Effects of Gypsum and Limestone on Early-Age Hydration and Strength Optimization in Belite Calcium Sulfoaluminate Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Mixture Designs
2.2. Characterization Techniques
2.3. Thermodynamic Modeling
3. Results and Discussion
3.1. XRD Analysis
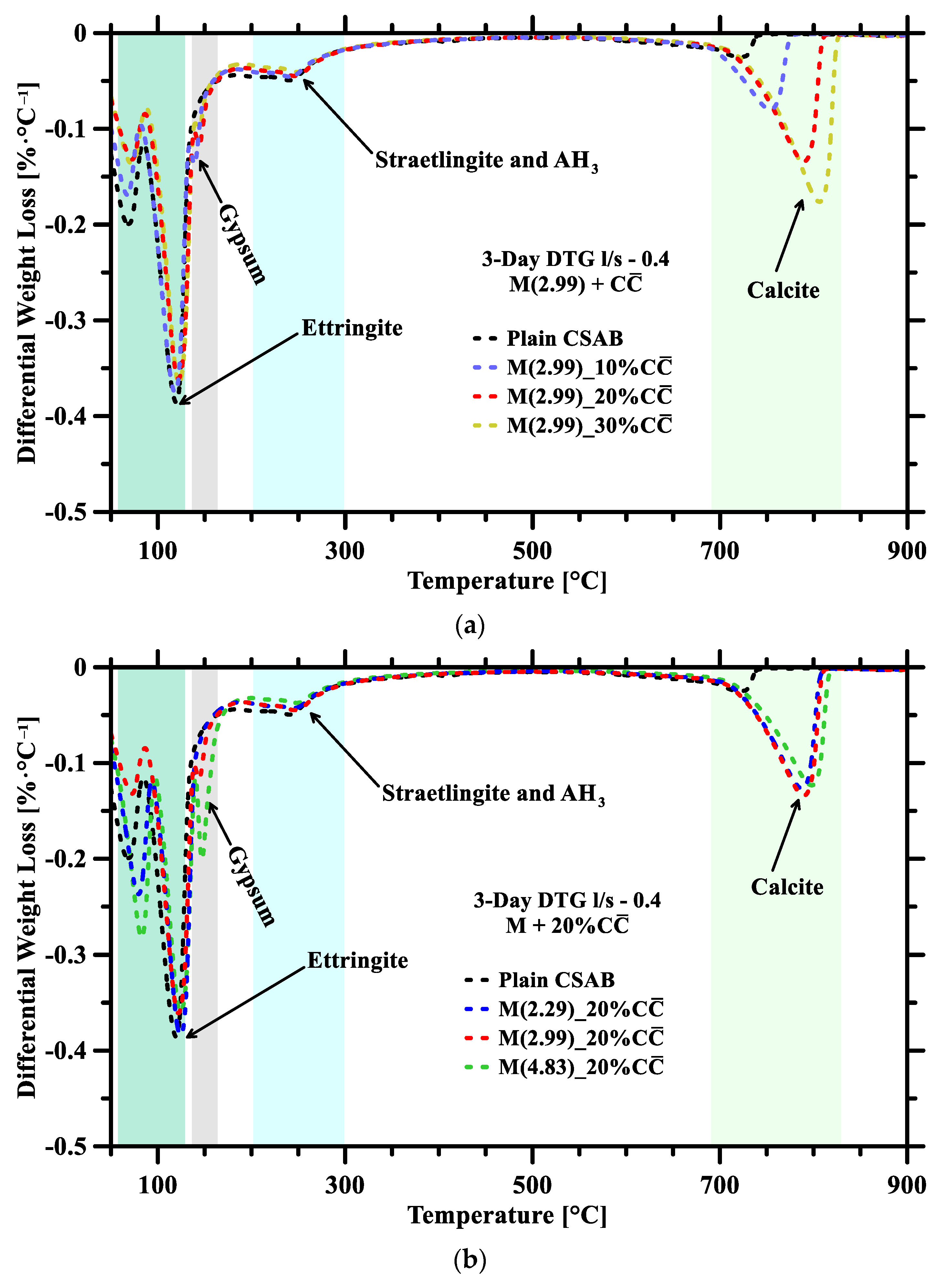
3.2. Thermogravimetric Analysis
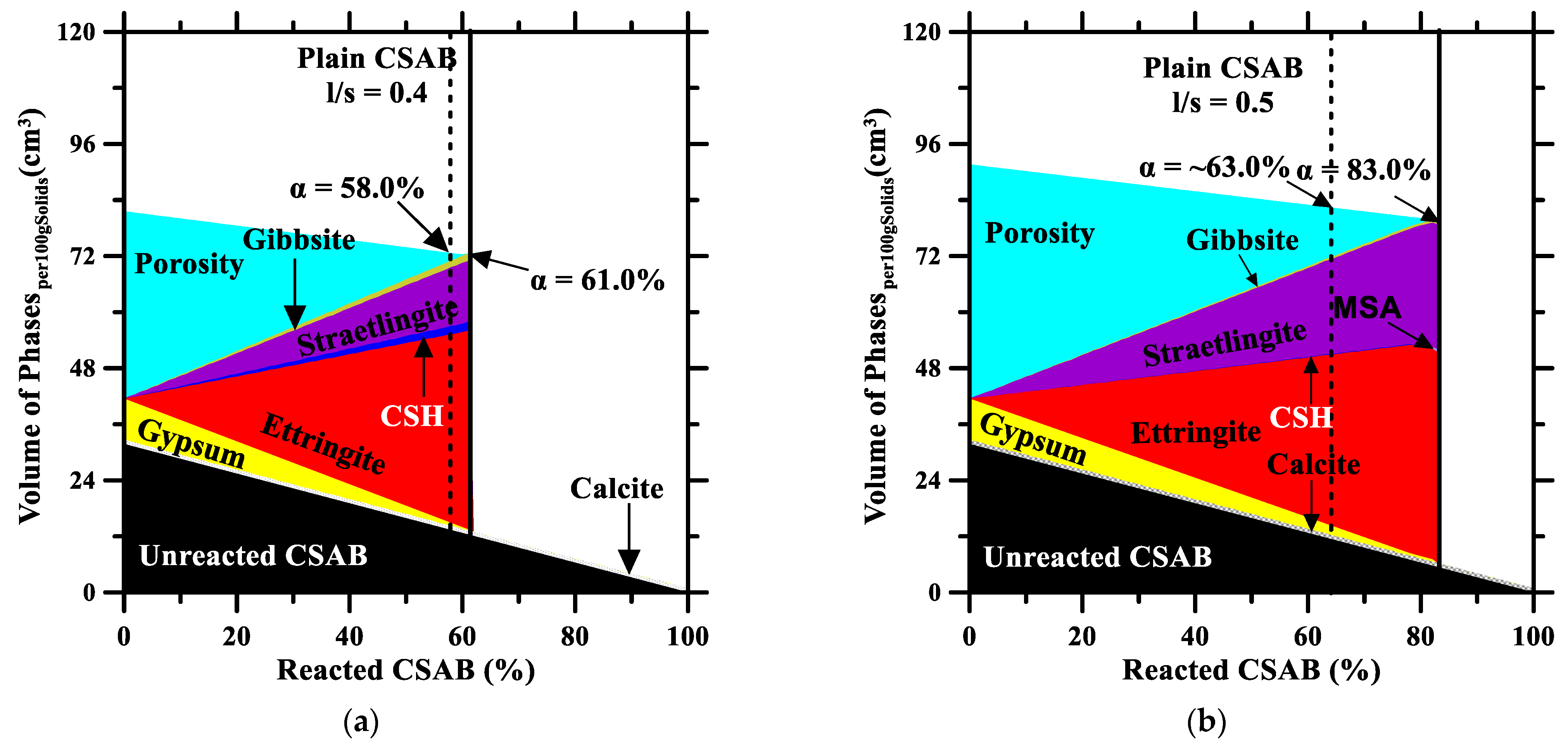
3.3. Thermodynamic Simulations
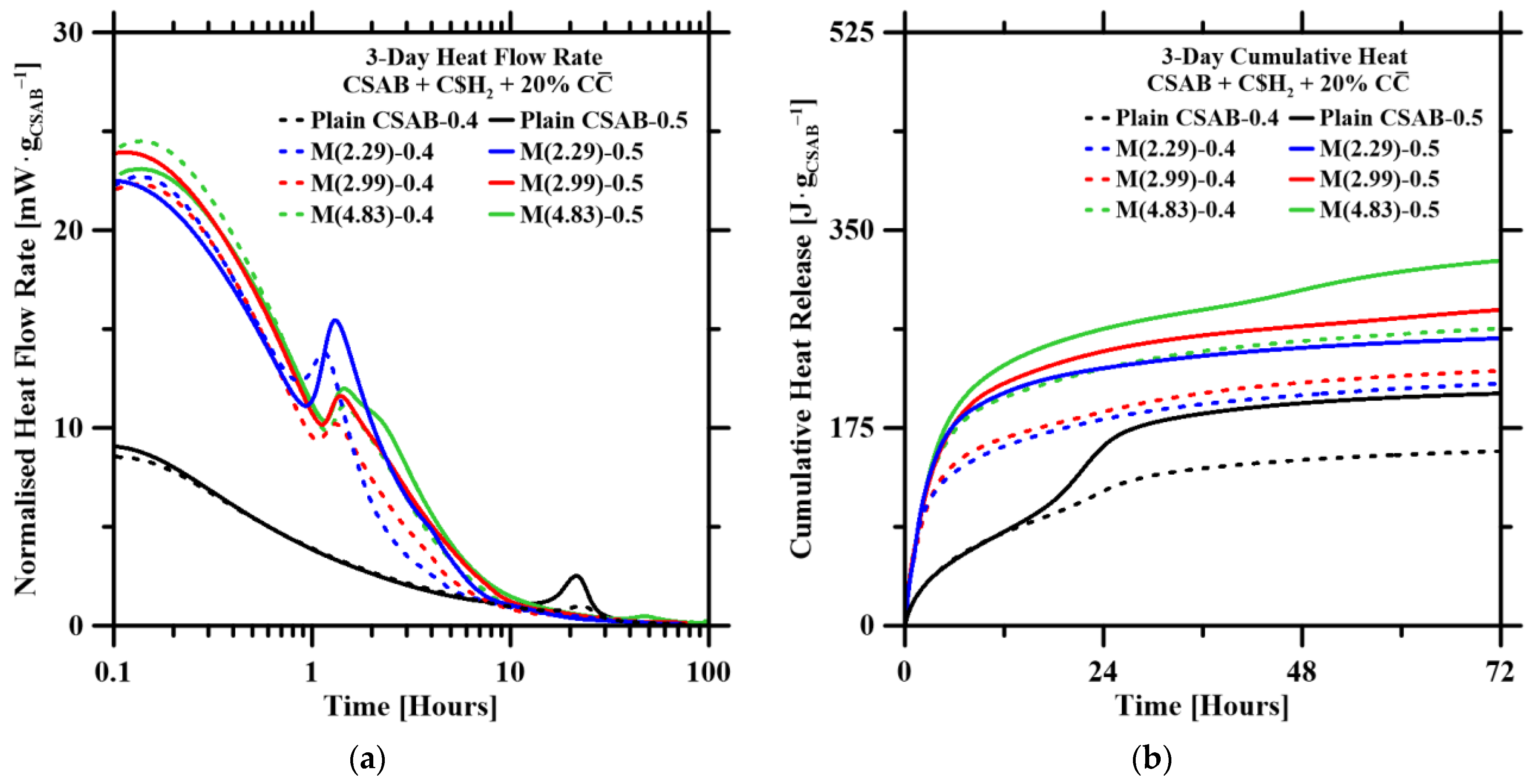
3.4. Hydration Kinetics
3.5. Compressive Strength
4. Conclusions
- Ettringite is the main hydration phase. The presence of other hydrates like CSH, straetlingite, and gibbsite are minimal when compared to ettringite. There is no formation of HCA/MCA in any other binders due to the minimal reactivity of .
- Higher replacement levels were found to enhance the CSAB hydration due to more free water for ye’elimite hydration. Due to the increase in C$H2, more ettringite is expected to form, and less AH3 appears in binders due to straetlingite formation. TGA confirms the average reactivity of C$H2 and at both the l/s ratios to be ~83.60% and ~1.25%, respectively.
- Isothermal calorimetry revealed that the addition of C$H2, , and water accelerated early-age hydration, leading to increased cumulative heat release. However, C$H2, , and water could reduce compressive strength due to the dilution effect.
- It was found that CSAB cement with an M-ratio of 2.99 and 20% replacement level were the optimal mixture design, achieving high carbon reduction and maintaining high compressive strength (i.e., 39 MPa).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- World Energy Outlook 2021—Analysis, IEA. 2021. Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 12 April 2024).
- Cheng, D.; Reiner, D.M.; Yang, F.; Cui, C.; Meng, J.; Shan, Y.; Liu, Y.; Tao, S.; Guan, D. Projecting future carbon emissions from cement production in developing countries. Nat. Commun. 2023, 14, 8213. [Google Scholar] [CrossRef] [PubMed]
- 4 Ways to Make the Cement Industry More Sustainable. World Economic Forum. 2024. Available online: https://www.weforum.org/agenda/2024/09/cement-production-sustainable-concrete-co2-emissions/ (accessed on 7 October 2024).
- Barbhuiya, S.; Bhusan Das, B.; Adak, D. Roadmap to a net-zero carbon cement sector: Strategies, innovations and policy imperatives. J. Environ. Manag. 2024, 359, 121052. [Google Scholar] [CrossRef]
- Liao, S.; Wang, D.; Xia, C.; Tang, J. China’s provincial process CO2 emissions from cement production during 1993–2019. Sci. Data 2022, 9, 165. [Google Scholar] [CrossRef]
- Wirthwein, C.R.; Emberger, B. Burners for alternative fuels utilization-Optimisation of kiln firing systems for advanced alternative fuel co-firing. Cem. Int. 2010, 8, 42–46. [Google Scholar]
- Technology Roadmap—Carbon Capture and Storage 2009—Analysis, IEA. 2009. Available online: https://www.iea.org/reports/technology-roadmap-carbon-capture-and-storage-2009 (accessed on 2 July 2024).
- Tsiligiannis, A.; Tsiliyannis, C. Renewable energy in cement manufacturing: A quantitative assessment of energy and environmental efficiency of food residue biofuels. Renew. Sustain. Energy Rev. 2019, 107, 568–586. [Google Scholar]
- Making Concrete Change: Innovation in Low-Carbon Cement and Concrete | Chatham House—International Affairs Think Tank. 2018. Available online: https://www.chathamhouse.org/2018/06/making-concrete-change-innovation-low-carbon-cement-and-concrete (accessed on 2 July 2024).
- Wood, J. 5 Innovations Building a More Sustainable Future for Cement, Spectra. 2024. Available online: https://spectra.mhi.com/5-innovations-building-a-more-sustainable-future-for-cement (accessed on 2 July 2024).
- Truscott, J. New Low-Carbon Innovations in Cement and Concrete Production. CarbonCure Technologies Inc. 2020. Available online: https://www.carboncure.com/concrete-corner/new-low-carbon-innovations-in-cement-and-concrete-production/ (accessed on 2 July 2024).
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Lothenbach, B.; Albert, B.; Morin, V.; Gartner, E. Hydration of Belite-Ye’elimite-Ferrite cements: Thermodynamic modeling. In Proceedings of the 14th International Congress on the Chemistry of Cement (ICCC), Beijing, China, 13–16 October 2015. [Google Scholar]
- Glasser, F.P.; Zhang, L. High Performance Cement Matrices Based on Calcium Sulfoaluminate—Belite Compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Cuberos, A.J.M.; De la Torre, Á.G.; Álvarez-Pinazo, G.; Martín-Sedeño, M.C.; Schollbach, K.; Pöllmann, H.; Aranda, M.A.G. Active Iron-Rich Belite Sulfoaluminate Cements: Clinkering and Hydration. Environ. Sci. Technol. 2010, 44, 6855–6862. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Compos. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Sahu, S.; Majling, J. Preparation of sulphoaluminate belite cement from fly ash. Cem. Concr. Res. 1994, 24, 1065–1072. [Google Scholar] [CrossRef]
- Arjunan, P.; Silsbee, M.R.; Della, M. Roy Sulfoaluminate-belite cement from low-calcium fly ash and sulfur-rich and other industrial by-products. Cem. Concr. Res. 1999, 29, 1305–1311. [Google Scholar] [CrossRef]
- Wu, K.; Shi, H.; Guo, X. Utilization of municipal solid waste incineration fly ash for sulfoaluminate cement clinker production. Waste Manag. 2011, 31, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Beretka, J.; de Vito, B.; Santoro, L.; Sherman, N.; Valenti, G.L. Hydraulic behaviour of calcium sulfoaluminate-based cements derived from industrial process wastes. Cem. Concr. Res. 1993, 23, 1205–1214. [Google Scholar] [CrossRef]
- Singh, M.; Upadhayay, S.N.; Prasad, P.M. Preparation of special cements from red mud. Waste Manag. 1996, 16, 665–670. [Google Scholar] [CrossRef]
- Use of Fluidized Bed Combustion Wastes for the Synthesis of Low-Energy Cements. Available online: https://iris.unibas.it/handle/11563/17078 (accessed on 26 April 2024).
- Pace, M.L.; Telesca, A.; Marroccoli, M.; Valenti, G.L. Use of Industrial Byproducts as Alumina Sources for the Synthesis of Calcium Sulfoaluminate Cements. Environ. Sci. Technol. 2011, 45, 6124–6128. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Dillard, R.J.; Murray, C.D.; Deschenes, R.A. Belitic calcium sulfoaluminate cement subjected to sulfate attack and sulfuric acid. Constr. Build. Mater. 2022, 343, 128089. [Google Scholar] [CrossRef]
- Bescher, E.; Rice, E.K.; Ramseyer, C.; Roswurm, S. Sulfate resistance of calcium sulphoaluminate cement. J. Struct. Integr. Maint. 2016, 1, 131–139. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.G.; De la Torre, Á.G. Hydration of belite–ye’elimite–ferrite cements with different calcium sulfate sources. Adv. Cem. Res. 2016, 28, 529–543. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase compositions. J. Mater. Sci. 2011, 46, 2568–2577. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.; Müller, C.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cem. Concr. Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial use of limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Paul, G.; Boccaleri, E.; Buzzi, L.; Canonico, F.; Gastaldi, D. Friedel’s salt formation in sulfoaluminate cements: A combined XRD and 27Al MAS NMR study. Cem. Concr. Res. 2015, 67, 93–102. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Irico, S.; Paul, G.; Canonico, F. Behavior of blends of CSA and Portland cements in high chloride environment. Constr. Build. Mater. 2020, 262, 120852. [Google Scholar] [CrossRef]
- Damidot, D.; Lothenbach, B.; Herfort, D.; Glasser, F.P. Thermodynamics and cement science. Cem. Concr. Res. 2011, 41, 679–695. [Google Scholar] [CrossRef]
- Moser, R.D.; Singh, P.M.; Kahn, L.F.; Kurtis, K.E. Chloride-induced corrosion resistance of high-strength stainless steels in simulated alkaline and carbonated concrete pore solutions. Corros. Sci. 2012, 57, 241–253. [Google Scholar] [CrossRef]
- Shakouri, M.; Ahmed, A.A.; Teymouri, M.; Trejo, D. Corrosion Resistance of Calcium Sulfoaluminate Cementitious Systems. J. Mater. Civ. Eng. 2023, 35, 04023238. [Google Scholar] [CrossRef]
- Bullard, J.W.; Scherer, G.W.; Thomas, J.J. Time dependent driving forces and the kinetics of tricalcium silicate hydration. Cem. Concr. Res. 2015, 74, 26–34. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Beltagui, H.; Jen, G.; Whittaker, M.; Imbabi, M.S. The influence of variable gypsum and water content on the strength and hydration of a belite-calcium sulphoaluminate cement. Adv. Appl. Ceram. 2017, 116, 199–206. [Google Scholar] [CrossRef]
- Development of the Use of Sulfo- and Ferroaluminate Cements in China | Advances in Cement Research. Available online: https://www.icevirtuallibrary.com/doi/10.1680/adcr.1999.11.1.15 (accessed on 6 December 2024).
- Berger, S.; Coumes, C.C.D.; Le Bescop, P.; Damidot, D. Influence of a thermal cycle at early age on the hydration of calcium sulphoaluminate cements with variable gypsum contents. Cem. Concr. Res. 2011, 41, 149–160. [Google Scholar] [CrossRef]
- García-Maté, M.; Santacruz, I.; De la Torre, Á.G.; León-Reina, L.; Aranda, M.A.G. Rheological and hydration characterization of calcium sulfoaluminate cement pastes. Cem. Concr. Compos. 2012, 34, 684–691. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Compos. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens). Available online: https://www.astm.org/c0109_c0109m-20b.html (accessed on 30 September 2022).
- Ponduru, S.A.; Han, T.; Huang, J.; Neithalath, N.; Sant, G.; Kumar, A. Understanding roles and evaluating reactivity of fly ashes in calcium aluminate binders. Constr. Build. Mater. 2024, 414, 135062. [Google Scholar] [CrossRef]
- Bhat, R.; Han, T.; Sant, G.; Neithalath, N.; Kumar, A. A comprehensive analysis of hydration kinetics and compressive strength development of fly ash-Portland cement binders. J. Build. Eng. 2024, 88, 109191. [Google Scholar] [CrossRef]
- Lapeyre, J.; Ponduru, S.A.; Okoronkwo, M.; Ma, H.; Kumar, A. Hydration of high-alumina calcium aluminate cements with carbonate and sulfate additives. J. Therm. Anal. Calorim. 2022, 147, 5575–5587. [Google Scholar] [CrossRef]
- Ponduru, S.A.; Han, T.; Huang, J.; Kumar, A. Predicting Compressive Strength and Hydration Products of Calcium Aluminate Cement Using Data-Driven Approach. Materials 2023, 16, 654. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.; Moon, J. Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Borštnar, M.; Lengauer, C.L.; Dolenec, S. Quantitative in Situ X-ray Diffraction Analysis of Early Hydration of Belite-Calcium Sulfoaluminate Cement at Various Defined Temperatures. Minerals 2021, 11, 297. [Google Scholar] [CrossRef]
- Palomino-Ore, S.B.; Rimstidt, J.D.; Chermak, J.A.; Schreiber, M.E.; Seal, R.R. Aluminum hydroxide coatings in limestone drains. Appl. Geochem. 2019, 103, 23–30. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. (Eds.) A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-22849-7. [Google Scholar]
- Chitvoranund, N.; Lothenbach, B.; Londono-Zuluaga, D.; Winnefeld, F.; Scrivener, K. Influence of temperature on phase assemblages of belite-ye’elimite cement. Cem. Concr. Res. 2023, 174, 107339. [Google Scholar] [CrossRef]
- Brien, J.V.; Henke, K.R.; Mahboub, K.C. Observations of Peak Strength Behavior in CSA Cement Mortars. J. Green Build. 2013, 8, 97–115. [Google Scholar] [CrossRef]
- Skalamprinos, S.; Galan, I.; Hanein, T.; Glasser, F. Enthalpy of formation of ye’elimite and ternesite. J. Therm. Anal. Calorim. 2018, 131, 2345–2359. [Google Scholar] [CrossRef]
- Burris, L.E.; Kurtis, K.E. Water-to-cement ratio of calcium sulfoaluminate belite cements: Hydration, setting time, and strength development. Cement 2022, 8, 100032. [Google Scholar] [CrossRef]
- Kleib, J.; Aouad, G.; Benzerzour, M.; Abriak, N.E.; Zakhour, M. Managing the Heat Release of Calcium Sulfoaluminate Cement by Modifying the Ye’elimite Content. Materials 2023, 16, 2470. [Google Scholar] [CrossRef]
- Beretka, J.; Marroccoli, M.; Sherman, N.; Valenti, G.L. The influence of C4A3S content and WS ratio on the performance of calcium sulfoaluminate-based cements. Cem. Concr. Res. 1996, 26, 1673–1681. [Google Scholar] [CrossRef]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Mrak, M.; Winnefeld, F.; Lothenbach, B.; Dolenec, S. The influence of calcium sulfate content on the hydration of belite-calcium sulfoaluminate cements with different clinker phase compositions. Mater. Struct. 2021, 54, 212. [Google Scholar] [CrossRef]
- Tambara, L.U.D.; Cheriaf, M.; Rocha, J.C.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis content on calcium sulfoaluminate (CSA) cement hydration. Cem. Concr. Res. 2020, 128, 105953. [Google Scholar] [CrossRef]
- Pérez-Bravo, R.; Morales-Cantero, A.; Cuesta, A.; Aranda, M.A.G.; Santacruz, I.; De la Torre, A.G. Early hydration of belite-ye’elimite-ferrite cements: Role of admixtures. Constr. Build. Mater. 2023, 371, 130765. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Boehm-Courjault, E.; Zajac, M.; Ben Haha, M.; Scrivener, K. Hydration reactions and stages of clinker composed mainly of stoichiometric ye’elimite. Cem. Concr. Res. 2019, 116, 120–133. [Google Scholar] [CrossRef]
- Borštnar, M.; Daneu, N.; Dolenec, S. Phase development and hydration kinetics of belite-calcium sulfoaluminate cements at different curing temperatures. Ceram. Int. 2020, 46, 29421–29428. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; T. Telford: London, UK, 1997; ISBN 978-0-7277-2592-9. [Google Scholar]
- Oey, T.; Kumar, A.; Bullard, J.W.; Neithalath, N.; Sant, G. The Filler Effect: The Influence of Filler Content and Surface Area on Cementitious Reaction Rates. J. Am. Ceram. Soc. 2013, 96, 1978–1990. [Google Scholar] [CrossRef]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Hydration of C3A–gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker [Einfluss von Calciumsulfat und Calciumhydroxid auf die Hydratation von Calciumsulfoaluminat-Klinker]. ZKG Int. 2009, 62, 42–53. [Google Scholar]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869–874. [Google Scholar] [CrossRef]
- Lian, C.; Zhuge, Y.; Beecham, S. The relationship between porosity and strength for porous concrete. Constr. Build. Mater. 2011, 25, 4294–4298. [Google Scholar] [CrossRef]
- Popovics, S. New Formulas for the Prediction of the Effect of Porosity on Concrete Strength. J. Am. Concr. Inst. 1985, 82, 136–146. [Google Scholar]
- Li, L.; Aubertin, M. A general relationship between porosity and uniaxial strength of engineering materials. Can. J. Civ. Eng. 2003, 30, 644–658. [Google Scholar] [CrossRef]
- Roy, D.M.; Gouda, G.R. Porosity-Strength Relation in Cementitious Materials with Very High Strengths. J. Am. Ceram. Soc. 1973, 56, 549–550. [Google Scholar] [CrossRef]
- Mehdipour, I.; Kumar, A.; Khayat, K.H. Rheology, hydration, and strength evolution of interground limestone cement containing PCE dispersant and high volume supplementary cementitious materials. Mater. Des. 2017, 127, 54–66. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]








| Oxides | %wt. | Phases | %wt. |
|---|---|---|---|
| CaO | 51.92 | Ye’elimite (C4A3$) | 48.50 |
| SiO2 | 11.86 | Anhydrite (C$) | 23.20 |
| Al2O3 | 20.45 | Belite (C2S) | 25.80 |
| SO3 | 9.86 | Calcite (CaCO3) | 2.51 |
| Fe2O3 | 3.34 | ||
| TiO2 | 0.57 | ||
| MgO | 1.12 | Particle Size d50 (µm) | ~8.45 |
| Other | 0.89 | SSA (g.cm−2) | 5500 |
| Sample ID | M-Ratio | CSAB (%wt.) | C$H2 (%wt.) | (%wt.) |
|---|---|---|---|---|
| Plain CSAB | 1.70 | 100 | 0 | 0 |
| M (2.29)_10% | 2.29 | 83.25 | 6.75 | 10 |
| M (2.29)_20% | 2.29 | 74.0 | 6.00 | 20 |
| M (2.29)_30% | 2.29 | 64.75 | 5.25 | 30 |
| M (2.99)_10% | 2.99 | 76.5 | 13.5 | 10 |
| M (2.99)_20% | 2.99 | 68.0 | 12.0 | 20 |
| M (2.99)_30% | 2.99 | 59.5 | 10.5 | 30 |
| M (4.83)_10% | 4.83 | 63.0 | 27.0 | 10 |
| M (4.83)_20% | 4.83 | 56.0 | 24.0 | 20 |
| M (4.83)_30% | 4.83 | 49.0 | 21.0 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponduru, S.A.; Aylas-Paredes, B.K.; Han, T.; Neithalath, A.; Neithalath, N.; Sant, G.; Kumar, A. Effects of Gypsum and Limestone on Early-Age Hydration and Strength Optimization in Belite Calcium Sulfoaluminate Cement. Infrastructures 2025, 10, 27. https://doi.org/10.3390/infrastructures10020027
Ponduru SA, Aylas-Paredes BK, Han T, Neithalath A, Neithalath N, Sant G, Kumar A. Effects of Gypsum and Limestone on Early-Age Hydration and Strength Optimization in Belite Calcium Sulfoaluminate Cement. Infrastructures. 2025; 10(2):27. https://doi.org/10.3390/infrastructures10020027
Chicago/Turabian StylePonduru, Sai Akshay, Bryan K. Aylas-Paredes, Taihao Han, Advaith Neithalath, Narayanan Neithalath, Gaurav Sant, and Aditya Kumar. 2025. "Effects of Gypsum and Limestone on Early-Age Hydration and Strength Optimization in Belite Calcium Sulfoaluminate Cement" Infrastructures 10, no. 2: 27. https://doi.org/10.3390/infrastructures10020027
APA StylePonduru, S. A., Aylas-Paredes, B. K., Han, T., Neithalath, A., Neithalath, N., Sant, G., & Kumar, A. (2025). Effects of Gypsum and Limestone on Early-Age Hydration and Strength Optimization in Belite Calcium Sulfoaluminate Cement. Infrastructures, 10(2), 27. https://doi.org/10.3390/infrastructures10020027






