A Sustainable Decision Support System for Drinking Water Systems: Resiliency Improvement against Cyanide Contamination
Abstract
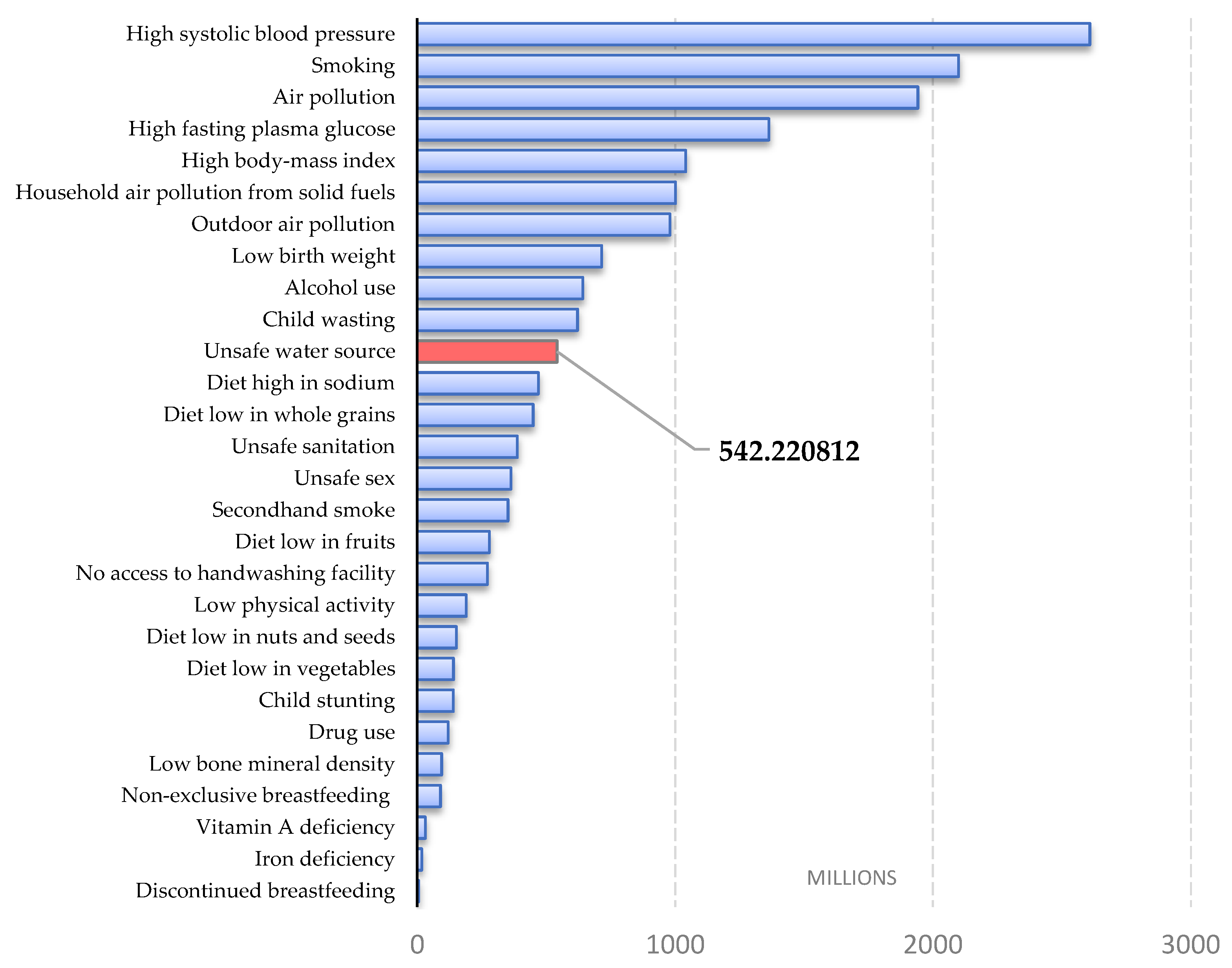
:1. Introduction
- Experimental practices due to the evaluation of cyanide interactions with injected chlorine in the water treatment plant.
- Predicting the residual cyanide with mathematical computations and finding the best regression model.
- Optimizing the proposed model for residual cyanide with the application of a Genetic Algorithm (GA).
- Implementation of machine learning (ML) computations as an artificial intelligence technique for soft sensor design in the water treatment plant.
- Perform a SDGs assessment analysis.
2. Materials and Methods
2.1. Determining the Optimal Concentrations of Chlorine
2.2. Reagents and Materials
2.3. Modelling the Residual Cyanide (RCN)
2.4. Calibration of Model using Genetic Algorithm (GA)
2.5. Artificial Intelligence (AI)
3. Results and Discussion
3.1. Experimental and Mathematical Modelling
3.2. AI and Soft-Sensor Design
3.3. SDGs Assessment
4. Conclusions and Future Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y. China’s water security: Current status, emerging challenges and future prospects. Environ. Sci. Policy 2015, 54, 106–125. [Google Scholar] [CrossRef]
- Fathollahi-Fard, A.M.; Ahmadi, A.; Al-e-Hashem, S.M. Sustainable closed-loop supply chain network for an integrated water supply and wastewater collection system under uncertainty. J. Environ. Manag. 2020, 275, 111277. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, S.; Shafieezadeh, A. Temporal global sensitivity analysis of concrete sewer pipes under compounding corrosion and heavy traffic loads. Struct. Infrastruct. Eng. 2021, 1–14. [Google Scholar] [CrossRef]
- Gheibi, M.; Pouresmaeil, H.; Akrami, M.; Kian, Z.; Takhtravan, A.; Mohammadi, M. Presenting a novel approach for designing chlorine contact reactors by combination of genetic algorithm with nonlinear condition functions, simulated annealing algorithm, pattern search algorithm and experimental efforts. Ann. Environ. Sci. Toxicol. 2021, 5, 12–17. [Google Scholar]
- Shahsavar, M.M.; Akrami, M.; Kian, Z.; Gheibi, M.; Fathollahi-Fard, A.M.; Hajiaghaei-Keshteli, M.; Behzadian, K.J. Bio-recovery of municipal plastic waste management based on alan integrated decision-making framework. J. Ind. Eng. Chem. 2022, 108, 215–234. [Google Scholar] [CrossRef]
- Singgalen, Y.A.; Sasongko, G.; Wiloso, P.G. Community participation in regional tourism development: A case study in North Halmahera Regency-Indonesia. Insights Into Reg. Dev. 2019, 1, 318–333. [Google Scholar] [CrossRef]
- Wolde, Z.; Wu, W.; Ketema, H.; Karikari, B.; Liu, X. Quantifying Sustainable Land-Water-Energy-Food Nexus: The Case of Sustainable Livelihoods in an East African Rift Valley. Atmosphere 2022, 13, 638. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, Y.; Chen, S.; Li, W.; Zhang, A. The Effect of the Water Tower of Typhoon Mangkhut (2018). Atmosphere 2022, 13, 636. [Google Scholar] [CrossRef]
- Mirabi, M.; Karrabi, M.; Gheibi, M. An economic analysis of industrial wastewater treatment systems using multi-attribute decision-making methods (case study: Toos Industrial Estate, Mashhad, Iran). Desalination Water Treat. 2019, 146, 131–140. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Saxena, G.; Chowdhary, P. Constructed wetlands: An emerging phytotechnology for degradation and detoxification of industrial wastewaters. In Environmental Pollutants and Their Bioremediation Approaches; CRC Press: Boca Raton, FL, USA, 2017; pp. 397–426. [Google Scholar]
- Amasha, R.H.; Aly, M.M. Removal of dangerous heavy metal and some human pathogens by dried green algae collected from Jeddah coast. Pharmacophores 2019, 10, 5–13. [Google Scholar]
- Haseena, M.; Malik, M.F.; Javed, A.; Arshad, S.; Asif, N.; Zulfiqar, S.; Hanif, J. Water pollution and human health. Environ. Risk Assess. Remediat. 2017, 1. [Google Scholar] [CrossRef]
- Shahsavar, M.M.; Najafzadeh, M.; Akrami, M.; Kian, Z.; Gheibi, M. Qualitative evaluation of surface water resources using iran water quality index (IRWQSC) and national sanitation foundation water quality index (Case Study: Kardeh Dam, Mashhad, Iran). Environ. Sci. Toxicol. 2021, 5, 30–037. [Google Scholar]
- Su, Y.; Gao, W.; Guan, D.; Zuo, T. Achieving urban water security: A review of water management approach from technology perspective. Water Resour. Manag. 2020, 34, 4163–4179. [Google Scholar] [CrossRef]
- Cheraghalipour, A.; Paydar, M.; Hajiaghaei-Keshteli, M. An integrated approach for collection center selection in reverse logistics. Int. J. Eng. 2017, 30, 1005–1016. [Google Scholar]
- Cheraghalipour, A.; Paydar, M.M.; Hajiaghaei-Keshteli, M. Applying a hybrid BWM-VIKOR approach to supplier selection: A case study in the Iranian agricultural implements industry. Int. J. Appl. Decis. Sci. 2018, 11, 274–301. [Google Scholar] [CrossRef]
- Liao, Y.; Kaviyani-Charati, M.; Hajiaghaei-Keshteli, M.; Diabat, A. Designing a closed-loop supply chain network for citrus fruits crates considering environmental and economic issues. J. Manuf. Syst. 2020, 55, 199–220. [Google Scholar] [CrossRef]
- Sadeghi-Moghaddam, S.; Hajiaghaei-Keshteli, M.; Mahmoodjanloo, M. New approaches in metaheuristics to solve the fixed charge transportation problem in a fuzzy environment. Neural Comput. Appl. 2019, 31, 477–497. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, J.; Fan, J.; Wei, L.; He, S.; Wu, H. Tracing dissolved organic matter in inflowing rivers of Nansi Lake as a storage reservoir: Implications for water-quality control. Chemosphere 2022, 286, 131624. [Google Scholar] [CrossRef]
- Eftekhari, M.; Akrami, M.; Gheibi, M.; Azizi-Toupkanloo, H.; Fathollahi-Fard, A.M.; Tian, G. Cadmium and copper heavy metal treatment from water resources by high-performance folic acid-graphene oxide nanocomposite adsorbent and evaluation of adsorptive mechanism using computational intelligence, isotherm, kinetic, and thermodynamic analyses. Environ. Sci. Pollut. Res. 2020, 27, 43999–44021. [Google Scholar] [CrossRef]
- Mohammadi, M.; Gheibi, M.; Fathollahi-Fard, A.M.; Eftekhari, M.; Kian, Z.; Tian, G. A hybrid computational intelligence approach for bioremediation of amoxicillin based on fungus activities from soil resources and aflatoxin B1 controls. J. Environ. Manag. 2021, 299, 113594. [Google Scholar] [CrossRef] [PubMed]
- Kwaansa-Ansah, E.E.; Amenorfe, L.P.; Armah, E.K.; Opoku, F. Human health risk assessment of cyanide levels in water and tuber crops from Kenyasi, a mining community in the Brong Ahafo Region of Ghana. Int. J. Food Contam. 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Ubalua, A.O. Cyanogenic glycosides and the fate of cyanide in soil. Aust. J. Crop Sci. 2010, 4, 223–237. [Google Scholar]
- Zhou, Z.; Hu, H.; Xia, L.; Li, G.; Xiao, X. A bisspiropyran fluorescent probe for the selective and rapid detection of cyanide anion in liqueurs. New J. Chem. 2022, 46, 7992–7998. [Google Scholar] [CrossRef]
- Onyutha, C.; Kwio-Tamale, J.C. Modelling chlorine residuals in drinking water: A review. J. Environ. Sci. Technol. 2022, 1–18. [Google Scholar] [CrossRef]
- Pirmoradi, M.; Hashemian, S.; Shayesteh, M.R. Kinetics and thermodynamics of cyanide removal by ZnO@ NiO nanocrystals. Trans. Nonferrous Met. Soc. China 2017, 27, 1394–1403. [Google Scholar] [CrossRef]
- Parga, J.; Shukla, S.; Carrillo-Pedroza, F. Destruction of cyanide waste solutions using chlorine dioxide, ozone and titania sol. Waste Manag. 2003, 23, 183–191. [Google Scholar] [CrossRef]
- Dash, R.R.; Balomajumder, C.; Kumar, A. Removal of cyanide from water and wastewater using granular activated carbon. Chem. Eng. J. 2009, 146, 408–413. [Google Scholar] [CrossRef]
- Arbabi, M.; Masoudipour, N.; Amiri, M.J. Negative effects of cyanide on health and its removal options from industrial wastewater. Int. J. Epidemiol. Res. 2015, 2, 44–49. [Google Scholar]
- Uppal, H.; Tripathy, S.S.; Chawla, S.; Sharma, B.; Dalai, M.; Singh, S.; Singh, S.; Singh, N. Study of cyanide removal from contaminated water using zinc peroxide nanomaterial. J. Environ. Sci. 2017, 55, 76–85. [Google Scholar] [CrossRef]
- Rasoulzadeh, H.; Sheikhmohammadi, A.; Abtahi, M.; Alipour, M.; Roshan, B.J. Predicting the capability of diatomite magnano composite boosted with polymer extracted from brown seaweeds for the adsorption of cyanide from water solutions using the response surface methodology: Modelling and optimisation. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Singh, N.; Balomajumder, C. Phytoremediation potential of water hyacinth (Eichhornia crassipes) for phenol and cyanide elimination from synthetic/simulated wastewater. Appl. Water Sci. 2021, 11, 144. [Google Scholar] [CrossRef]
- Tirado-Muñoz, O.; Tirado-Ballestas, I.; Barbosa Lopez, A.L.; Colina-Marquez, J. Heterogeneous Photocatalytic Pilot Plant for Cyanide decontamination: A novel solar rotary photoreactor. J. Sol. Energy Eng. 2022, 144, 51005. [Google Scholar]
- Preis, A.; Ostfeld, A. Multiobjective contaminant sensor network design for water distribution systems. J. Water Resour. Plan. Manag. 2008, 134, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Rosario, C.G.A.; Vallenas-Arévalo, A.T.; Arévalo, S.J.; Espinosa, D.C.R.; Tenório, J.A.S. Biodegradation of cyanide using a Bacillus subtilis strain isolated from artisanal gold mining tailings. Braz. J. Chem. Eng. 2022, 1–8. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Chen, J.; Cui, S.; Yang, Y.; Shen, X.; Liu, K.; Han, Q. Purifying cyanide-bearing wastewaters by electrochemical precipitate process using sacrificial Zn anode. Sep. Purif. Technol. 2022, 284, 120250. [Google Scholar] [CrossRef]
- Verma, V.; Ghosh, P.; Singh, S.B.; Gupta, V.; Chaudhari, P.K. Kinetics of catalytic treatment of coking wastewater (COD, phenol and cyanide) using wet air oxidation. Int. J. Chem. React. Eng. 2022, 20, 325–341. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L. Enhanced photocatalytic oxidation degradability for real cyanide wastewater by designing photocatalyst GO/TiO2/ZSM-5: Performance and mechanism research. Chem. Eng. J. 2022, 428, 131257. [Google Scholar] [CrossRef]
- Kalboussi, N.; Biard, Y.; Pradeleix, L.; Rapaport, A.; Sinfort, C.; Ait-Mouheb, N. Life cycle assessment as decision support tool for water reuse in agriculture irrigation. Sci. Total Environ. 2022, 836, 155486. [Google Scholar] [CrossRef]
- Pouresmaeil, H.; Faramarz, M.G.; ZamaniKherad, M.; Gheibi, M.; Fathollahi-Fard, A.M.; Behzadian, K.; Tian, G. A decision support system for coagulation and flocculation processes using the adaptive neuro-fuzzy inference system. Int. J. Environ. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Bilger, E.; Wolf, H. Method for the Colorimetric Determination of the Cyanide Concentration of Aqueous Solutions. U.S. Patent No. 4,871,681, 3 October 1989. [Google Scholar]
- Gheibi, M.; Emrani, N.; Eftekhari, M.; Akrami, M.; Abdollahi, J.; Ramezani, M.; Sedghian, A. Experimental investigation and mathematical modeling for microbial removal using potassium permanganate as an oxidant—case study: Water treatment plant No. 1, Mashhad, Iran. Environ. Monit. Assess. 2019, 191, 141. [Google Scholar] [CrossRef]
- De Jong, K.A. An Analysis of the Behavior of a Class of Genetic Adaptive Systems; University of Michigan: Ann Arbor, MI, USA, 1975. [Google Scholar]
- Azé, J.; Bourquard, T.; Hamel, S.; Poupon, A.; Ritchie, D.W. Using Kendall-τ meta-bagging to improve protein-protein docking predictions. In Proceedings of the IAPR International Conference on Pattern Recognition in Bioinformatics, Delf, The Netherlands, 2–4 November 2011; pp. 284–295. [Google Scholar]
- Al-Rousan, N.; Al-Najjar, H.; Alomari, O. Assessment of predicting hourly global solar radiation in Jordan based on rules, trees, meta, lazy and function prediction methods. Sustain. Energy Technol. Assess. 2021, 44, 100923. [Google Scholar] [CrossRef]
- Alnuaim, A.A.; Zakariah, M.; Shukla, P.K.; Alhadlaq, A.; Hatamleh, W.A.; Tarazi, H.; Sureshbabu, R.; Ratna, R. Human-Computer Interaction for Recognizing Speech Emotions Using Multilayer Perceptron Classifier. J. Healthc. Eng. 2022, 2022, 6005446. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, H.; Fathollahi-Fard, A.M.; Fazlollahtabar, H.; Charles, V. Fuzzy data-driven scenario-based robust data envelopment analysis for prediction and optimisation of an electrical discharge machine’s parameters. Expert Syst. Appl. 2022, 193, 116419. [Google Scholar] [CrossRef]
- Botz, M.M. Overview of cyanide treatment methods. Min. Environ. Manag. Min. J. 2001, 28, 30. [Google Scholar]
- Manyuchi, M.; Sukdeo, N.; Stinner, W. Potential to remove heavy metals and cyanide from gold mining wastewater using biochar. Phys. Chem. Earth Parts A/B/C 2022, 126, 103110. [Google Scholar] [CrossRef]
- Alvillo-Rivera, A.J.; Garrido-Hoyos, S.E.; Buitrón, G.J.E.S.; Research, P. Cyanide treatment of mining tailings using suspended biomass and moving bed biomass reactors. Environ. Sci. Pollut. Res. 2022, 29, 37458–37470. [Google Scholar] [CrossRef]
- Geng, B.; Fan, J.; Shi, M.; Zhang, S.; Li, J.J.C. Control of maximum water age based on total chlorine decay in secondary water supply system. Chemosphere 2022, 287, 132198. [Google Scholar] [CrossRef]
- Okoji, C.N.; Okoji, A.I.; Ibrahim, M.S.; Obinna, O. Comparative analysis of adaptive neuro-fuzzy inference system (ANFIS) and RSRM models to predict DBP (trihalomethanes) levels in the water treatment plant. Arab. J. Chem. 2022, 15, 103794. [Google Scholar] [CrossRef]
- Liu, J.-L.; Han, X.; Zhang, J.; Wang, H.-J.; Zhou, M.-X.; Li, S.-W.; Ma, X.; Wang, Y.; Liu, A.-L. Total organic halogen in two drinking water supply systems: Occurrence, variations, and relationship with trihalomethanes. Chemosphere 2022, 288, 132541. [Google Scholar] [CrossRef]
- Amiri, S.A.H.S.; Zahedi, A.; Kazemi, M.; Soroor, J.; Hajiaghaei-Keshteli, M. Determination of the optimal sales level of perishable goods in a two-echelon supply chain network. Comput. Ind. Eng. 2020, 139, 106156. [Google Scholar] [CrossRef]
- Golmohamadi, S.; Tavakkoli-Moghaddam, R.; Hajiaghaei-Keshteli, M. Solving a fuzzy fixed charge solid transportation problem using batch transferring by new approaches in meta-heuristic. Electron. Notes Discret. Math. 2017, 58, 143–150. [Google Scholar] [CrossRef]
- Hajiaghaei-Keshteli, M.; Sajadifar, S.M.; Haji, R.J. Determination of the economical policy of a three-echelon inventory system with (R, Q) ordering policy and information sharing. Int. J. Adv. Manuf. Technol. 2011, 55, 831–841. [Google Scholar] [CrossRef]
- Hajiaghaei-Keshteli, M.; Sajadifar, S.M. Deriving the cost function for a class of three-echelon inventory system with N-retailers and one-for-one ordering policy. Int. J. Adv. Manuf. Technol. 2010, 50, 343–351. [Google Scholar] [CrossRef]
- Yazdani, M.; Jolai, F. Lion optimization algorithm (LOA): A nature-inspired metaheuristic algorithm. J. Comput. Des. Eng. 2016, 3, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Fathollahi-Fard, A.M.; Hajiaghaei-Keshteli, M.; Tavakkoli-Moghaddam, R. The social engineering optimizer (SEO). Eng. Appl. Artif. Intell. 2018, 72, 267–293. [Google Scholar] [CrossRef]
- Fathollahi-Fard, A.M.; Hajiaghaei-Keshteli, M.; Tavakkoli-Moghaddam, R. Red deer algorithm (RDA): A new nature-inspired meta-heuristic. Soft Comput. 2020, 24, 14637–14665. [Google Scholar] [CrossRef]










| Test Stages | Description |
|---|---|
| 1 | Pour 25 mL of the testing solution in the beaker |
| 2 | Add 5 mL of Na2CO3 0.5 mol L−1 |
| 3 | Add 5 mL Picric acid (1% w/v) into the beaker |
| 4 | Heat the container to near boiling point to get the color changes |
| 5 | Let the samples to cool at room temperature |
| 6 | Measure the absorptions of the standard and testing samples at the wavelength of 520 nm |
| Name | Formula/Model of Instrument | Source | |
|---|---|---|---|
| Raw materials | Sodium carbonate | Na2CO3 | Merck, Germany |
| Sodium hypochlorite | NaOCl | Merck, Germany | |
| Picric acid | C6H3N3O7 | Merck, Germany | |
| Potassium cyanide | KCN | Merck, Germany | |
| Instruments | UV-visible Spectroscopy System | Agilent 8453 | Agilent Technologies, United States |
| Model’s Name | General Form | Scenario Num. | Typical Content | SSE | RMSE | R2 |
|---|---|---|---|---|---|---|
| Exponential | 1 | 0.01 | 0.05 | 0.97 | ||
| 2 | 0.02 | 0.08 | 0.91 | |||
| 3 | 0.01 | 0.03 | 0.95 | |||
| Fourier | 1 | 0.01 | 0.07 | 0.96 | ||
| 2 | 0.01 | 0.06 | 0.97 | |||
| 3 | 0.00 | 0.01 | 0.99 | |||
| Gaussian | 1 | 0.01 | 0.01 | 0.93 | ||
| 2 | 0.01 | 0.01 | 0.97 | |||
| 3 | 0.00 | 0.01 | 0.98 | |||
| Rational | 1 | 0.01 | 0.08 | 0.96 | ||
| 2 | 0.01 | 0.04 | 0.97 | |||
| 3 | 0.00 | 0.01 | 0.99 | |||
| Polynomial | 1 | 0.01 | 0.04 | 0.96 | ||
| 2 | 0.02 | 0.02 | 0.96 | |||
| 3 | 0.00 | 0.01 | 0.99 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value (Prob > F) | |
|---|---|---|---|---|---|---|
| Model | 46.10124 | 6 | 7.683541 | 61.54065 | <0.0001 | significant |
| A-pH | 0.01776 | 1 | 0.01776 | 0.142247 | 0.7092 | |
| B-VDS | 0.083866 | 1 | 0.083866 | 0.671721 | 0.4202 | |
| C-T | 0.291519 | 1 | 0.291519 | 2.3349 | 0.1391 | |
| D-Initial CN | 16.59342 | 1 | 16.59342 | 132.9036 | <0.0001 | |
| E-Ammonia | 0.26992 | 1 | 0.26992 | 2.161901 | 0.1539 | |
| F-Cl2 | 32.6946 | 1 | 32.6946 | 261.8646 | <0.0001 | |
| Residual | 3.121328 | 25 | 0.124853 | |||
| Cor Total | 49.22257 | 31 |
| Statistical parameters | Meta Bagging | Meta Random Committee | Functions Multilayer Perceptron |
|---|---|---|---|
| Correlation coefficient | 0.90 | 0.84 | 0.99 |
| Mean absolute error | 0.61 | 0.81 | 0.3 |
| Root mean squared error | 0.81 | 1.12 | 0.36 |
| Relative absolute error | 62.37% | 70.88% | 26.49 % |
| Root relative squared error | 72.73% | 83.78% | 26.98% |
| Description | Equation (S1) | Equation (S2) | Equation (S3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheibi, M.; Eftekhari, M.; Akrami, M.; Emrani, N.; Hajiaghaei-Keshteli, M.; Fathollahi-Fard, A.M.; Yazdani, M. A Sustainable Decision Support System for Drinking Water Systems: Resiliency Improvement against Cyanide Contamination. Infrastructures 2022, 7, 88. https://doi.org/10.3390/infrastructures7070088
Gheibi M, Eftekhari M, Akrami M, Emrani N, Hajiaghaei-Keshteli M, Fathollahi-Fard AM, Yazdani M. A Sustainable Decision Support System for Drinking Water Systems: Resiliency Improvement against Cyanide Contamination. Infrastructures. 2022; 7(7):88. https://doi.org/10.3390/infrastructures7070088
Chicago/Turabian StyleGheibi, Mohammad, Mohammad Eftekhari, Mehran Akrami, Nima Emrani, Mostafa Hajiaghaei-Keshteli, Amir M. Fathollahi-Fard, and Maziar Yazdani. 2022. "A Sustainable Decision Support System for Drinking Water Systems: Resiliency Improvement against Cyanide Contamination" Infrastructures 7, no. 7: 88. https://doi.org/10.3390/infrastructures7070088
APA StyleGheibi, M., Eftekhari, M., Akrami, M., Emrani, N., Hajiaghaei-Keshteli, M., Fathollahi-Fard, A. M., & Yazdani, M. (2022). A Sustainable Decision Support System for Drinking Water Systems: Resiliency Improvement against Cyanide Contamination. Infrastructures, 7(7), 88. https://doi.org/10.3390/infrastructures7070088










