Phase Transformation by 100 keV Electron Irradiation in Partially Stabilized Zirconia
Abstract
:1. Introduction
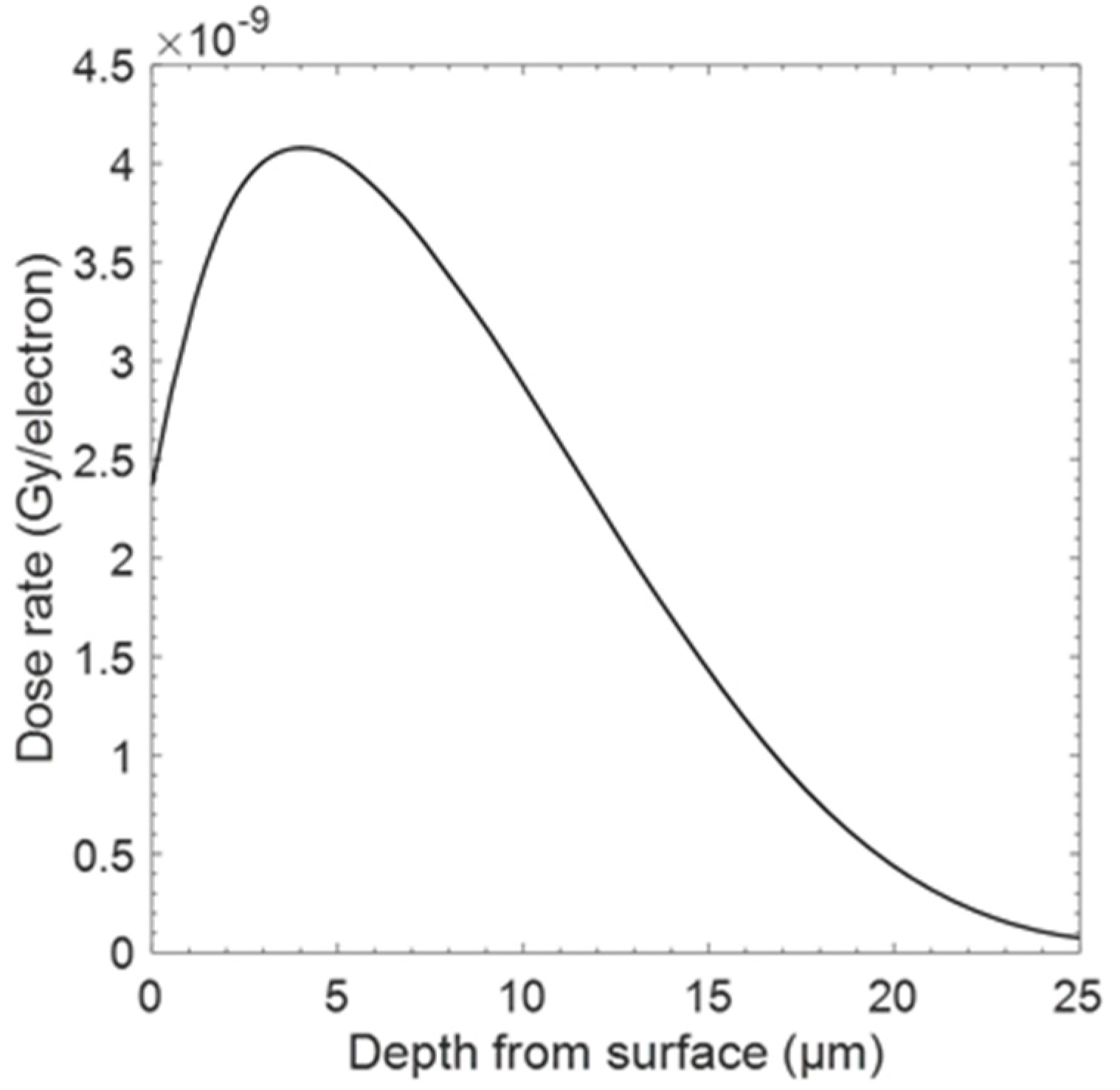
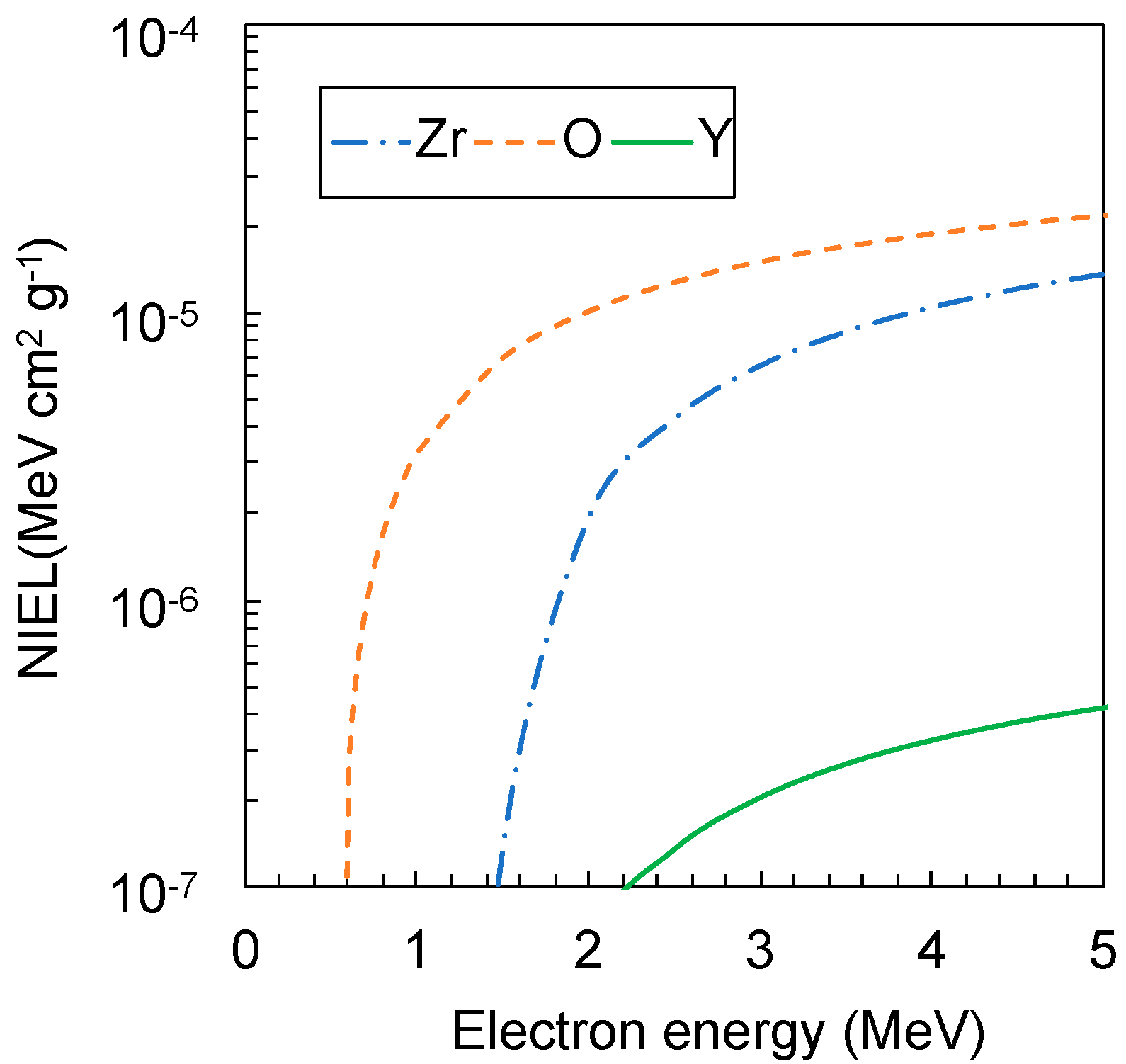
2. Materials and Methods
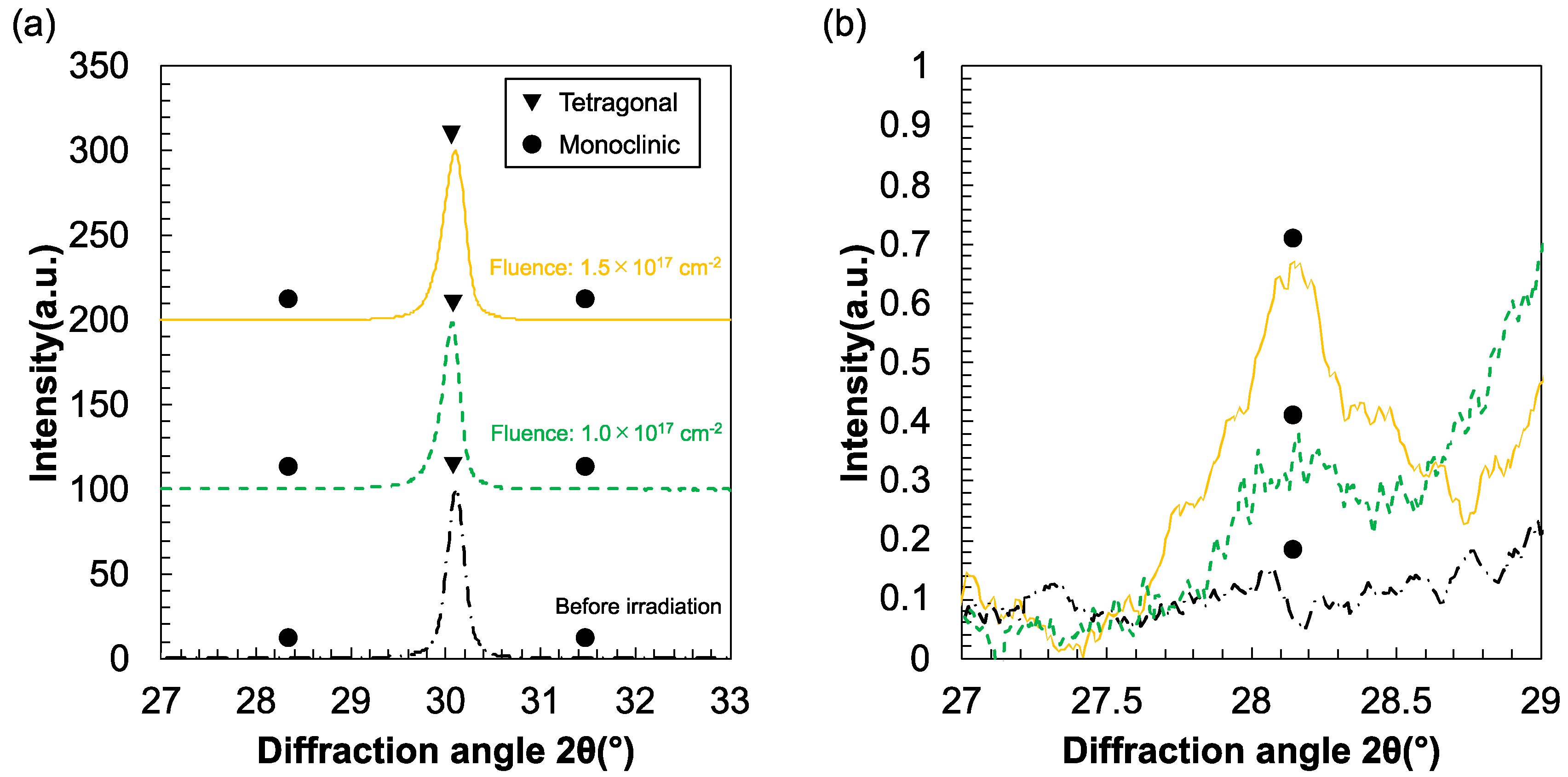
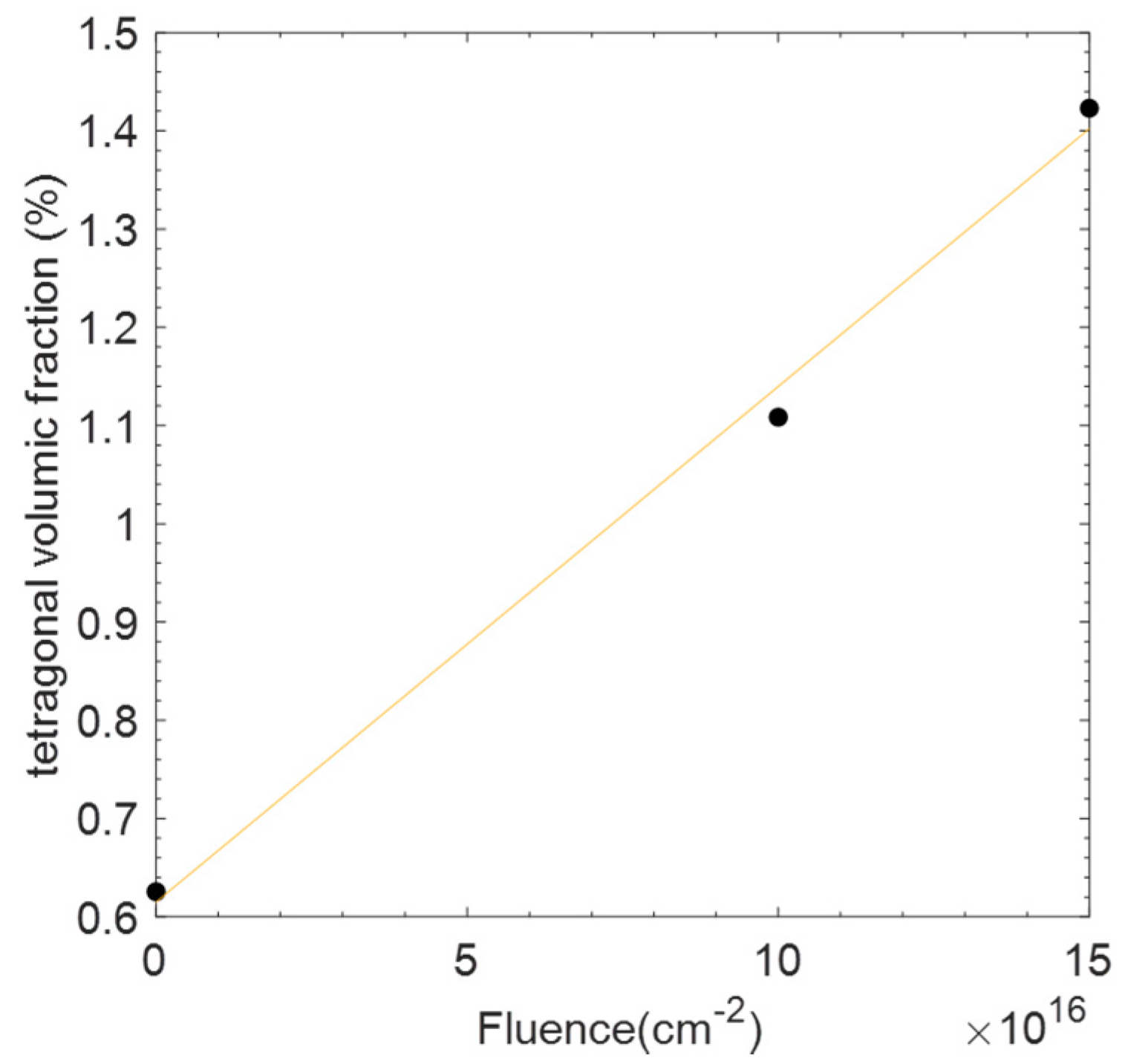
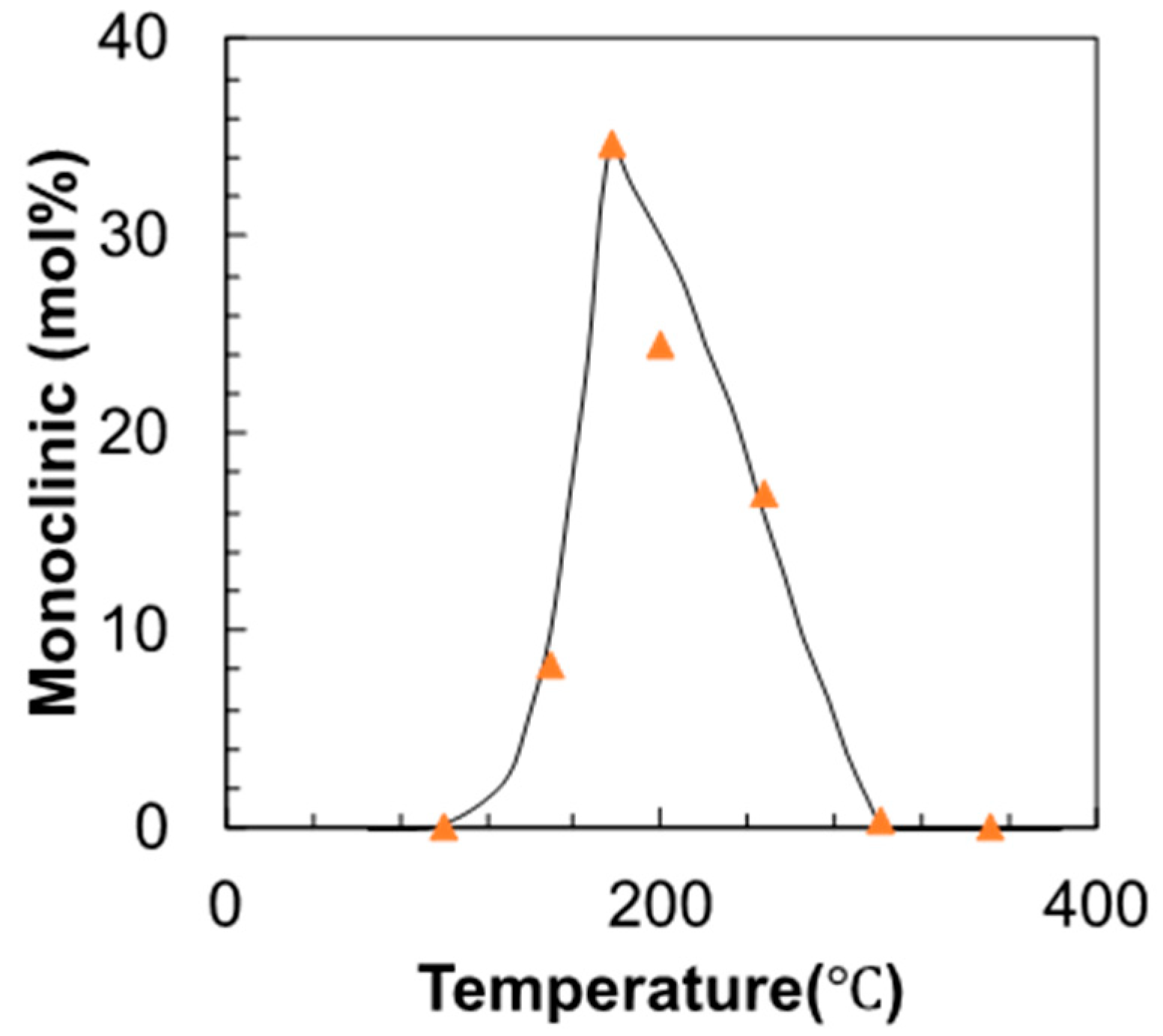
3. Results
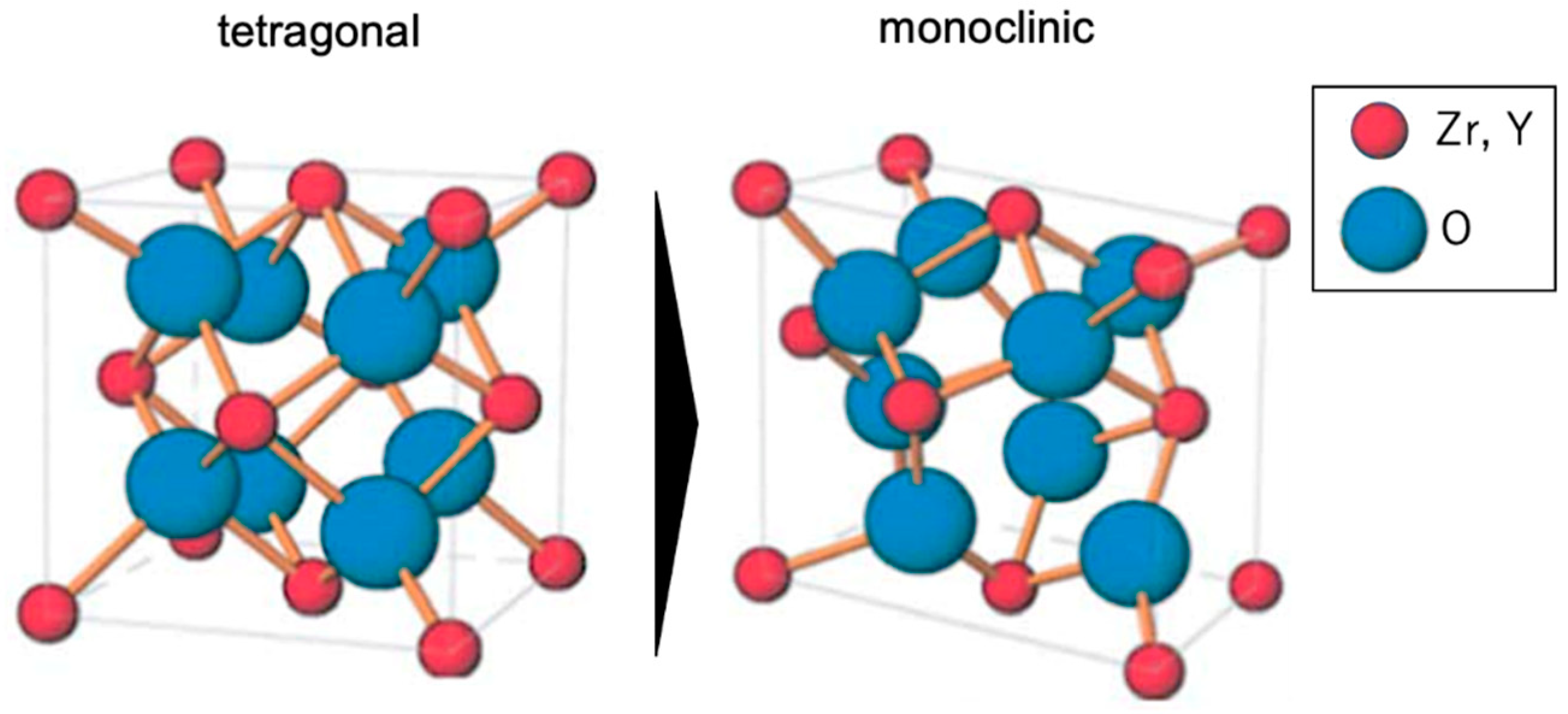
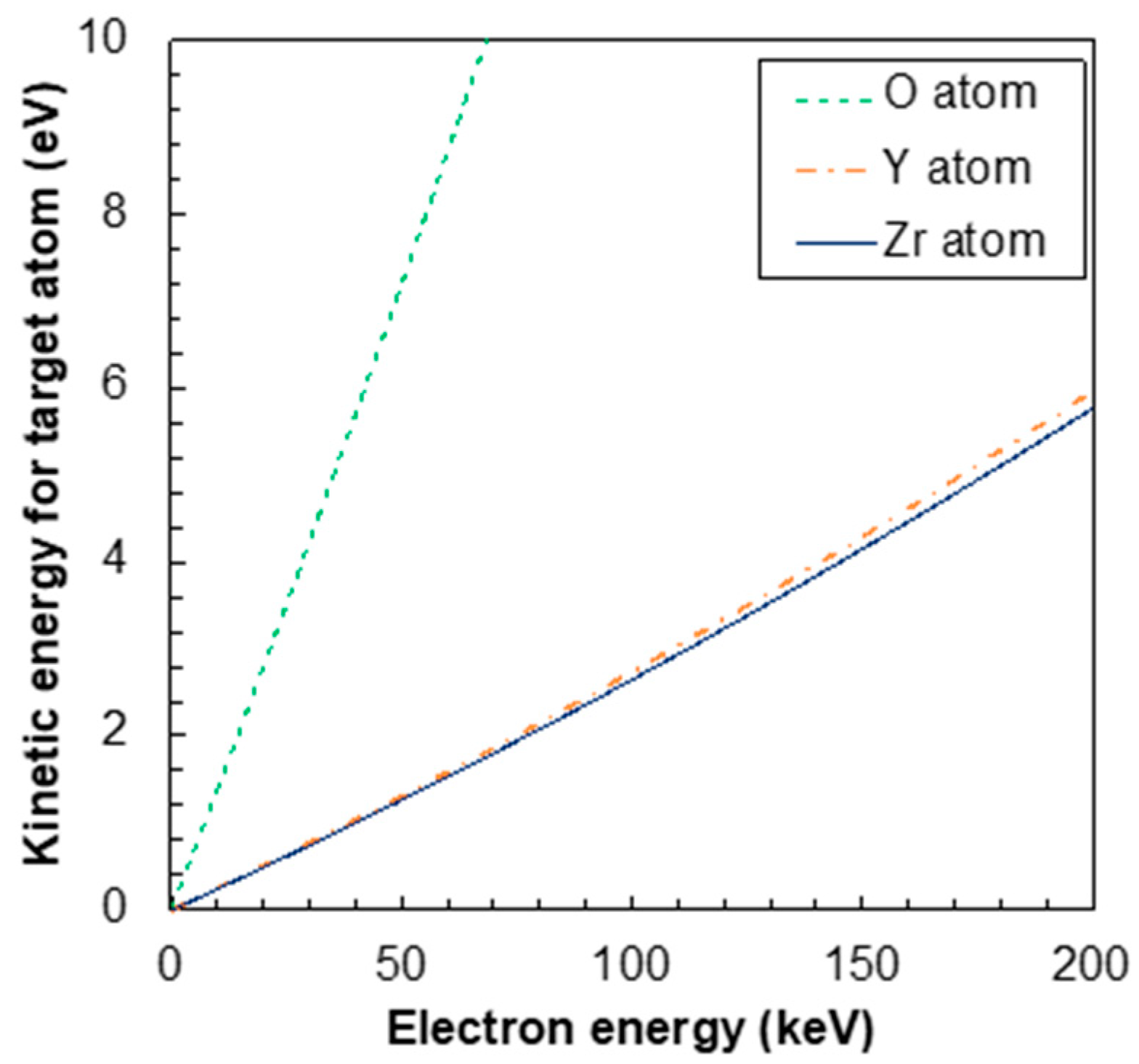
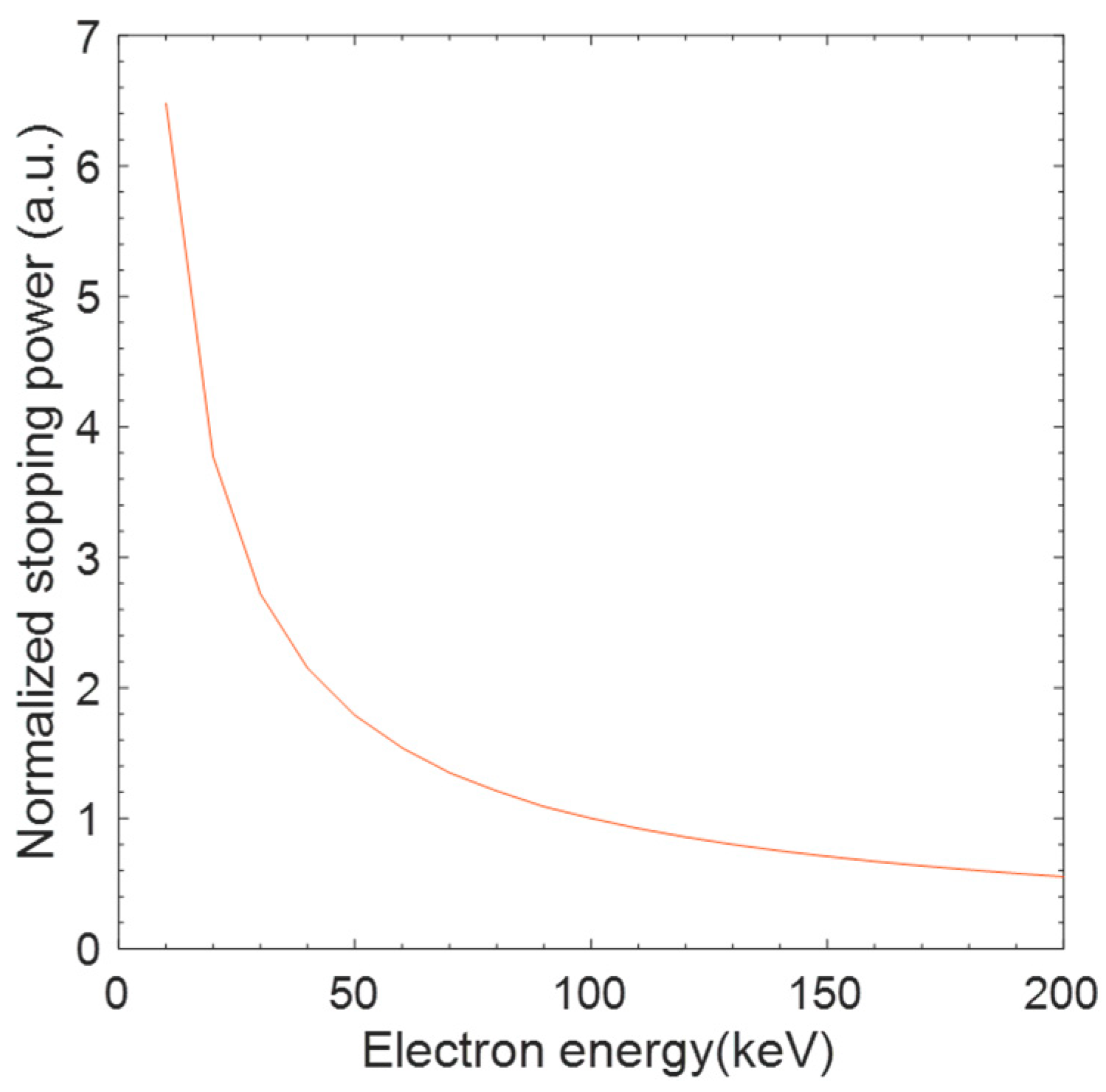
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsujimoto, K.; Oigawa, H.; Ouchi, N.; Kikuchi, K.; Kurata, Y.; Mizumoto, M.; Sasa, T.; Saito, S.; Nishihara, K.; Umeno, M.; et al. Research and Development Program on Accelerator Driven Subcritical System in JAEA. J. Nucl. Sci. Technol. 2007, 44, 483. [Google Scholar] [CrossRef]
- Oigawa, H.; Tsujimoto, K.; Nishihara, K.; Sugawara, T.; Kurata, Y.; Takei, H.; Saito, S.; Sasa, T.; Obayashi, H. Role of ADS in the back-end of the fuel cycle strategies and associated design activities: The case of Japan. J. Nucl. Mater. 2011, 415, 229. [Google Scholar] [CrossRef]
- Kurata, Y. Corrosion experiments and materials developed for the Japanese HLM systems. J. Nucl. Mater. 2011, 415, 254. [Google Scholar] [CrossRef]
- Yeliseyeva, O.; Tsisar, V.; Benamati, G. Influence of temperature on the interaction mode of T91 and AISI 316L steels with Pb–Bi melt saturated by oxygen. Corros. Sci. 2008, 50, 1672. [Google Scholar] [CrossRef]
- Schroer, C.; Skrypnik, A.; Wedemeyer, O.; Konys, J. Oxidation and dissolution of iron in flowing lead–bismuth eutectic at 450 °C. Corros. Sci. 2012, 61, 63. [Google Scholar] [CrossRef]
- Deloffre, P.; Terlain, A.; Barbier, F. Corrosion and deposition of ferrous alloys in molten lead–bismuth. J. Nucl. Mater. 2002, 301, 35–39. [Google Scholar] [CrossRef]
- Fernandez, J.A.; Abella, J.; Barcelo, J.; Victori, L. Development of an oxygen sensor for molter 44.5% lead–55.5% bismuth alloy. J. Nucl. Mater. 2002, 301, 47–52. [Google Scholar] [CrossRef]
- Schroer, C.; Konys, J.; Verdaguer, A.; Abellà, J.; Gessi, A.; Kobzova, A.; Babayan, S.; Courouau, J.-L. Design and testing of electrochemical oxygen sensors for service in liquid lead alloys. J. Nucl. Mater. 2011, 415, 338–347. [Google Scholar] [CrossRef]
- Chevalier, J.; Cremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Amer. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Ramos, C.M.; Tabata, A.S.; Cesar, P.F.; Rubo, J.H.; Fracisconi, P.A.S.; Borges, A.F.S. Application of Micro-Raman Spectroscopy to the Study of Yttria-Stabilized Tetragonal Zirconia Polycrystal (Y-TZP) Phase Transformation. Appl. Spectrosc. 2015, 69, 810–814. [Google Scholar] [CrossRef]
- Paul, A.; Vaidhyanathan, B.; Binner, J. Micro-Raman spectroscopy of indentation induced phase transformation in nanozirconia ceramics. Adv. Appl. Ceram. 2011, 110, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Melk, L.; Turon-Vinas, M.; Roa, J.J.; Antti, M.L.; Anglada, M. The influence of unshielded small cracks in the fracture toughness of yttria and of ceria stabilised zirconia. J. Eur. Ceram. Soc. 2016, 36, 147. [Google Scholar] [CrossRef]
- Simeone, D.; Bechade, J.L.; Gosset, D.; Chevarier, A.; Daniel, P.; Pilliaire, H.; Baldinozzi, G. Investigation on the zirconia phase transition under irradiation. J. Nucl. Mater. 2000, 281, 171–181. [Google Scholar] [CrossRef]
- Simeone, D.; Gossset, D.; Bechade, J.L.; Chevarier, A. Analysis of the monoclinic–tetragonal phase transition of zirconia under irradiation. J. Nucl. Mater. 2002, 300, 27–38. [Google Scholar] [CrossRef]
- Simeone, D.; Baldinozzi, G.; Gosset, D.; Dutheil, M.; Bulou, A.; Hansen, T. Monoclinic to tetragonal semireconstructive phase transition of zirconia. Phys. Rev. B 2003, 67, 1–8. [Google Scholar] [CrossRef]
- Leib, E.W.; Vainio, U.; Pasquarelli, R.M.; Kus, J.; Czaschke, C.; Walter, N.; Janssen, R.; Müller, M.; Schreyer, A.; Weller, H.; et al. Synthesis and thermal stability of zirconia and yttria-stabilized zirconia microspheres. J. Colloid Interface Sci. 2015, 448, 582. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, N.; Sonoda, T.; Okamoto, Y.; Sawabe, T.; Takegahara, K.; Kosugi, S.; Iwase, A. X-ray Study of Radiation Damage in UO2 Irradiated with High-Energy Heavy Ions. J. Nucl. Mater. 2011, 419, 392–396. [Google Scholar] [CrossRef]
- Blanton, T.N.; Barnes, C.L.; Lelental, M. The effect of X-ray penetration depth on structural characterization of multiphase Bi-Sr-Ca-Cu-O thin films by X-ray diffraction techniques. Phys. C 1991, 173, 3–4. [Google Scholar] [CrossRef]
- Sato, T.; Iwamoto, Y.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.; Kai, T.; Tsai, P.; Matsuda, N.; Iwase, H.; et al. Features of Particle and Heavy Ion Transport code System (PHITS) version 3.02. J. Nucl. Sci. Technol. 2018, 55, 684. [Google Scholar] [CrossRef] [Green Version]
- Messenger, S.R.; Burke, E.A.; Xapsos, M.A.; Walters, R.J.; Jackson, E.M.; Weaver, B.D. Nonionizing Energy Loss (NIEL) for Heavy Ions. IEEE Trans. Nucl. Sci. 1999, 46, 1595. [Google Scholar] [CrossRef]
- Jun, I.; Xapsos, M.A.; Messenger, S.R.; Burke, E.A.; Walters, R.J.; Summers, G.P.; Jordan, T. Proton nonionizing energy loss (NIEL) for device applications. IEEE Trans. Nucl. Sci. 2003, 50, 1924. [Google Scholar]
- Summers, G.P.; Burke, E.A.; Xapsos, M.A. Displacement damage analogs to ionizing radiation effects. Radiat. Meas. 1995, 24, 1–8. [Google Scholar] [CrossRef]
- Sato, T.; Ohtaki, S.; Shimada, M. Transformation of yttria partially stabilized zirconia by low temperature annealing in air. J. Mater. Sci. 1985, 20, 1466. [Google Scholar] [CrossRef]
- Brog, J.P.; Chanez, A.C.; Crochet, A.; Fromm, K.M. Polymorphism, what it is and how to identify it: A systematic review. RSC Adv. 2013, 3, 16905. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.; Wayman, C.M. Fundamentals of martensitic reactions. In Metallurgical Treatises; Tien, J.K., Elliott, J.F., Eds.; TMS-AIME: Warrendale, PA, USA, 1983; pp. 445–468. [Google Scholar]
- Ge, Q.L.; Lei, T.C.; Mao, J.F.; Zhou, Y. In situ transmission electron microscopy observations of the tetragonal-to-monoclinic phase transformation of zirconia in AI2Oa-ZrO2 (2 mol % Y2O3) composite. J. Mater. Sci. Lett. 1993, 12, 819–822. [Google Scholar] [CrossRef]
- Lamas, D.G.; Walsöe de Reca, N.E. Oxygen ion displacement in compositionally homogeneous, nanocrystalline ZrO2–12 mol% Y2O3 powders. Mater. Lett. 1999, 41, 204–208. [Google Scholar] [CrossRef]
- Nakamura, R.; Ishimaru, M.; Yasuda, H.; Nakajima, H. Atomic rearrangements in amorphous Al2O3 under electron-beam irradiation. J. Appl. Phys. 2013, 113, 064312. [Google Scholar] [CrossRef] [Green Version]
- Meldrum, A.; Boatner, L.A.; Ewing, R.C. Electron-irradiation-induced nucleation and growth in amorphous LaPO4, ScPO4, and zircon. J. Mater. Res. 1997, 12, 1816. [Google Scholar] [CrossRef]
- Nguyen-Truong, H.T. Modified Bethe formula for low-energy electron stopping power without fitting parameters. Ultramicroscopy 2015, 149, 26–33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuno, Y.; Okubo, N. Phase Transformation by 100 keV Electron Irradiation in Partially Stabilized Zirconia. Quantum Beam Sci. 2021, 5, 20. https://doi.org/10.3390/qubs5030020
Okuno Y, Okubo N. Phase Transformation by 100 keV Electron Irradiation in Partially Stabilized Zirconia. Quantum Beam Science. 2021; 5(3):20. https://doi.org/10.3390/qubs5030020
Chicago/Turabian StyleOkuno, Yasuki, and Nariaki Okubo. 2021. "Phase Transformation by 100 keV Electron Irradiation in Partially Stabilized Zirconia" Quantum Beam Science 5, no. 3: 20. https://doi.org/10.3390/qubs5030020
APA StyleOkuno, Y., & Okubo, N. (2021). Phase Transformation by 100 keV Electron Irradiation in Partially Stabilized Zirconia. Quantum Beam Science, 5(3), 20. https://doi.org/10.3390/qubs5030020




