Preparation and Performance Evaluation of a Low-Fume Asphalt Binder
Abstract
1. Introduction
2. Material and Methods
2.1. Base Asphalt Binder
2.2. Inhibitors
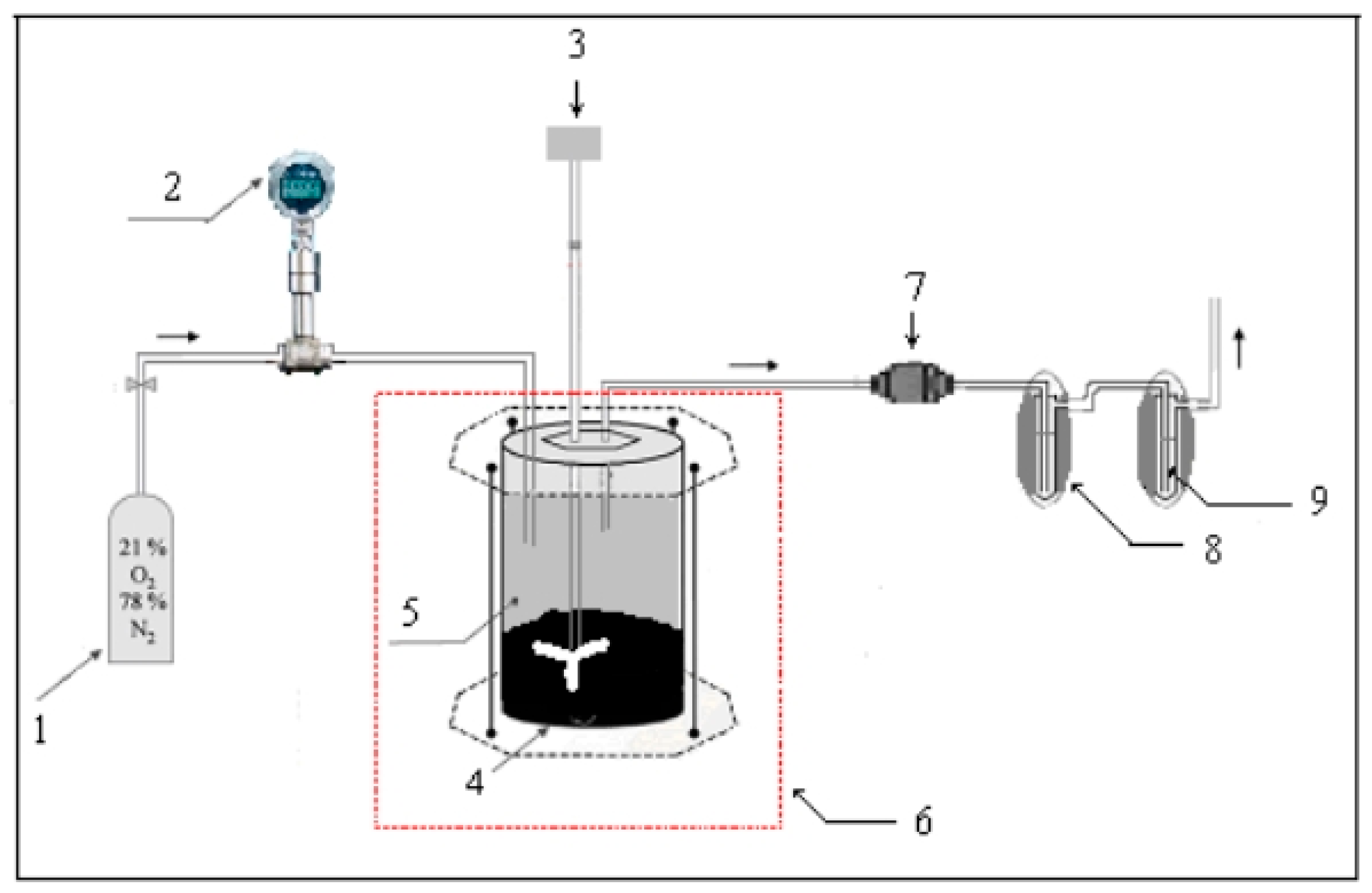
2.3. Asphalt Fume Preparation and Collection Equipment
2.4. Experimental Methods
Methods for Generating and Enriching Asphalt Fume
2.5. Calculation Methods for Asphalt Fume Quality
2.5.1. Gravimetric Method
- W1—Mass of glass fiber filter cartridge, g;
- W2—Total mass of glass fiber filter cartridge and asphalt fume particles, g;
- M—Mass of asphalt used in the experiment, g.
2.5.2. UV Spectrophotometry
- X—UV absorbance of asphalt fume cyclohexane solution;
- Y—The concentration of asphalt fume;
- M—Mass of asphalt used in the experiment, g, and the total amount of asphalt fume MA = M1 + M2.
2.6. Experiments
2.6.1. Research on the Mechanism of Asphalt Fume Production
2.6.2. Simulation of the Mixing Aging Conditions
2.6.3. Preparation of the Low-Fume Asphalt Binder
2.6.4. Performance Evaluation of the Low-Fume Asphalt
3. Results and Discussion
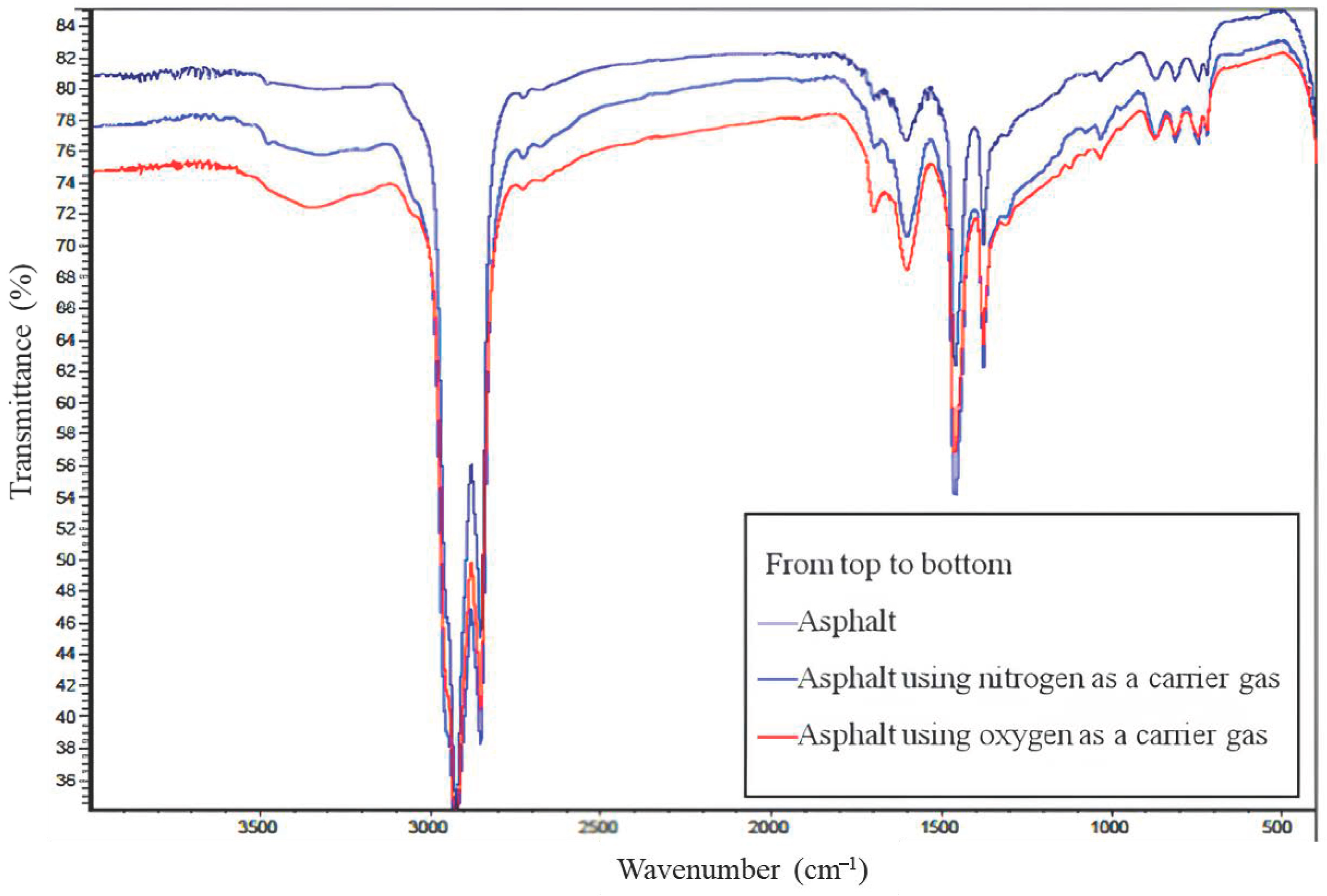
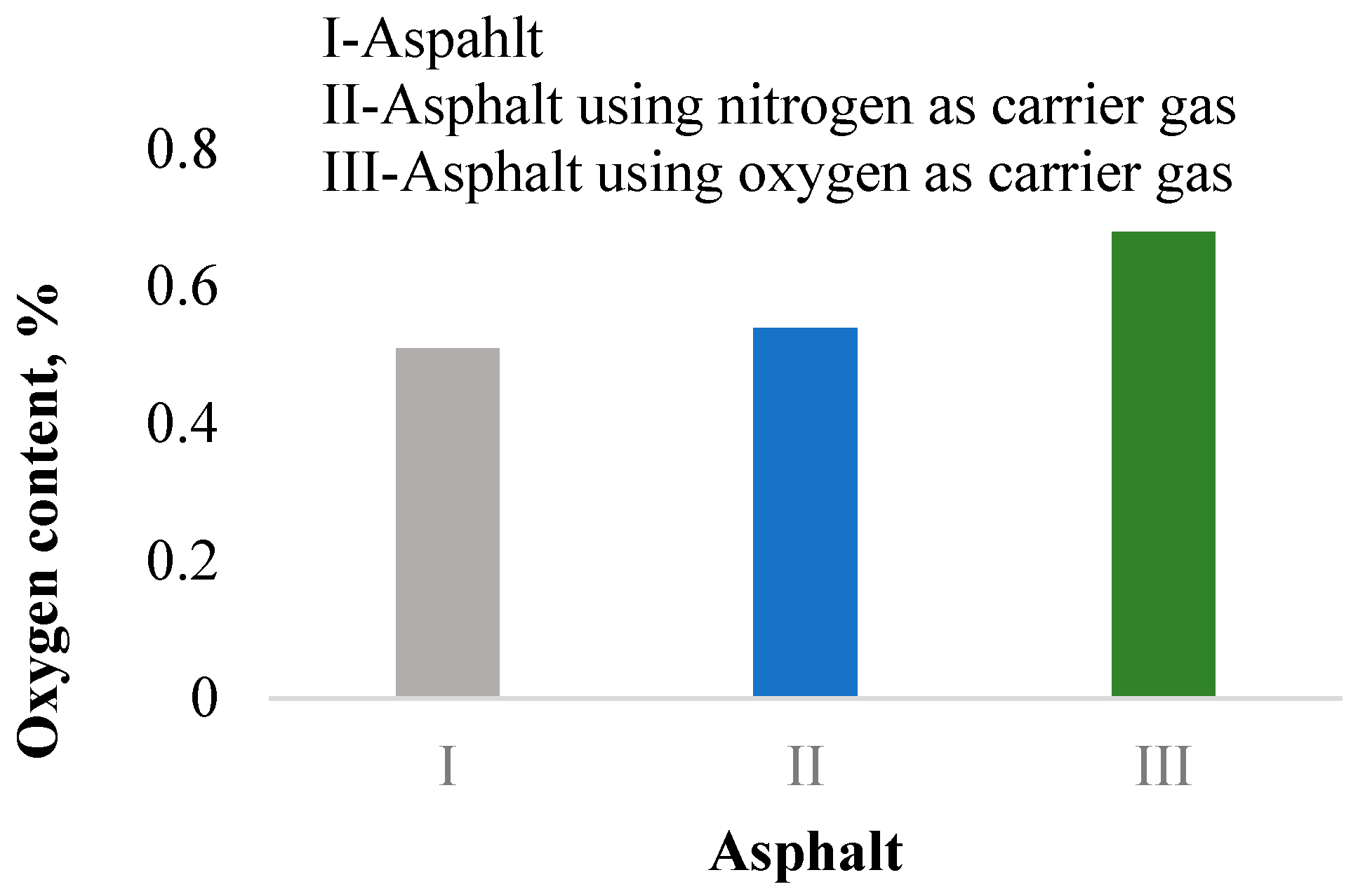
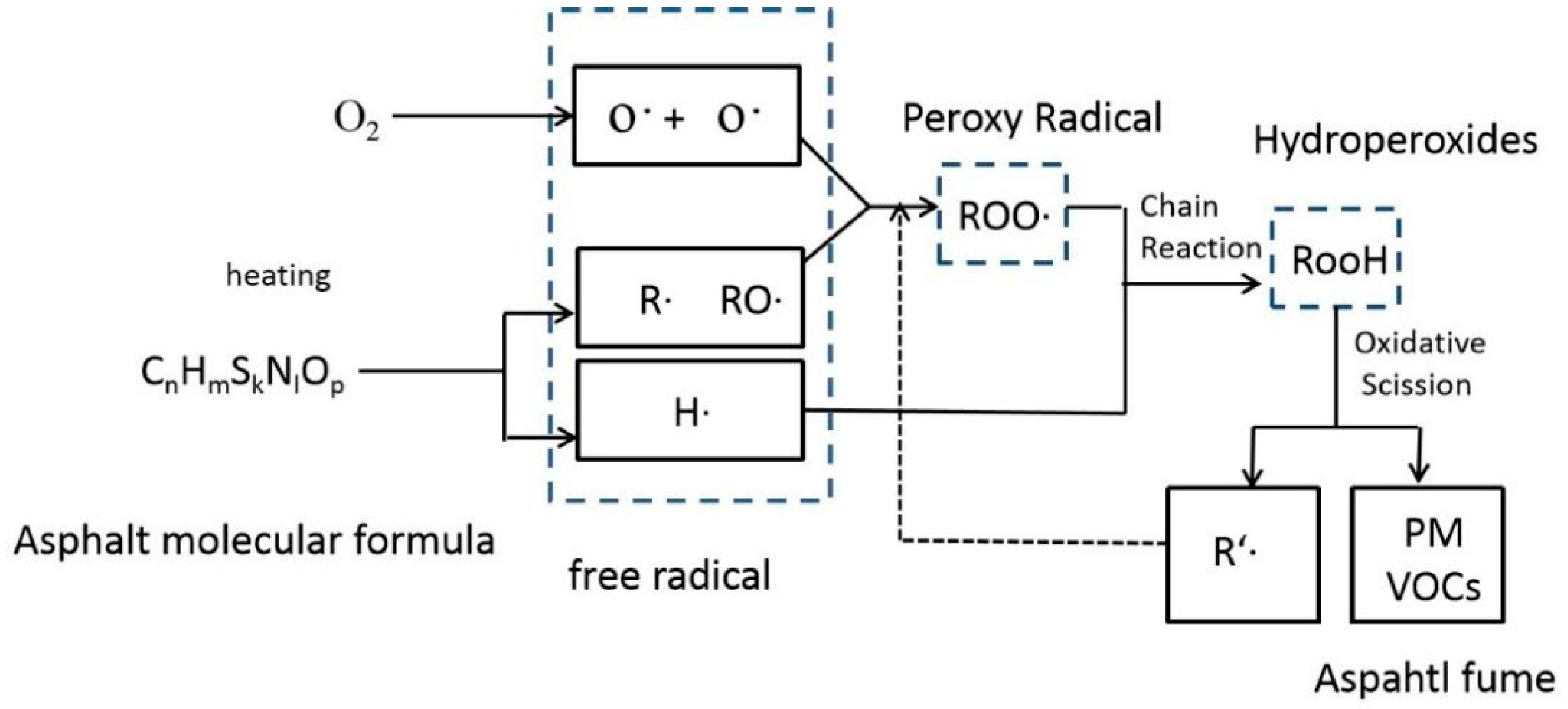
3.1. Research on the Mechanism of Asphalt Fume Generation
- (1)
- Thermal decomposition of asphalt
- (2)
- Formation of peroxy radicals and intermediate products
- (3)
- Secondary reactions and byproduct formation
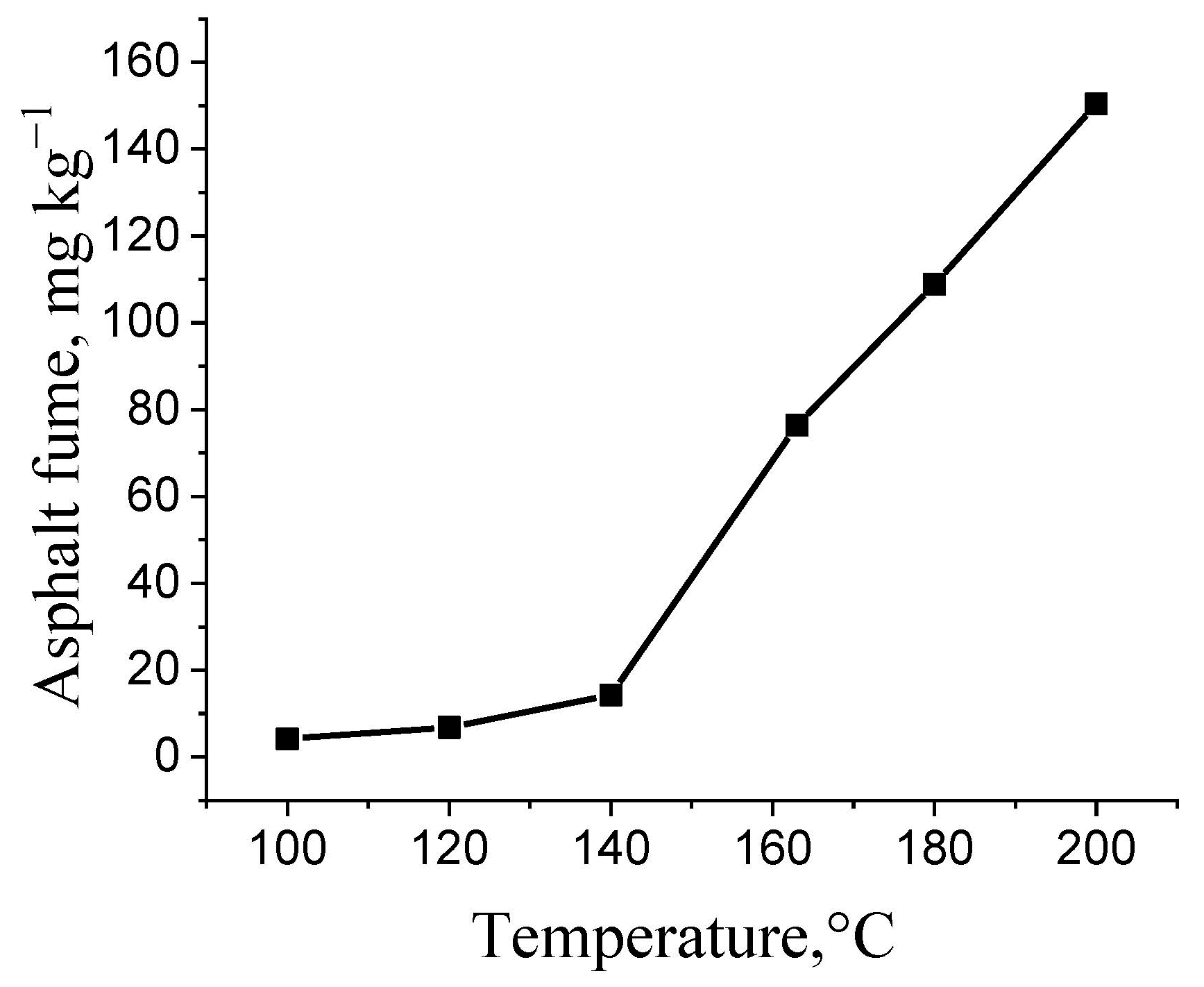
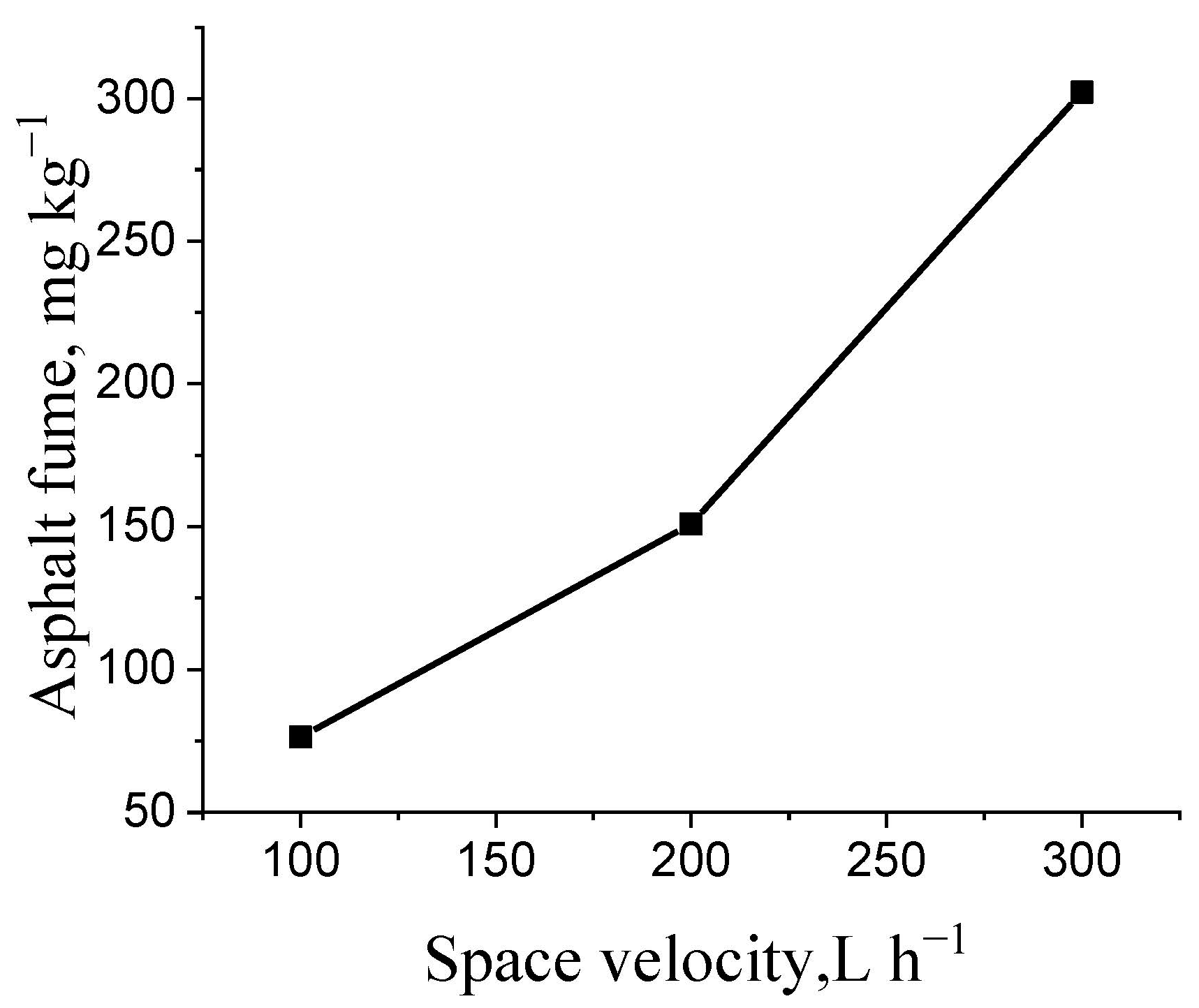
3.2. Study on Simulated Mixing Conditions
3.3. Influence of Different Types of Inhibitors
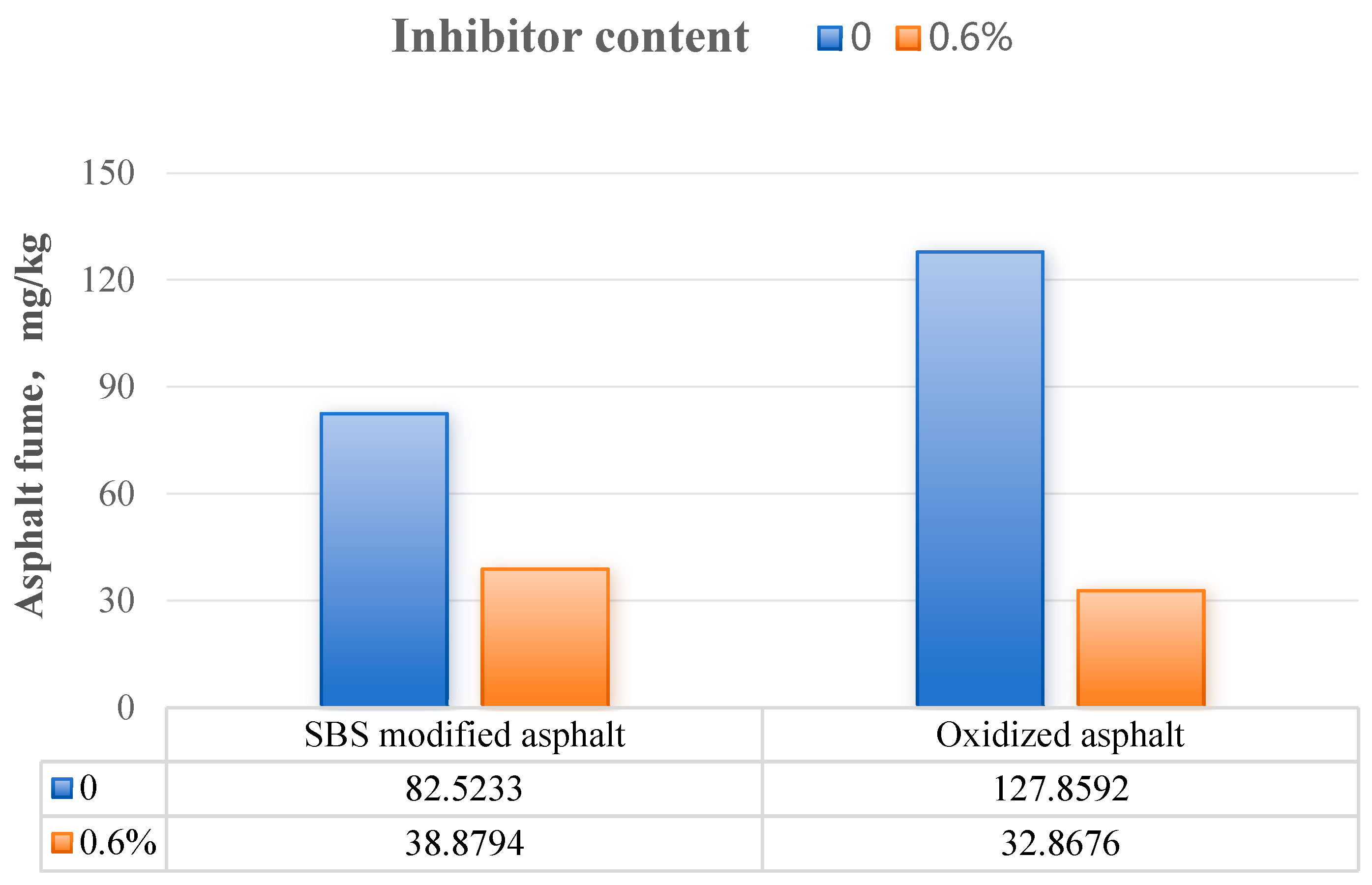
3.4. Influence of Inhibitor Content
3.5. Mechanisms of Asphalt Fume Inhibitors
3.5.1. DTBHQ
3.5.2. CuCl
3.5.3. FeCl3
- (1)
- Redox Cycling
- (2)
- Lewis Acidity
3.6. Performance Evaluation of the Low-Fume Asphalt Binder
4. Conclusions and Findings
- (1)
- The oxidation of the asphalt binder is the main reason for asphalt fume generation.
- (2)
- To simulate the on-site mixing conditions, preparation parameters for asphalt fumes were determined to be as follows: temperature 200 °C, air velocity 300 L/h, time 12 h, and mixing rate 180 rpm.
- (3)
- The best inhibition effect on asphalt fume was achieved with the following inhibitor recipe: mass percentages of cuprous chloride, ditert butylhydroquinone, and ferric chloride at a ratio of 40%, 40%, and 20%. When the inhibitor was added at a dosage of 0.6%, it could effectively reduce 50% of the production of asphalt fumes.
- (4)
- Inhibitors can enhance the antioxidant capacity of the asphalt binder and improve the properties after short-term laboratory aging.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TFOT | Thin-film oven aging test |
| VOCs | Volatile organic compounds |
| PAHs | Polycyclic aromatic hydrocarbons |
| TG-FTIR | Thermogravimetric analysis-Fourier transform infrared spectroscopy |
| SBS | Styrene-butadiene-styrene block copolymer |
| GC-MS | Gas chromatography–mass spectrometry |
| DTBHQ | 2,5-Di-tert-butylhydroquinone |
| PM | Particulate matter |
| SARA | Saturates, aromatics, resins, asphaltenes |
| HAT | Hydrogen atom transfer |
| SET | Single-electron transfer mechanism |
| ROS | reactive oxygen species |
References
- Tang, B.; Isacsson, U. Chemical characterization of oil-based asphalt release agents and their emissions. Fuel 2006, 85, 1232–1241. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Thives, L.P.; Ghisi, E. Asphalt mixtures emission and energy consumption: A review. Renew. Sust. Energ. Rev. 2017, 72, 473–484. [Google Scholar] [CrossRef]
- Partanen, T.; Boffetta, P. Cancer risk in asphalt workers and roofers: Review and meta-analysis of epidemiologic studies. Am. J. Ind. Med. 1994, 26, 721–740. [Google Scholar] [CrossRef]
- Boffetta, P.; Burstyn, I.; Partanen, T.; Kromhout, H.; Svane, O.; Langård, S.; Järvholm, B.; Frentzel-Beyme, R.; Kauppinen, T.; Stücker, I.; et al. Cancer mortality among European asphalt workers: An international epidemiological study. II. Exposure to bitumen fume and other agents. Am. J. Ind. Med. 2003, 43, 28–39. [Google Scholar] [CrossRef]
- Xu, Y.; Kåredal, M.; Nielsen, J.; Adlercreutz, M.; Bergendorf Strandberg Bo Antonsson, A.; Tinnerberg, H.; Albin, M. Exposure, respiratory symptoms, lung function and inflammation response of road-paving asphalt workers. Occup. Environ. Med. 2018, 75, 494–500. [Google Scholar] [CrossRef]
- Autelitano, F.; Bianchi, F.; Giuliani, F. Airborne emissions of asphalt/wax blends for warm mix asphalt production. J. Clean. Prod. 2017, 164, 749–756. [Google Scholar] [CrossRef]
- Shi, J. Environmental Issues and Prevention Measures Arising from the Use of Asphalt in Road Construction. Inn. Mong. Environ. Prot. 2006, 18, 37–40. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.; Wang, Y.; Niu, X.; Xu, Q.; Li, R. Research Progress on the Composition of Petroleum Asphalt Fumes and Smoke Suppression Technologies. Appl. Chem. Ind. 2023, 52, 256–260+265. [Google Scholar] [CrossRef]
- Irha, N.; Slet, J.; Petersell, V. Effect of heavy metals and PAH on soil assessed via dehydrogenase assay. Environ. Int. 2003, 28, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Cheng, P.; Lin, M.; Wang, M. Effect of tourmaline/organic montmorillonite on fume generation and suppression characteristics of asphalt binder and mixture. Fuel 2025, 381, 133540. [Google Scholar] [CrossRef]
- Yang, C.; Xie, J.; Zhou, X.; Wang, L. Temperature-dependent emission characteristics of volatile organic compounds from asphalt binders. Fuel 2019, 235, 1604–1611. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Zhang, H.; Liu, Y. Temperature threshold characteristics of fume generation in SBS-modified asphalt based on closed-loop test system. China J. Highw. Transp. 2021, 34, 45–53. [Google Scholar] [CrossRef]
- Wang, Y.; Leng, Z.; Li, X.; Hu, C. Chemical characterization and toxicity assessment of asphalt fumes generated at different temperatures through synchronous spectroscopy and chromatography. J. Hazard. Mater. 2020, 387, 121701. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Wang, K.; Zhang, L. Temperature-dependent fume suppression mechanism of Sasobit-modified asphalt binders through rheological and chemical characterization. Constr. Build. Mater. 2023, 400, 132887. [Google Scholar] [CrossRef]
- Almeida-Costa, R.; Fernandes, S.R.M.; Pais, J.C.; Pereira, P.A.A. Montmorillonite nanoclay as an effective fume suppressant for paving-grade asphalts: Adsorption mechanisms and environmental benefits. Fuel 2024, 357, 129803. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Wu, S.; Xie, J.; Yang, C.; Li, N.; Cui, P. Road performance, VOCs emission and economic benefit evaluation of asphalt mixture by incorporating steel slag and SBS/CR composite modified asphalt. Case Stud. Constr. Mater. 2023, 18, e01929. [Google Scholar] [CrossRef]
- Qiao, Z.; Chen, Q.; Wang, Z.; Niu, C.; Guo, T. Study on new tourmaline composite material and its adsorption properties for asphalt fumes. J. Chongqing Jiaotong Univ. (Nat. Sci. Ed.) 2021, 40, 126–131+149. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Chen, Q.; Zhang, L. Basic performance and asphalt smoke absorption effect of environment-friendly asphalt to improve pavement construction environment. J. Clean. Prod. 2022, 333, 130142. [Google Scholar] [CrossRef]
- Yang, X.; Shen, A.; Su, Y.; Zhao, W. Effects of alumina trihydrate (ATH) and organic montmorillonite (OMMT) on asphalt fume emission and flame retardancy properties of SBS-modified asphalt. Constr. Build. Mater. 2020, 236, 117576. [Google Scholar] [CrossRef]
- Peng, X.Y.; Qian, S.L.; Xiao, F.; Zhang, P.; Li, Z.Y. The experimental study on the road asphalt fumes suppressive effect of SBS and nano calcium carbonate. Adv. Mater. Res. 2011, 233, 507–511. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, N.; Zhu, H.; Xi, Y.; Sheng, W.; Li, Z. Short-term emission behavior and evolution law of organic emissions from asphalt binder materials. Constr. Build. Mater. 2024, 433, 136498. [Google Scholar] [CrossRef]
- Mo, S.; Wang, Y.; Xiong, F.; Ai, C.; Wang, D.; Tan, G.Y.A. Changes of asphalt fumes in hot-mix asphalt pavement recycling. J. Clean. Prod. 2020, 258, 120586. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, L.; Liu, J.; Li, Y. Evaluation of Smoke Suppression Effect and Performance of Smoke Suppressant Asphalt. Pet. Asph. 2023, 37, 19–23. [Google Scholar] [CrossRef]
- Kurek, J.T.; Kriech, A.J.; Wissel, H.L.; Osborn, L.V.; Blackburn, G.R. Laboratory generation and evaluation of paving asphalt fumes. J. Transp. Res. Board 2007, 1661, 35–40. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation: Perspectives for the future. Food Chem. 2010, 118, 915–916. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Li, R.; Wang, H. Effects of antioxidants on the rheological and anti-aging properties of asphalt binders: A review. J. Road Eng. 2022, 2, 118–129. [Google Scholar] [CrossRef]
- Ali, M.F.; Ahmed, S.; Qureshi, M.S. Catalytic coprocessing of coal and petroleum residues with waste plastics to produce transportation fuels. Fuel Process. Technol. 2011, 92, 1109–1120. [Google Scholar] [CrossRef]
- Sun, G.; Ning, W.; Jiang, X.; Qiu, K.; Cao, Z.; Ding, Y. A comprehensive review on asphalt fume suppression and energy saving technologies in asphalt pavement industry. Sci. Total Environ. 2024, 913, 169726. [Google Scholar] [CrossRef]
- Shi, K.; Ma, F.; Falchetto, A.C.; Fu, Z.; Yuan, D.; Song, R.; Dai, J.; Wang, H. Comprehensive review on the composition, influence, and inhibition of asphalt fumes. J. Traffic Transp. Eng. (Engl. Ed.) 2025, 12, 926–964. [Google Scholar] [CrossRef]
- Alugoju, P.; Dinesh, B.J.; Latha, P. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Petersen, R.C. Free-radicals and advanced chemistries involved in cell membrane organization influence oxygen diffusion and pathology treatment. AIMS Biophys. 2017, 2, 240–283. [Google Scholar] [CrossRef] [PubMed]
- Zubanova, E.M.; Golubeva, E.N.; Zhidomirov, G.M. Two mechanisms of chlorocuprate reactions with alkyl radicals: Dramatic role of nuclearity. Organometallics 2014, 33, 121–128. [Google Scholar] [CrossRef]
- Hammond, G.S.; Mahoney, L.R.; Nandi, U.S. Effects of Metallic Compounds in Oxidation Systems. I. Ferric Chloride as an Inhibitor. J. Am. Chem. Soc. 1963, 85, 737–741. [Google Scholar] [CrossRef]

| Item | Test Result | Test Method |
|---|---|---|
| Penetration (25 °C, 100 g, 5 s), 1/10 mm | 65 | ASTM D5/D5M-20 |
| Penetration index | −1.34 | ASTM D5/D5M-20 |
| Ductility (15 °C, 5 cm/min), cm | 150+ | ASTM D113/D113M-17 |
| Ductility (10 °C, 5 cm/min), cm | 31.4 | ASTM D113/D113M-17 |
| Soft point, °C | 48.6 | ASTM D36/D36M-14 |
| Flash point, °C | 329 | ASTM D92-18 |
| Wax content, % | 1.8 | SH/T 0425-2003 |
| Solubility, % | 99.81 | ASTM D2042-22 |
| Density (15 °C), g/cm3 | 1.039 | ASTM D70/D70M-21 |
| Viscosity (60 °C), Pa·s | 261 | ASTM D4402/D4402M-22 |
| Thin-film oven test (163 °C, 5 h) | ASTM D1754/D1754M-20 | |
| Mass loss, % | −0.107 | ASTM D6/D6M-95 |
| Retained penetration, % | 67 | ASTM D5/D5M-20 |
| Ductility (10 °C, 5 cm/min), cm | 7.3 | ASTM D113/D113M-17 |
| Experimental Conditions | Saturates, % | Aromatics, % | Resin, % | Asphaltene, % | Asphalt Fume, mg·kg−1 |
|---|---|---|---|---|---|
| TFOT | 17.89 | 31.86 | 42.41 | 7.84 | / |
| 163 °C; 100 L/h; 6 h; 180 rpm | 19.59 | 39.26 | 33.18 | 7.97 | 76 |
| 200 °C; 200 L/h; 6 h; 180 rpm | 20.25 | 41.97 | 32.31 | 7.90 | 858 |
| 200 °C; 300 L/h; 6 h; 180 rpm | 20.85 | 40.19 | 32.89 | 8.24 | 1280 |
| 200 °C; 300 L/h; 12 h; 180 rpm | 17.89 | 33.43 | 40.25 | 7.79 | 1425 |
| Experimental Conditions | Softening Point, °C | Penetration (25 °C), 0.1 mm | Ductility (15 °C), cm |
|---|---|---|---|
| TFOT | 52.4 | 62 | >150 |
| 200 °C; 300 L/h; 12 h; 180 rpm | 51.6 | 66 | >150 |
| Inhibitor | Component Name | Chemical Composition/Formulation |
|---|---|---|
| Inhibitor 1 | cuprous chloride | CuCl |
| Inhibitor 2 | 2,5-di-tert-butyl-hydroquinone | C14H22O2(DTBHQ) |
| Inhibitor 3 | Ferric chloride | FeCl3 |
| Inhibitor 4 | Mixed Inhibitor | CuCl:DTBHQ = 1:1 |
| Inhibitor 5 | Mixed Inhibitor | FeCl3:DTBHQ = 1:1 |
| Inhibitor 6 | Mixed Inhibitor | CuCl:FeCl3 = 1:1 |
| Inhibitor 7 | Mixed Inhibitor | CuCl:DTBHQ:FeCl3 = 1:1:1 |
| Inhibitor 8 | Mixed Inhibitor | CuCl:DTBHQ:FeCl3 = 4:4:2 |
| Inhibitor | Asphalt Fume, mg/kg | ||
|---|---|---|---|
| M2 | M1 | MA | |
| Inhibitor 1 | 0.2753 | 117.6324 | 117.9074 |
| Inhibitor 2 | 0.3390 | 110.2171 | 110.5561 |
| Inhibitor 3 | 0.3472 | 106.3918 | 106.7390 |
| Inhibitor 4 | 0.4766 | 96.3966 | 96.8732 |
| Inhibitor 5 | 0.3529 | 94.2802 | 94.6331 |
| Inhibitor 6 | 0.5871 | 111.6963 | 112.2834 |
| Inhibitor 7 | 0.3110 | 74.7271 | 75.0381 |
| Inhibitor 8 | 0.2444 | 67.4961 | 67.7405 |
| Content of Inhibition 3, % | Asphalt Fume, mg/kg | Reduction, wt% | ||
|---|---|---|---|---|
| M2 | M1 | MA | ||
| 0 | 0.2406 | 137.9852 | 138.2258 | — — |
| 0.2 | 0.2460 | 120.8674 | 121.1134 | 12.4 |
| 0.4 | 0.2396 | 88.4523 | 88.6919 | 35.8 |
| 0.6 | 0.2444 | 67.4961 | 67.7405 | 50.1 |
| 0.8 | 0.2899 | 64.8647 | 65.1546 | 52.9 |
| Item | Low-Fume Asphalt Binder | Base Asphalt Binder | Test Method |
|---|---|---|---|
| Penetration (25 °C, 100 g, 5 s), 1/10 mm | 67 | 65 | ASTM D5/D5M-20 |
| Penetration index | −1.39 | −1.34 | ASTM D5/D5M-20 |
| Ductility (15 °C, 5 cm/min), cm | 150+ | 150+ | ASTM D113/D113M-17 |
| Ductility (10 °C, 5 cm/min), cm | 32.6 | 31.4 | ASTM D113/D113M-17 |
| Soft point, °C | 48.5 | 48.6 | ASTM D36/D36M-14 |
| Flash point, °C | 338 | 329 | ASTM D92-18 |
| Wax content, % | 1.6 | 1.8 | SH/T 0425-2003 |
| Solubility, % | 99.89 | 99.81 | ASTM D2042-22 |
| Density (15 °C), g/cm3 | 1.038 | 1.039 | ASTM D70/D70M-21 |
| Viscosity (60 °C), Pa·s | 260 | 261 | ASTM D4402/D4402M-22 |
| Thin-film oven test (163 °C,5h) | ASTM D1754/D1754M-20 | ||
| Mass loss, % | −0.115 | −0.107 | ASTM D6/D6M-95 |
| Retained penetration, % | 73 | 67 | ASTM D5/D5M-20 |
| Ductility (10 °C, 5 cm/min), cm | 8.9 | 7.3 | ASTM D113/D113M-17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Li, R.; Zhang, Y.; Xiao, J. Preparation and Performance Evaluation of a Low-Fume Asphalt Binder. Infrastructures 2025, 10, 244. https://doi.org/10.3390/infrastructures10090244
Cai H, Li R, Zhang Y, Xiao J. Preparation and Performance Evaluation of a Low-Fume Asphalt Binder. Infrastructures. 2025; 10(9):244. https://doi.org/10.3390/infrastructures10090244
Chicago/Turabian StyleCai, Hongmei, Rui Li, Yuzhen Zhang, and Junrui Xiao. 2025. "Preparation and Performance Evaluation of a Low-Fume Asphalt Binder" Infrastructures 10, no. 9: 244. https://doi.org/10.3390/infrastructures10090244
APA StyleCai, H., Li, R., Zhang, Y., & Xiao, J. (2025). Preparation and Performance Evaluation of a Low-Fume Asphalt Binder. Infrastructures, 10(9), 244. https://doi.org/10.3390/infrastructures10090244









