Radioactive Waste Immobilization Using Vitreous Materials for Facilities in a Safe and Resilient Infrastructure Classified by Multivariate Exploratory Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Data on Glass Composition
2.2. Hierarchical and Non-Hierarchical Clustering Methods
2.3. Principal Component Analysis
2.4. Execution of Numerical Multivariate Computations
3. Results and Discussion
3.1. Hierarchical Clustering
3.2. Non-Hierarchical Data Analysis
3.3. Principal Component Analysis
3.4. Analysis of the Borosilicate Cluster
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilisation, 1st ed.; Elsevier: Oxford, UK, 2014; p. 362. [Google Scholar]
- Lutze, W.; Ewing, R.C. Radioactive Waste Forms for the Future, 1st ed.; North Holland: Amsterdam, The Netherlands, 1988; p. 791. [Google Scholar]
- Zanotto, E.D.; Coutinho, F.A.B. How Many Non-Crystalline Solids Can be Made from All the Elements of the Periodic Table? J. Non-Cryst. Sol. 2004, 347, 285–288. [Google Scholar] [CrossRef]
- Vernaz, E.; Bruezière, J. History of Nuclear Waste Glass in France. Procedia Mater. Sci. 2014, 7, 3–9. [Google Scholar] [CrossRef]
- Watson, L.C.; Aikin, A.M.; Bancroft, A.R. The Permanent Disposal of Highly Radioactive Wastes by Incorporation into Glass. In International Atomic Energy Agency. Disposal of Radioactive Wastes, Proceedings of the Conference Jointly Sponsored by IAEA and UNESCO, IAEA, Monaco, Vienna, Austria, 16–21 November 1959; IAEA: Monaco, Vienna, Austria, 1960; pp. 373–393. [Google Scholar]
- Robbins, R.A.; Ojovan, M. Vitreous Materials for Nuclear Waste Immobilisation and IAEA Support Activities. MRS Adv. 2017, 2017, 4201–4206. [Google Scholar] [CrossRef]
- Priven, A.I.; Mazurin, O.V. Glass Property Databases: Their History, Present State, and Prospects for Further Development. Adv. Mat. Res. 2008, 39, 147–152. [Google Scholar] [CrossRef]
- Lemmens, K.; van Iseghem, P. The long-term dissolution behavior of the pamela borosilicate glass SM527—Application of SA/V as accelerating parameter. Mater. Res. Soc. Symp. Proc. 1992, 257, 49–56. [Google Scholar] [CrossRef]
- Vienna, J.D.; Hrma, P.; Schweiger, M.J.; Langowski, M.H. Compositional dependence of elemental release from HLW glasses by the product consistency test: One-component-at-a-time study. Ceram. Trans. 1996, 72, 307–315. [Google Scholar]
- Bakel, A.J.; Ebert, W.L.; Strachan, D.M. Glass dissolution at 20, 40, 70 and 90 °C: Short-term effects of solution chemistry and long-term Na release. Ceram. Trans. 1996, 72, 271–278. [Google Scholar]
- Feng, X.; Pegg, I.L. A glass dissolution model for the effects of S/V on leachate pH. J. Non-Cryst. Solids 1994, 175, 281–293. [Google Scholar] [CrossRef]
- Li, H.; Vienna, J.D.; Hrma, P.; Schweiger, M.J.; Smith, D.E.; Gong, M. Borosilicate based glasses for immobilization of plutonium-bearing materials. Ceram. Trans. 1996, 72, 399–408. [Google Scholar]
- Langowski, M.H.; Li, H.; Hrma, P.; Schweiger, M.J.; Smith, D.E. The effect of phosphate on crystallization, viscosity, and chemical durability of simulated Hanford Site high-level radioactive waste glasses. Ceram. Trans. 1996, 72, 291–298. [Google Scholar]
- Feng, X.; Hrma, P.; Peeler, D.K.; Kim, D.; Schweiger, M.J.; Chang, C.; Li, H.; Bakel, A.J.; Ebert, W.L. Glass durability evaluation using product consistency, single-pass flow-through, and vapor hydration tests. Ceram. Trans. 1996, 72, 93–102. [Google Scholar]
- Piepel, G.; Redgate, T. Mixture experiment techniques for reducing the number of components applied for modeling waste glass sodium release. J. Am. Ceram. Soc. 1997, 80, 3038–3044. [Google Scholar] [CrossRef]
- Hyun, S.-H.; Song, W.-S. Chemical durability of simulated waste glasses. J. Korean Ceram. Soc. 1989, 27, 521–531. [Google Scholar]
- Li, H.; Vienna, J.D.; Hrma, P.; Smith, D.E.; Schweiger, M.J. Nepheline precipitation in high-level waste glasses: Compositional effects and impact on the waste form acceptability. Mater. Res. Soc. Symp. Proc. 1997, 465, 261–268. [Google Scholar] [CrossRef]
- Bulkley, S.A.; Vienna, J.D. Composition effects on viscosity and chemical durability of simulated plutonium residue glasses. Mater. Res. Soc. Symp. Proc. 1997, 465, 1243–1250. [Google Scholar] [CrossRef]
- Chaoying, R.; Hong, L.; Hrma, P.R.; Cho, H.M. Spectroscopic investigation of simulated low-level nuclear waste glasses. Ceram. Trans. 1996, 72, 505–511. [Google Scholar]
- Wei, J.; van Iseghem, P. The effect of humic acids on the element release from high level waste glass. Mater. Res. Soc. Symp. Proc. 1997, 465, 189–196. [Google Scholar] [CrossRef]
- Dong-Sang, K.; Hrma, P.; Palmer, S.E.; Smith, D.E.; Schweiger, M.J. Effect of B2O3, CaO, and Al2O3 on the chemical durability of silicate glasses for hanford low-level waste immobilization. Ceram. Trans. 1995, 61, 531–538. [Google Scholar]
- Bakel, A.J.; Ebert, W.L.; Luo, J.S. Long-term performance of glasses for hanford low-level waste. Ceram. Trans. 1995, 61, 515–522. [Google Scholar]
- Hong, L.; Tomozawa, M. Chemical durability of simulated nuclear waste glasses containing water. Ceram. Trans. 1995, 61, 539–546. [Google Scholar]
- Ebert, W.L.; Tam, S.-W. Dissolution rates of DWPF glasses from long-term PCT. Mater. Res. Soc. Symp. Proc. 1997, 465, 149–156. [Google Scholar] [CrossRef]
- Ebert, W.L. The effects of the leachate pH and the ratio of glass surface area to leachant volume on glass reactions. Phys. Chem. Glasses 1993, 34, 58–65. [Google Scholar]
- Pickering, S. Kinetics of surface-layer formation on simulated nuclear waste glass. J. Am. Ceram. Soc. 1980, 63, 558–562. [Google Scholar] [CrossRef]
- Ebert, W.L. Laboratory testing of West Valley reference 6 glass. Ceram. Trans. 1995, 61, 471–478. [Google Scholar]
- Feng, X.; Pegg, I.L.; Barkatt, A.; Macedo, P.B.; Cucinell, S.J.; Lai, S. Correlation between composition effects on glass durability and the structural role of the constituent oxides. Nuclear Technol. 1989, 85, 334–345. [Google Scholar] [CrossRef]
- Clark, D.E.; Urwongse, L.; Maurer, C. Application of glass corrosion concepts to nuclear waste immobilization. Nuclear Technol. 1982, 56, 212–225. [Google Scholar] [CrossRef]
- McGrail, B.P.; Martin, P.F.; Lindenmeier, C.W. Accelerated testing of waste forms using a novel pressurized unsaturated flow (PUF) method. Mater. Res. Soc. Symp. Proc. 1997, 465, 253–260. [Google Scholar] [CrossRef]
- Buechele, A.C.; Muller, I.S.; Pegg, I.L.; Kim, C.-W.; Yaschenko, E. Properties of glasses for Idaho mixed waste vitrification. Ceram. Trans. 1995, 61, 203–211. [Google Scholar]
- Fu, S.S.; Gan, H.; Muller, I.S.; Pegg, I.L.; Macedo, P.B. Optimization of Savannah River M-Area mixed waste for vitrification. Mater. Res. Soc. Symp. Proc. 1997, 465, 139–146. [Google Scholar] [CrossRef]
- Yan, Q.; Buechele, A.C.; Hu, S.; Wang, E.; Fu, S.S. Effect of crystallization on the durability of mixed waste glasses. Ceram. Trans. 1995, 61, 167–176. [Google Scholar]
- Oversby, V.M.; Phinney, D.L. The development of surface alteration layers on SRL-165 nuclear waste glasses. J. Nucl. Mater. 1992, 190, 247–268. [Google Scholar] [CrossRef]
- Pederson, L.R.; Buckwalter, C.Q.; McVay, G.L.; Riddle, B.L. Glass surface area to solution volume ratio and its implications to accelerated leach testing. Mater. Res. Soc. Symp. Proc. 1983, 15, 45–54. [Google Scholar] [CrossRef]
- Moir, D.L.; Chatt, A. Studies on leaching behavior of sodium borosilicate glasses by neutron activation: Effects of groundwater composition, pH, surface area to volume ratio, and temperature. J. Radioanal. Nucl. Chem. 1992, 161, 503–526. [Google Scholar] [CrossRef]
- Ishiguro, K.; Kawanishi, N.; Sasaki, N.; Nagaki, H.; Yamamoto, M. Growth of surface layer during the leaching of the simulated waste glass and its barrier effects on the leaching. Mater. Res. Soc. Symp. Proc. 1983, 15, 135–142. [Google Scholar] [CrossRef]
- Hermansson, H.-P.; Christensen, H.; Clark, D.E.; Werme, L. Effects of solution chemistry and atmosphere on leaching of alkali borosilicate glass. Mater. Res. Soc. Symp. Proc. 1983, 15, 143–150. [Google Scholar] [CrossRef]
- Heimann, R.B.; Wood, D.D.; Hamon, R.F. Multicomponent leach tests in standard canadian shield saline solution on glasses containing simulated nuclear waste. Mater. Res. Soc. Symp. Proc. 1984, 26, 191–200. [Google Scholar] [CrossRef]
- Chick, L.A.; Pederson, L.R. The relationship between reaction layer thickness and leach rate for nuclear waste glasses. Mater. Res. Soc. Symp. Proc. 1984, 26, 635–642. [Google Scholar] [CrossRef]
- Fillet, S.; Nogues, J.L.; Vernaz, E.; Jacquet-Francillon, N. Leaching of actinides from the french LWR reference glass. Mater. Res. Soc. Symp. Proc. 1985, 50, 211–218. [Google Scholar] [CrossRef]
- Jantzen, C.M.; Bibler, N.E. The role of groundwater oxidation potential and radiolysis on waste glass performance in crystalline repository environments. Mater. Res. Soc. Symp. Proc. 1985, 50, 219–230. [Google Scholar] [CrossRef]
- Dwivedi, A.; Berta, Y.; Speyer, R.F. Effect of controlled crystallization on the chemical durability of a lead-containing waste glass. J. Mater. Sci. 1994, 29, 2304–2308. [Google Scholar] [CrossRef]
- Elliot, M.N.; Auty, D.B. The durability of glass for the disposal of highly radioactive waste. Discussion of method and effect of leaching conditions. Glass Technol. 1968, 9, 5–13. [Google Scholar]
- Sales, B.C.; Boatner, L.A. Lead phosphate glass as a stable medium for the immobilization and disposal of high-level nuclear waste. Mater. Lett. 1984, 2, 301–304. [Google Scholar] [CrossRef]
- Sales, B.C.; Boatner, L.A. Physical and chemical characteristics of lead-iron phosphate nuclear waste glasses. J. Non-Cryst. Solids 1986, 79, 83–116. [Google Scholar] [CrossRef]
- Kamizono, H.; Hayakawa, I.; Muraoka, S. Effects of some glass additives on nuclear waste glass durability in water. J. Mater. Sci. Lett. 1991, 10, 423–425. [Google Scholar] [CrossRef]
- Marasinghe, G.K.; Karabulut, M.; Ray, C.S.; Day, D.E.; Shuh, D.K.; Allen, P.G.; Saboungi, M.L.; Grimsditch, M.; Haeffner, D. Properties and structure of vitrified iron phosphate nuclear wasteforms. J. Non-Cryst. Solids 2000, 263–264, 146–154. [Google Scholar] [CrossRef]
- Prokin, E.S.; Kuptsov, V.S.; Ananina, T.N.; Ermolaev, E.E. Characteristics of borosilicate glass in simulation of alpha-radiation and thermal conditions for storage of vitrified highly radioactive wastes. Radiokhimiya 1988, 30, 694–698. [Google Scholar]
- Sales, B.C.; White, C.W.; Begun, G.M.; Boatner, L.A. Surface layer formation on corroded nuclear waste glasses. J. Non-Cryst. Solids 1984, 67, 245–264. [Google Scholar] [CrossRef]
- Kamizono, H. Leaching behaviour of simulated high-level waste glass in groundwater. J. Nucl. Mater. 1985, 127, 242–246. [Google Scholar] [CrossRef]
- Hara, S.; Terai, R.; Yamanaka, H. Chemical durability of borosilicate glasses containing simulated high-level nuclear wastes (I). Bull. Governm. Ind. Res. Inst. Osaka 1983, 34, 1–8. [Google Scholar]
- Bibler, N.E.; Ramsey, W.G.; Meaker, T.F.; Pareizs, J.M. Durabilities and microstructures of radioactive glasses for immobilization of excess actinides at the savannah river site. Mater. Res. Soc. Symp. Proc. 1996, 412, 65–72. [Google Scholar] [CrossRef]
- Li, H.; Schweiger, M.J.; Hrma, P.; Feng, X. Surface characterization of simulated low-level radioactive waste glasses corroded in water and LiOH buffer solution. Mater. Res. Soc. Symp. Proc. 1996, 412, 213–220. [Google Scholar] [CrossRef]
- Mazer, J.J.; Bates, J.K.; Biwer, B.M.; Bradley, C.R. AEM analyses of SRL 131 glass altered as a function of SA/V. Mater. Res. Soc. Symp. Proc. 1992, 257, 73–81. [Google Scholar] [CrossRef]
- Gin, S.; Godon, N.; Mestre, J.P.; Vernaz, E.Y.; Beaufort, D. Experimental investigation of aqueous corrosion of R7T7 nuclear glass at 90C in the presence of humic acids: A kinetic approach. Mater. Res. Soc. Symp. Proc. 1994, 333, 565–572. [Google Scholar] [CrossRef]
- Knauss, K.G.; Bourcier, W.L.; McKeegan, K.D.; Marzbacher, C.I.; Nguyen, S.N.; Ryerson, F.J.; Smith, D.K.; Weed, H.C.; Newton, L. Dissolution kinetics of a simple analogue nuclear waste glass as a function of pH, time and temperature. Mater. Res. Soc. Symp. Proc. 1990, 176, 371–381. [Google Scholar] [CrossRef]
- Inagaki, Y.; Furuya, H.; Idemitsu, K.; Maeda, T.; Sakai, A. Corrosion behavior of a powdered simulated nuclear waste glass under anoxic condition. Mater. Res. Soc. Symp. Proc. 1995, 353, 23–30. [Google Scholar] [CrossRef]
- Ebert, W.L.; Bates, J.K. A comparison of glass reaction at high and low glass surface/solution volume. Nuclear Technol. 1993, 104, 372–384. [Google Scholar] [CrossRef]
- Feng, X.; Pegg, I.L.; Guo, Y.; Barkatt, A.; Macedo, P.B. Effects of surface area-to-solution volume ratio on chemical durability of nuclear waste glasses. Mater. Res. Soc. Symp. Proc. 1990, 176, 383–392. [Google Scholar] [CrossRef]
- Raman, S.V. Analysis of the hydrated zone in nuclear waste glass forms by electron microprobe, Raman spectroscopy and diffusion models. Phys. Chem. Glasses 2001, 42, 27–41. [Google Scholar]
- Day, D.E.; Wu, Z.; Ray, C.S.; Hrma, P. Chemically durable iron phosphate glass wasteforms. J. Non-Cryst. Solids 1998, 241, 1–12. [Google Scholar] [CrossRef]
- Pinet, O.; Baudrey, E.; Dussossoy, J.L.; Fillet, C.; Hollebecque, J.F. Redox effect on waste containment glass properties: Case of a borosilicate glass containing 16 wt% MoO3. In Proceedings of the XIX International Congress Glass, Edinburgh, Scotland, 1–6 July 2001; Society of Glass Technology: Sheffield, UK, 2002; Volume 43C, pp. 158–161. [Google Scholar]
- Bates, J.K.; Ebert, W.L.; Feng, X.; Bourcier, W.L. Issues affecting the prediction of glass reactivity in an unsaturated environment. J. Nucl. Mater. 1992, 190, 198–227. [Google Scholar] [CrossRef]
- Suzuki, M.; Hara, S.; Nagaoka, K. Disposal method by the vitrification of wastes containing heavy metals. The collected particles from the electrostaic precipitator of cupola. Bull. Governm. Ind. Res. Inst. Osaka 1988, 39, 194–200. [Google Scholar]
- Kawamoto, T.; Terai, R.; Hara, S. Effects of crystallization on thermal properties and chemical durability of the glasses containing simulated high level radioactive wastes. Bull. Governm. Ind. Res. Inst. Osaka 1978, 29, 168–173. [Google Scholar]
- Helebrant, A.; Pekarkova, I. Kinetic of glass corrosion in acid solutions. Ber. Bunsenges. Phys. Chem. 1996, 100, 1519–1522. [Google Scholar] [CrossRef]
- Yi-Ming, P.; Jain, V.; Pensado, O. Degradation of high-level waste glass under simulated repository conditions. J. Non-Cryst. Solids 2003, 319, 74–88. [Google Scholar]
- Kim, C.-W.; Day, D.E. Immobilization of Hanford LAW in iron phosphate glasses. J. Non-Cryst. Solids 2003, 331, 20–31. [Google Scholar] [CrossRef]
- Godon, N.; Thomassin, J.H.; Touray, J.C.; Vernaz, E. Experimental alteration of R7T7 nuclear model glass in solutions with different salinities (90 °C, 1 bar): Implications for the selection of geological repositories. J. Mater. Sci. 1988, 23, 126–134. [Google Scholar] [CrossRef]
- McGrail, B.P.; Ebert, W.L.; Bakel, A.J.; Peeler, D.K. Measurement of kinetic rate law parameters on a Na-Ca-Al borosilicate glass for low-activity waste. J. Nucl. Mater. 1997, 249, 175–189. [Google Scholar] [CrossRef]
- Reis, S.T.; Karabulut, M.; Day, D.E. Structural features and properties of lead-iron-phosphate nuclear wasteforms. J. Nucl. Mater. 2002, 304, 87–95. [Google Scholar] [CrossRef]
- Kim, C.W.; Ray, C.S.; Zhu, D.; Day, D.E.; Gombert, D.; Aloy, A.; Mogus-Milankovic, A.; Karabulut, M. Chemically durable iron phosphate glasses for vitrifying sodium bearing waste (SBW) using conventional and cold crucible induction melting (CCIM) techniques. J. Nucl. Mater. 2003, 322, 152–164. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Ray, C.S.; Kim, C.W.; Reis, S.T.D. Properties and solubility of chrome in iron alumina phosphate glasses containing high level nuclear waste. Glass Sci. Technol. 2004, 77, 203–210. [Google Scholar]
- Raman, S.V. The effect of mixed modifiers on nuclear waste glass processing, leaching, and Raman spectra. J. Mater. Res. 1998, 13, 8–15. [Google Scholar] [CrossRef]
- Luckscheiter, B.; Nesovic, M. Long term corrosion behaviour of the WAK-HLW glass in salt solutions. Waste Manag. 1997, 17, 429–436. [Google Scholar] [CrossRef]
- Sheng, J. Examination of the leachability of PG-glass. Glass Technol. 2005, 46, 36–38. [Google Scholar]
- Choi, K.; Sheng, J.; Lee, M.-C.; Song, M.-J. Utilizing the KEP-A glass frit to vitrify low-level radioactive waste from Korean NPPs. Waste Manag. 2000, 20, 575–580. [Google Scholar] [CrossRef]
- Ji, H.; Rouxel, T.; Abdelouas, A.; Grambow, B.; Jollivet, P. Mechanical behavior of a borosilicate glass under aqueous corrosion. J. Am. Ceram. Soc. 2005, 88, 3256–3259. [Google Scholar] [CrossRef]
- Gauthier, A.; le Coustumer, P.; Motelica, M.; Donard, O.F.X. Real time alteration of a nuclear waste glass and remobilization of lanthanide into an interphase. Waste Manag. 2000, 20, 731–739. [Google Scholar] [CrossRef]
- Ferrand, K.; Abdelouas, A.; Grambow, B. Water diffusion in the simulated French nuclear waste glass SON 68 contacting silica rich solutions: Experimental and modeling. J. Nucl. Mater. 2006, 355, 54–67. [Google Scholar] [CrossRef]
- Yanagi, T.; Yoshizoe, M.; Kuramoto, K.I. Leach rates and thermal properties of lead-iron phosphate glass waste forms. J. Nucl. Sci. Technol. 1989, 26, 948–954. [Google Scholar] [CrossRef][Green Version]
- Crawford, C.L.; Marra, J.C.; Bibler, N.E. Glass fabrication and product consistency testing of lanthanide borosilicate glass for plutonium disposition. J. Alloys Compd. 2007, 444–445, 569–579. [Google Scholar] [CrossRef][Green Version]
- Barkatt, A.; Saad, E.E.; Adiga, R.; Sousanpour, W.; al Barkatt; Adel-Hadadi, M.A.; O’Keefe, J.A.; Alterescu, S. Leaching of natural and nuclear waste glasses in sea water. Appl. Geochem. 1989, 4, 593–603. [Google Scholar] [CrossRef]
- Gin, S.; Godon, N.; Mestre, J.P.; Vernaz, E.Y.; Beaufort, D. Experimental investigation of aqueous corrosion of R7T7 nuclear glass at 90 °C in the presence of organic species. Appl. Geochem. 1994, 9, 255–269. [Google Scholar] [CrossRef]
- Cassingham, N.J.; Bingham, P.A.; Hand, R.J.; Forder, S.D. Property modification of a high level nuclear waste borosilicate glass through the addition of Fe2O3. Glass Technol. Eur. J. Glass Sci. Technol. A 2008, 49, 21–26. [Google Scholar]
- Pierce, E.M.; Rodriguez, E.A.; Calligan, L.J.; Shaw, W.J.; McGrail, B.P. An experimental study of the dissolution rates of simulated aluminoborosilicate waste glasses as a function of pH and temperature under dilute conditions. Appl. Geochem. 2008, 23, 2559–2573. [Google Scholar] [CrossRef]
- Mesko, M.G.; Day, D.E. Immobilization of spent nuclear fuel in iron phosphate glass. J. Nucl. Mater. 1999, 273, 27–36. [Google Scholar] [CrossRef]
- Ezz-Eldin, F.M. Leaching and mechanical properties of cabal glasses developed as matrices for immobilization high-level wastes. Nucl. Instr. Methods Phys. Res. B 2001, 183, 285–300. [Google Scholar] [CrossRef]
- Wellman, D.M.; Icenhower, J.P.; Weber, W.J. Elemental dissolution study of Pu-bearing borosilicate glasses. J. Nucl. Mater. 2005, 340, 149–162. [Google Scholar] [CrossRef]
- Weber, W.J.; Wald, J.W.; McVay, G.L. Effects of (alfa)-radiolysis on leaching of a nuclear waste glass. J. Am. Ceram. Soc. 1985, 68, C-253. [Google Scholar] [CrossRef]
- Gan, X.Y.; Zhang, Z.T.; Yuan, W.Y.; Wang, L.; Bai, Y.; Ma, H. Long-term product consistency test of simulated 90-19/Nd HLW glass. J. Nucl. Mater. 2011, 408, 102–109. [Google Scholar] [CrossRef]
- Kim, C.-W.; Park, J.-K.; Hwang, T.-W. Analysis of leaching behavior of simulated LILW glasses by using the MCC-1 test method. J. Nucl. Sci. Technol. 2011, 48, 1108–1114. [Google Scholar] [CrossRef]
- Riley, B.J.; Rieck, B.T.; McCloy, J.S.; Crum, J.V.; Sundaram, S.K.; Vienna, J.D. Tellurite glass as a waste form for mixed alkali-chloride waste streams: Candidate materials selection and initial testing. J. Nucl. Mater. 2012, 424, 29–37. [Google Scholar] [CrossRef]
- Sokal, R.R. Distance as a Measure of Taxonomic Similarity. Syst. Zool. 1961, 10, 70–79. [Google Scholar] [CrossRef]
- Sokal, R.R.; Sneath, P.H.A. Principles of Numerical Taxonomy; W. H. Freeman and Company: San Francisco, CA, USA; London, UK, 1963; p. 359. [Google Scholar]
- MacQueen, J.B. Some Methods for Classification and Analysis of Multivariate Observations. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Oakland, CA, USA, 21 June–18 July 1965; University of California: California, CA, USA, 1967; pp. 281–297. [Google Scholar]
- Hair, J.F., Jr.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Cengage: Hampshire, UK, 2019; p. 813. [Google Scholar]
- Favero, L.P.; Belfiore, P. Data Science for Business and Decision Making; Academic Press: Cambridge, UK, 2019; p. 1170. [Google Scholar]
- Kopec, D. Classic Computer Science Problems in Python; Manning Publications, Co.: Shelter Island, NY, USA, 2019; p. 206. [Google Scholar]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis; John Wiley & Sons, Ltd.: West Sussex, UK, 2011; p. 330. [Google Scholar]
- Nascimento, M.L.F. In Search of Star Clusters: An Introduction to the K-Means Algorithm. J. Humanist. Math. 2022, 12, 243–255. [Google Scholar] [CrossRef]
- Pearson, K. On Lines and Planes of Closest Fit to Systems of Points in Space. Phil. Mag. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a Complex of Statistical Variables into Principal Components. J. Ed. Psych. 1933, 24, 498–520. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis; Springer: Berlin/Heidelberg, Germany, 2002; p. 518. [Google Scholar]
- Nascimento, M.L.F.; Aparicio, C. Viscosity of Strong and Fragile Glass-forming Liquids Investigated by Means of Principal Component Analysis. J. Phys. Chem. Solids 2007, 68, 104–110. [Google Scholar] [CrossRef]
- Manly, B.F.J.; Alberto, J.A.N. Multivariate Statistical Methods; Routledge: Abingdon, UK, 2016; p. 269. [Google Scholar]
- Bartlett, M.S. A Note on the Multiplying Factors for Various χ2 Approximations. J. Roy. Stat. Soc. Ser. B. 1954, 16, 296–298. [Google Scholar] [CrossRef]
- Nascimento, H.H.S.; Nascimento, M.L.F. Identifying silica types using viscosity data and principal component analysis. J. Phys. Chem. Solids 2021, 157, 110177. [Google Scholar] [CrossRef]
- Pearson, K., VII. Mathematical Contributions to the Theory of Evolution. III. Regression, Heredity, and Panmixia. Phil. Trans. R. Soc. Lond. 1896, 187, 253–318. [Google Scholar]
- Harrison, M.T. Vitrification of High Level Waste in the UK. Procedia Mat. Sci. 2014, 7, 10–15. [Google Scholar] [CrossRef]
- Raj, K.; Kaushik, C.P. Glass Matrices for Vitrification of Radioactive Waste—An Update on R & D Efforts. IOP Conf. Ser. Mater. Sci. Eng. 2009, 2, 012002. [Google Scholar]
- Anonymous. Vitrification Technologies for Treatment of Hazardous and Radioactive Waste: Handbook; United States Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 1992; p. 96.
- Barlet, M.; Delaye, J.-M.; Charpentier, T.; Gennisson, M.; Bonamy, D.; Rouxel, T.; Rountree, C.L. Hardness and Toughness of Sodium Borosilicate Glasses via Vickers’s Indentations. J. Non-Cryst. Solids 2015, 417, 66–79. [Google Scholar] [CrossRef]
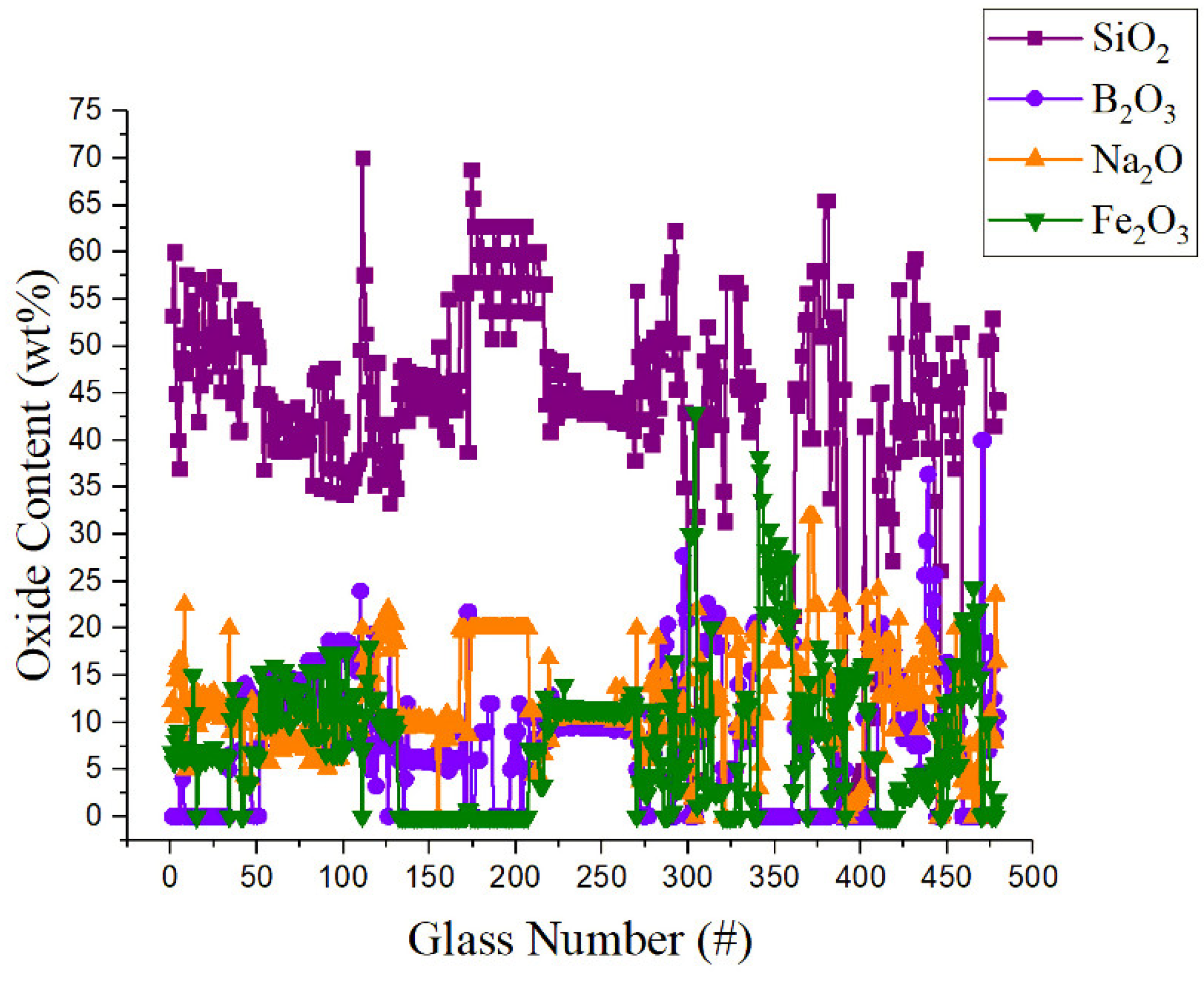
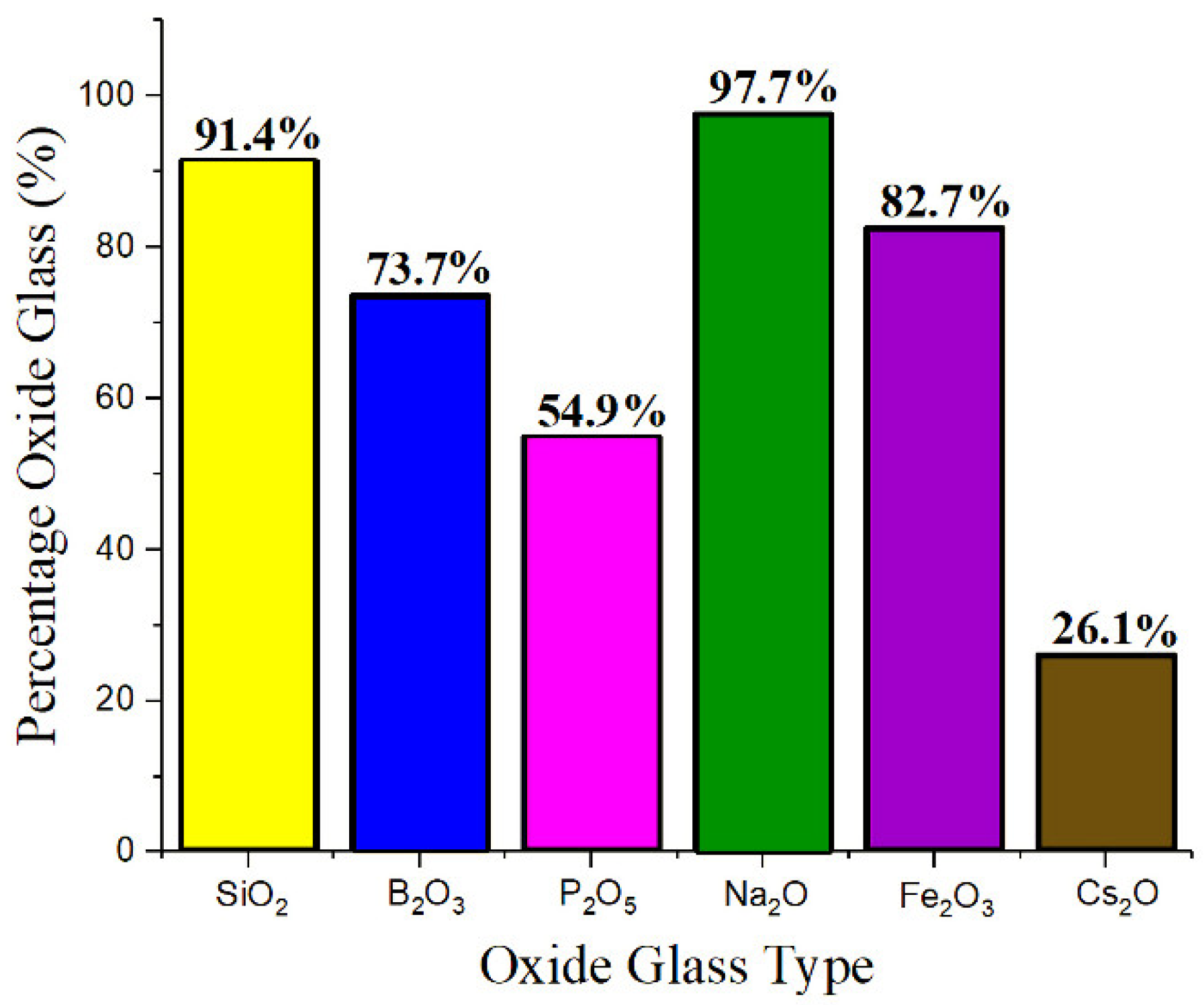


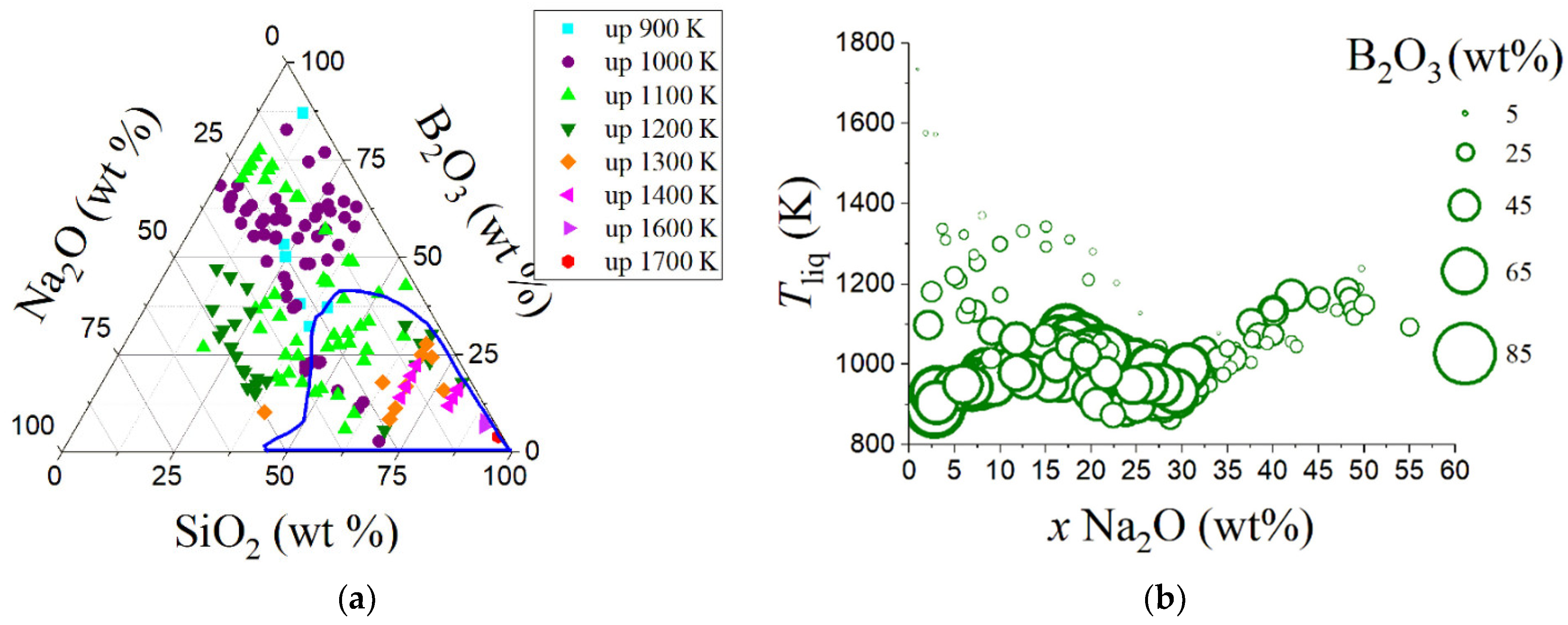
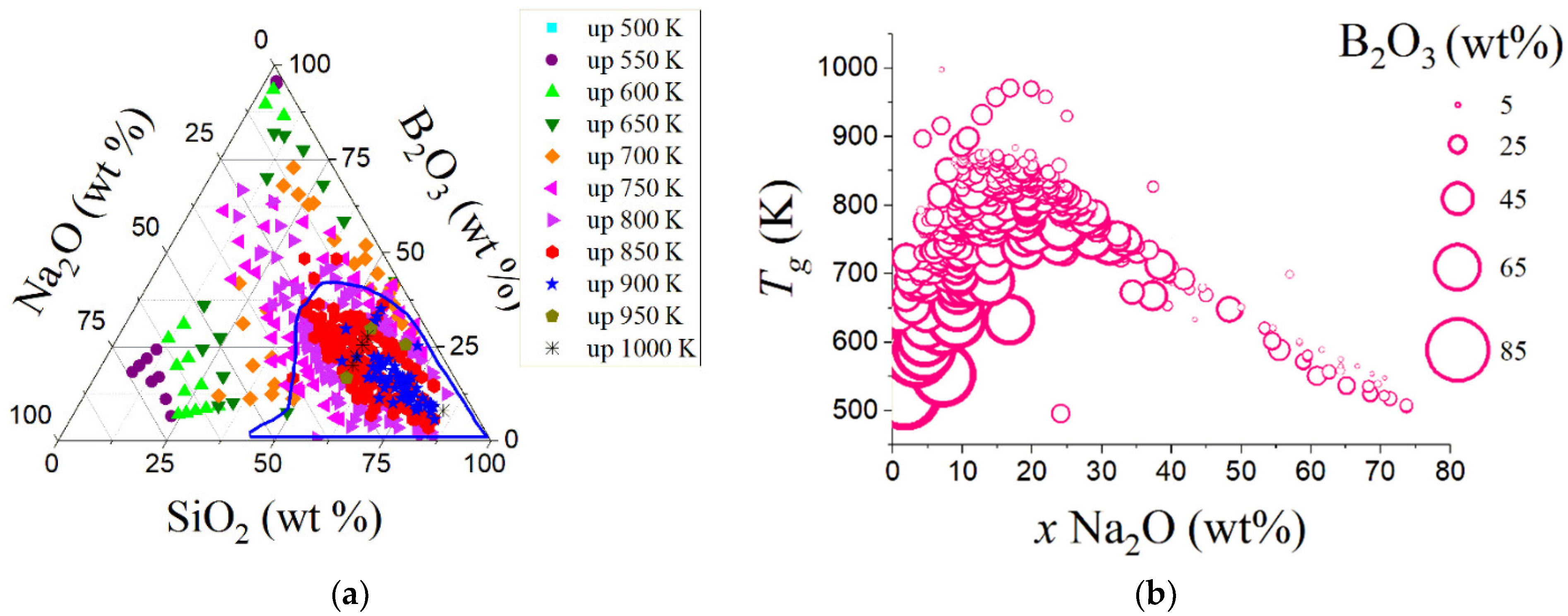

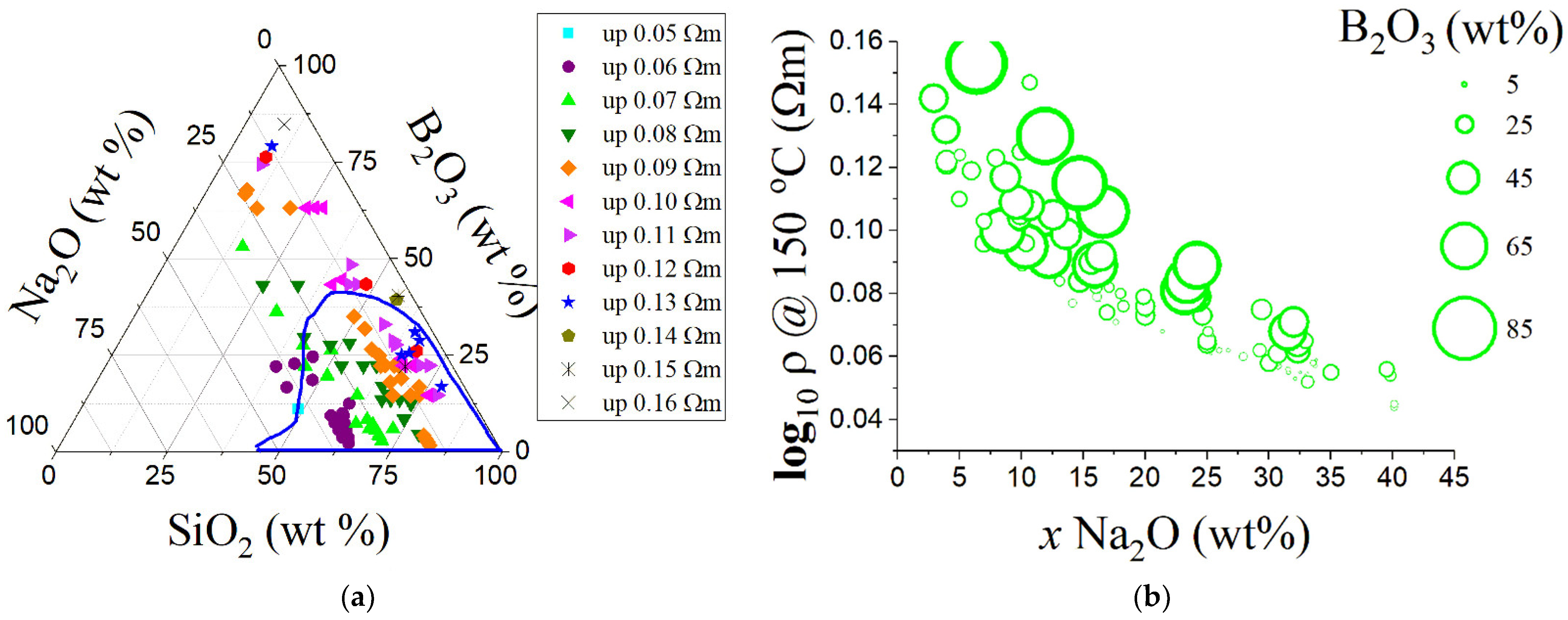
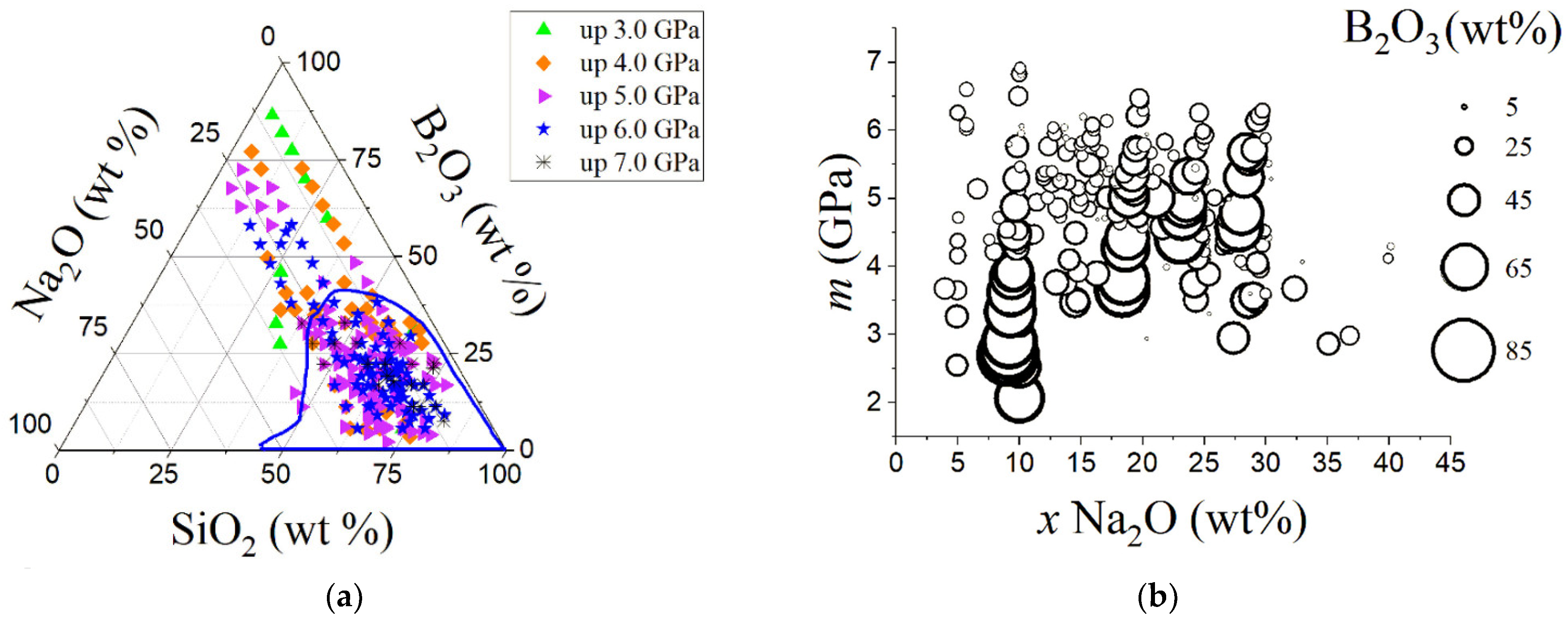
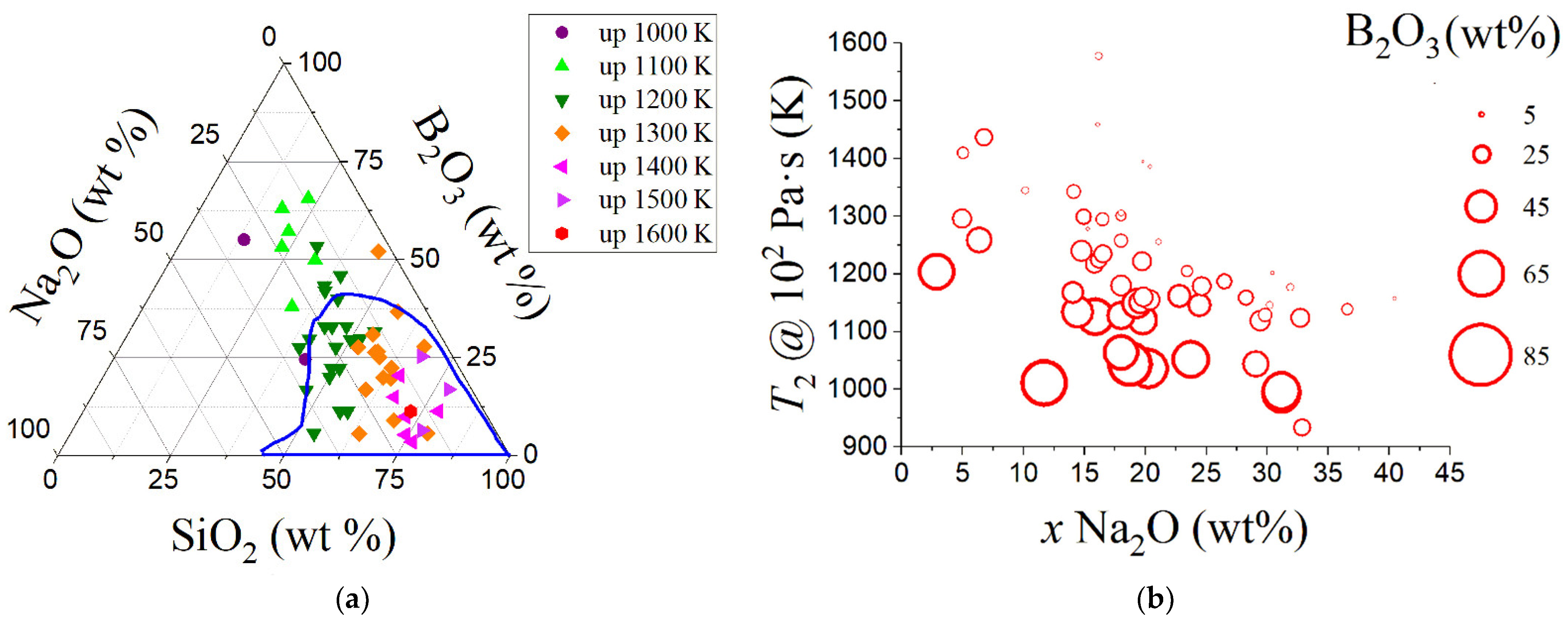

| Variable | Glass Component | Variable | Glass Component | Variable | Glass Component | Variable | Glass Component | Variable | Glass Component |
|---|---|---|---|---|---|---|---|---|---|
| X1 | SiO2 | X11 | CoO | X21 | K2O | X31 | PbO | X41 | ThO2 |
| X2 | B2O3 | X12 | Cr2O3 | X22 | La2O3 | X32 | Pr2O3 | X42 | TeO2 |
| X3 | Al2O3 | X13 | Cs2O | X23 | Li2O | X33 | PuO2 | X43 | TiO2 |
| X4 | BaO | X14 | CuO | X24 | MgO | X34 | Rb2O | X44 | UO2 |
| X5 | Bi2O3 | X15 | Eu2O3 | X25 | MnO2 | X35 | RuO2 | X45 | U3O8 |
| X6 | CaF | X16 | F | X26 | MoO3 | X36 | SO3 | X46 | WO3 |
| X7 | CaO | X17 | Fe2O3 | X27 | Na2O | X37 | Sm2O3 | X47 | Y2O3 |
| X8 | CdO | X18 | Gd2O3 | X28 | Nd2O3 | X38 | SnO | X48 | ZnO |
| X9 | CeO2 | X19 | GeO2 | X29 | NiO | X39 | SrO | X49 | ZrO2 |
| X10 | Cl | X20 | HfO2 | X30 | P2O5 | X40 | Tb2O3 | X50 | RmOn * |
| X51 | H2O ** |
| PC Axis | Eigenvalue | Total Percent (%) | Cumulative Percent (%) | PC Axis | Eigenvalue | Total Percent (%) | Cumulative Percent (%) |
|---|---|---|---|---|---|---|---|
| 1 | 508.65108 | 64.98 | 64.98 | 26 | 0.34794 | 0.04 | 99.85 |
| 2 | 68.65232 | 8.77 | 73.75 | 27 | 0.28107 | 0.04 | 99.89 |
| 3 | 55.65593 | 7.11 | 80.86 | 28 | 0.23713 | 0.03 | 99.92 |
| 4 | 42.67085 | 5.45 | 86.31 | 29 | 0.1585 | 0.02 | 99.94 |
| 5 | 24.12581 | 3.08 | 89.39 | 30 | 0.09625 | 0.01 | 99.95 |
| 6 | 20.35036 | 2.60 | 91.99 | 31 | 0.07913 | 0.01 | 99.96 |
| 7 | 13.92004 | 1.78 | 93.77 | 32 | 0.06779 | 0.01 | 99.97 |
| 8 | 12.0399 | 1.54 | 95.31 | 33 | 0.05762 | 0.01 | 99.98 |
| 9 | 8.54077 | 1.09 | 96.40 | 34 | 0.04461 | 0.01 | 99.98 |
| 10 | 4.17766 | 0.53 | 96.93 | 35 | 0.04065 | 0.01 | 99.99 |
| 11 | 3.41634 | 0.44 | 97.37 | 36 | 0.01975 | 0.00 | 99.99 |
| 12 | 2.62962 | 0.34 | 97.71 | 37 | 0.01468 | 0.00 | 99.99 |
| 13 | 2.33879 | 0.30 | 98.01 | 38 | 0.01165 | 0.00 | 100.00 |
| 14 | 2.16081 | 0.28 | 98.28 | 39 | 0.0088 | 0.00 | 100.00 |
| 15 | 1.79214 | 0.23 | 98.51 | 40 | 0.00633 | 0.00 | 100.00 |
| 16 | 1.62874 | 0.21 | 98.72 | 41 | 0.00536 | 0.00 | 100.00 |
| 17 | 1.45306 | 0.19 | 98.90 | 42 | 0.00265 | 0.00 | 100.00 |
| 18 | 1.38426 | 0.18 | 99.08 | 43 | 0.00242 | 0.00 | 100.00 |
| 19 | 1.14968 | 0.15 | 99.23 | 44 | 0.00194 | 0.00 | 100.00 |
| 20 | 1.12113 | 0.14 | 99.37 | 45 | 0.00142 | 0.00 | 100.00 |
| 21 | 0.96774 | 0.12 | 99.50 | 46 | 9.34 × 10−4 | 0.00 | 100.00 |
| 22 | 0.8684 | 0.11 | 99.61 | 47 | 7.79 × 10−4 | 0.00 | 100.00 |
| 23 | 0.66566 | 0.09 | 99.69 | 48 | 6.43 × 10−4 | 0.00 | 100.00 |
| 24 | 0.50162 | 0.06 | 99.76 | 49 | 3.25 × 10−4 | 0.00 | 100.00 |
| 25 | 0.42778 | 0.05 | 99.81 | 50 | 9.50 × 10−5 | 0.00 | 100.00 |
| 51 | 8.00 × 10−5 | 0.00 | 100.00 |
| PC Axis | Eigenvalue | Total Percent (%) | Cumulative Percent (%) | PC Axis | Eigenvalue | Total Percent (%) | Cumulative Percent (%) |
|---|---|---|---|---|---|---|---|
| X1 | SiO2 | 0.70438 | −0.40027 | X26 | MoO3 | 2.46 × 10−4 | 0.01802 |
| X2 | B2O3 | 0.10248 | 0.48875 | X27 | Na2O | 0.05085 | −0.34555 |
| X3 | Al2O3 | 0.0466 | −0.02062 | X28 | Nd2O3 | 6.63 × 10−4 | −0.04281 |
| X4 | BaO | 0.001 | 0.00743 | X29 | NiO | 0.00445 | −0.00317 |
| X5 | Bi2O3 | −0.01909 | −0.01048 | X30 | P2O5 | −0.64988 | −0.441 |
| X6 | CaF | −0.01763 | 0.00699 | X31 | PbO | −0.12033 | 0.50572 |
| X7 | CaO | 0.02779 | 0.05747 | X32 | Pr2O3 | −1.98 × 10−4 | 0.00321 |
| X8 | CdO | 1.73 × 10−4 | 0.00372 | X33 | PuO2 | −1.23 × 10−4 | 0.00692 |
| X9 | CeO2 | 0.003 | −0.0024 | X34 | Rb2O | −6.57 × 10−6 | 8.36 × 10−5 |
| X10 | Cl | −4.14 × 10−5 | −6.86 × 10−4 | X35 | RuO2 | −3.66 × 10−5 | −4.06 × 10−5 |
| X11 | CoO | 2.31 × 10−4 | 8.89 × 10−4 | X36 | SO3 | 5.32 × 10−4 | −0.0058 |
| X12 | Cr2O3 | −0.00351 | −0.00788 | X37 | Sm2O3 | −1.52 × 10−4 | 0.0092 |
| X13 | Cs2O | 0.00117 | 0.00769 | X38 | SnO | 1.93 × 10−4 | 5.44 × 10−4 |
| X14 | CuO | 2.23 × 10−4 | 1.94 × 10−4 | X39 | SrO | 1.12 × 10−4 | 0.00444 |
| X15 | Eu2O3 | 1.65 × 10−5 | 5.99 × 10−4 | X40 | Tb2O3 | −7.36 × 10−5 | 3.15 × 10−4 |
| X16 | F | 2.21 × 10−4 | −0.00361 | X41 | ThO2 | 0.00302 | 0.03535 |
| X17 | Fe2O3 | −0.21738 | −0.07948 | X42 | TeO2 | 1.55 × 10−4 | 0.00128 |
| X18 | Gd2O3 | 0.0069 | 0.01948 | X43 | TiO2 | 0.00697 | 0.01526 |
| X19 | GeO2 | 0.0012 | 0.00683 | X44 | UO2 | −0.031 | −0.03633 |
| X20 | HfO2 | −1.07 × 10−4 | 0.00242 | X45 | U3O8 | −0.00441 | −0.00248 |
| X21 | K2O | 0.01541 | 0.06735 | X46 | WO3 | 1.42 × 10−4 | −7.56 × 10−4 |
| X22 | La2O3 | −0.00159 | 0.01509 | X47 | Y2O3 | 7.81 × 10−5 | 3.44 × 10−4 |
| X23 | Li2O | 0.02854 | 0.05249 | X48 | ZnO | 0.00659 | −0.02766 |
| X24 | MgO | 0.00998 | 0.02401 | X49 | ZrO2 | 0.01827 | 0.01158 |
| X25 | MnO2 | 0.006 | −0.01138 | X50 | RmOn | 0.0191 | 0.07827 |
| X51 | H2O | 1.95 × 10−4 | 1.49 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.L.F.; Cassar, D.R.; Ciolini, R.; Souza, S.d.O.; d’Errico, F. Radioactive Waste Immobilization Using Vitreous Materials for Facilities in a Safe and Resilient Infrastructure Classified by Multivariate Exploratory Analyses. Infrastructures 2022, 7, 120. https://doi.org/10.3390/infrastructures7090120
Nascimento MLF, Cassar DR, Ciolini R, Souza SdO, d’Errico F. Radioactive Waste Immobilization Using Vitreous Materials for Facilities in a Safe and Resilient Infrastructure Classified by Multivariate Exploratory Analyses. Infrastructures. 2022; 7(9):120. https://doi.org/10.3390/infrastructures7090120
Chicago/Turabian StyleNascimento, Marcio Luis Ferreira, Daniel Roberto Cassar, Riccardo Ciolini, Susana de Oliveira Souza, and Francesco d’Errico. 2022. "Radioactive Waste Immobilization Using Vitreous Materials for Facilities in a Safe and Resilient Infrastructure Classified by Multivariate Exploratory Analyses" Infrastructures 7, no. 9: 120. https://doi.org/10.3390/infrastructures7090120
APA StyleNascimento, M. L. F., Cassar, D. R., Ciolini, R., Souza, S. d. O., & d’Errico, F. (2022). Radioactive Waste Immobilization Using Vitreous Materials for Facilities in a Safe and Resilient Infrastructure Classified by Multivariate Exploratory Analyses. Infrastructures, 7(9), 120. https://doi.org/10.3390/infrastructures7090120










