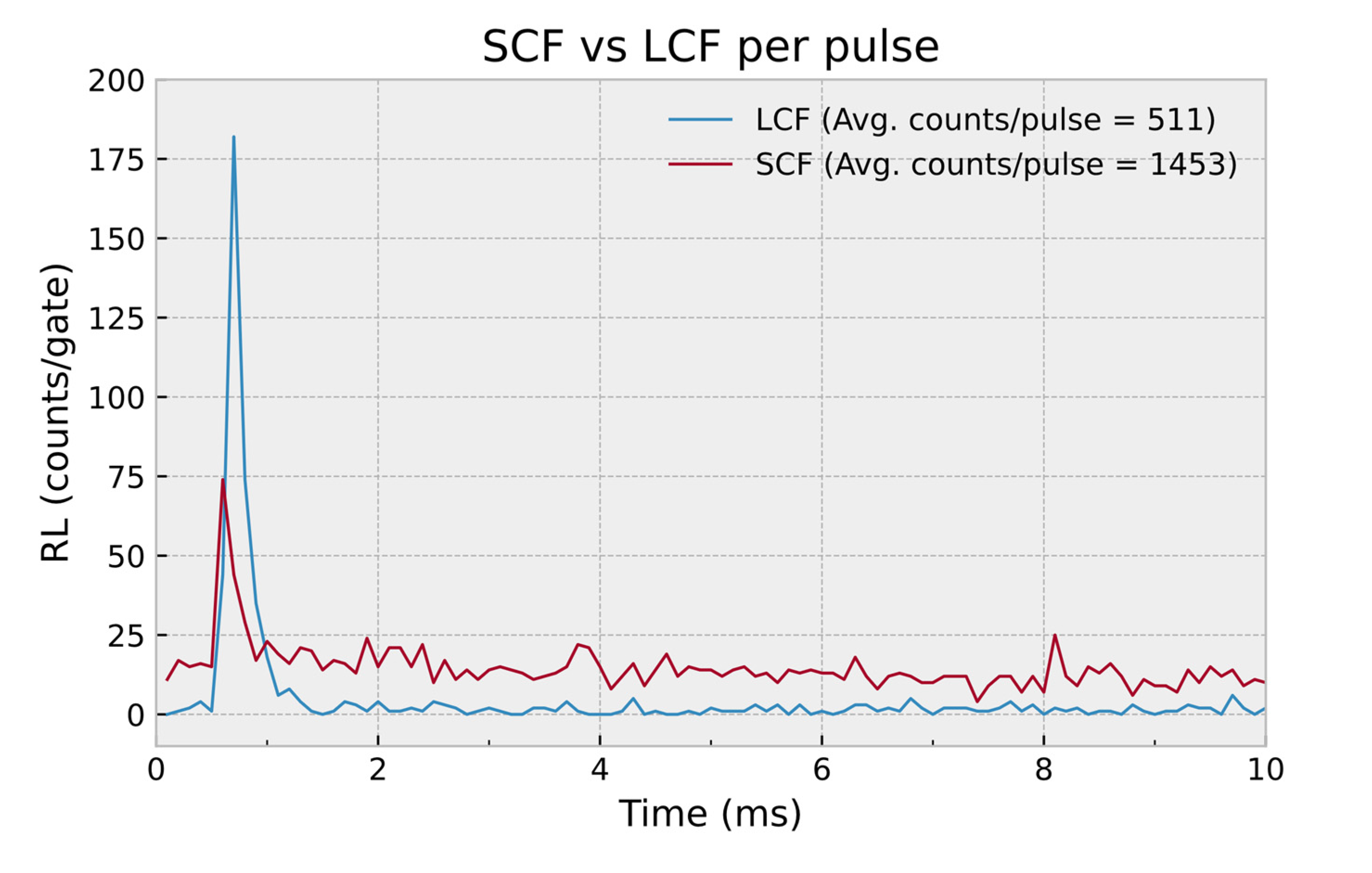
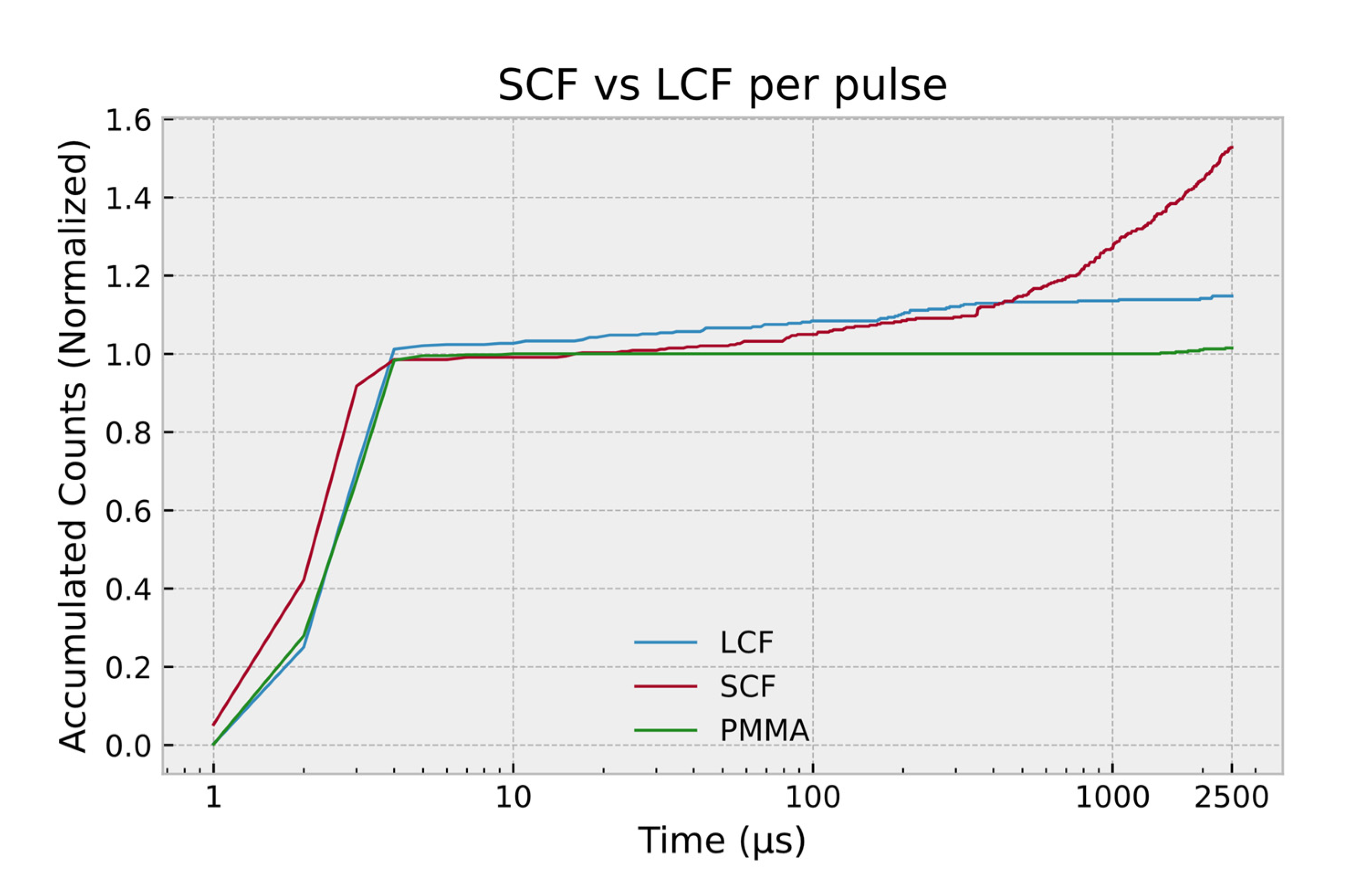
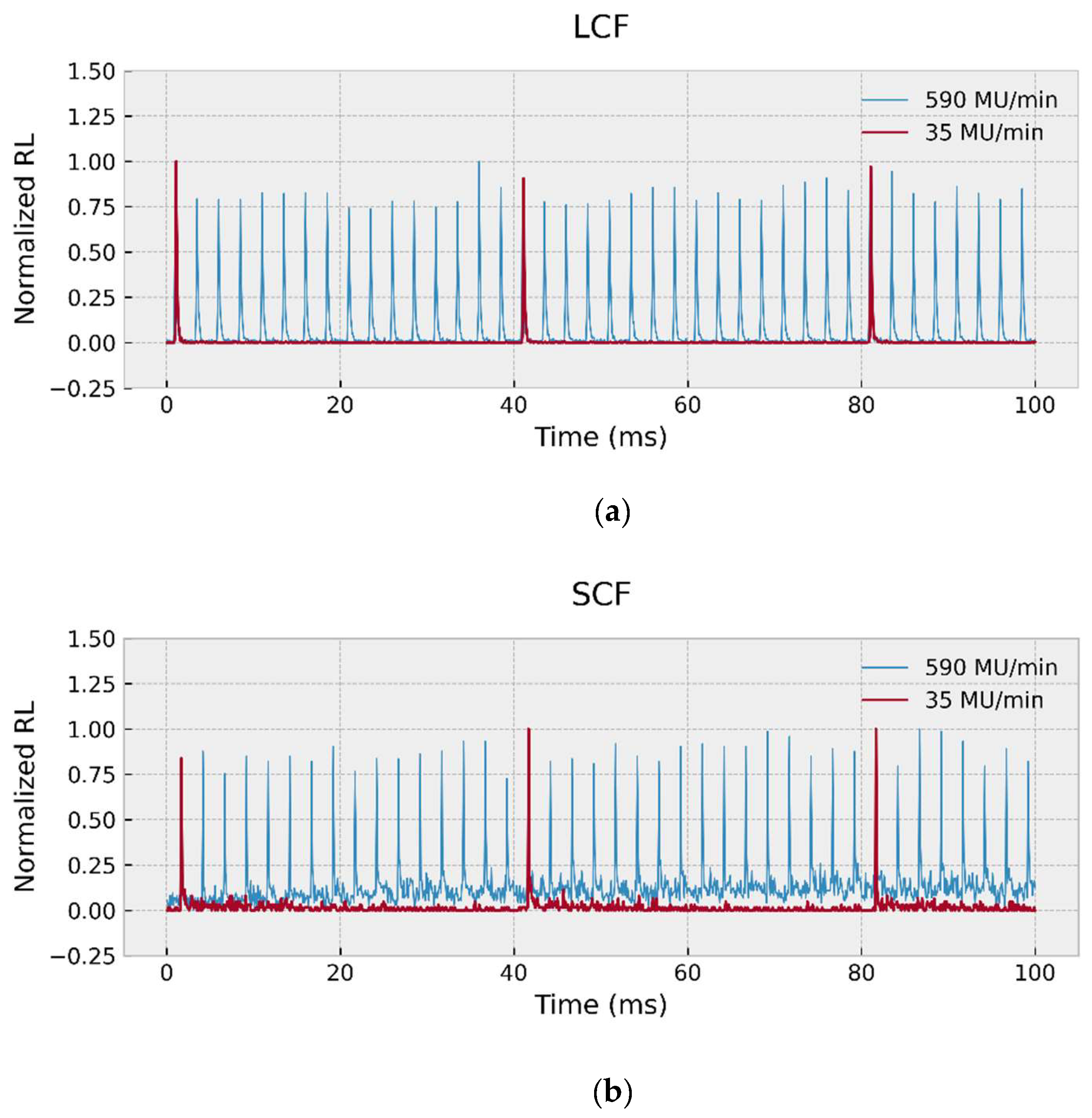
| Core Type | Sample Codename | Outer Diameter (μm) | Inner Diameter (μm) | Core to Cladding Ratio | Germanium Concentration (wt%) |
|---|---|---|---|---|---|
| Small core | SCF 500 | 477 | 34.6 | 0.073 | 0.85 |
| SCF 700 | 713 | 52.6 | 0.074 | 0.91 | |
| SCF 1000 | 978 | 64.5 | 0.066 | 0.95 | |
| Large core | LCF 500 | 500 | 449 | 0.898 | 3.05 |
| LCF 640 | 649 | 582 | 0.897 | 3.17 | |
| LCF 1000 | 994 | 888 | 0.893 | 3.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarif, Z.H.; Oresegun, A.; Abubakar, A.; Basaif, A.; Zin, H.M.; Choo, K.Y.; Ibrahim, S.A.; Abdul-Rashid, H.A.; Bradley, D.A. Time-Resolved Radioluminescence Dosimetry Applications and the Influence of Ge Dopants In Silica Optical Fiber Scintillators. Quantum Beam Sci. 2022, 6, 15. https://doi.org/10.3390/qubs6020015
Tarif ZH, Oresegun A, Abubakar A, Basaif A, Zin HM, Choo KY, Ibrahim SA, Abdul-Rashid HA, Bradley DA. Time-Resolved Radioluminescence Dosimetry Applications and the Influence of Ge Dopants In Silica Optical Fiber Scintillators. Quantum Beam Science. 2022; 6(2):15. https://doi.org/10.3390/qubs6020015
Chicago/Turabian StyleTarif, Zubair H., Adebiyi Oresegun, Auwal Abubakar, Azmi Basaif, Hafiz M. Zin, Kan Yeep Choo, Siti A. Ibrahim, Hairul Azhar Abdul-Rashid, and David A. Bradley. 2022. "Time-Resolved Radioluminescence Dosimetry Applications and the Influence of Ge Dopants In Silica Optical Fiber Scintillators" Quantum Beam Science 6, no. 2: 15. https://doi.org/10.3390/qubs6020015
APA StyleTarif, Z. H., Oresegun, A., Abubakar, A., Basaif, A., Zin, H. M., Choo, K. Y., Ibrahim, S. A., Abdul-Rashid, H. A., & Bradley, D. A. (2022). Time-Resolved Radioluminescence Dosimetry Applications and the Influence of Ge Dopants In Silica Optical Fiber Scintillators. Quantum Beam Science, 6(2), 15. https://doi.org/10.3390/qubs6020015







