Caffeine–Acrylic Resin DLP-Manufactured Composite as a Modern Biomaterial
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment Processes
2.3. Composites DLP Manufacturing
2.4. Mechanical Testing
2.5. Caffeine Release Test
2.5.1. Gravimetric Method
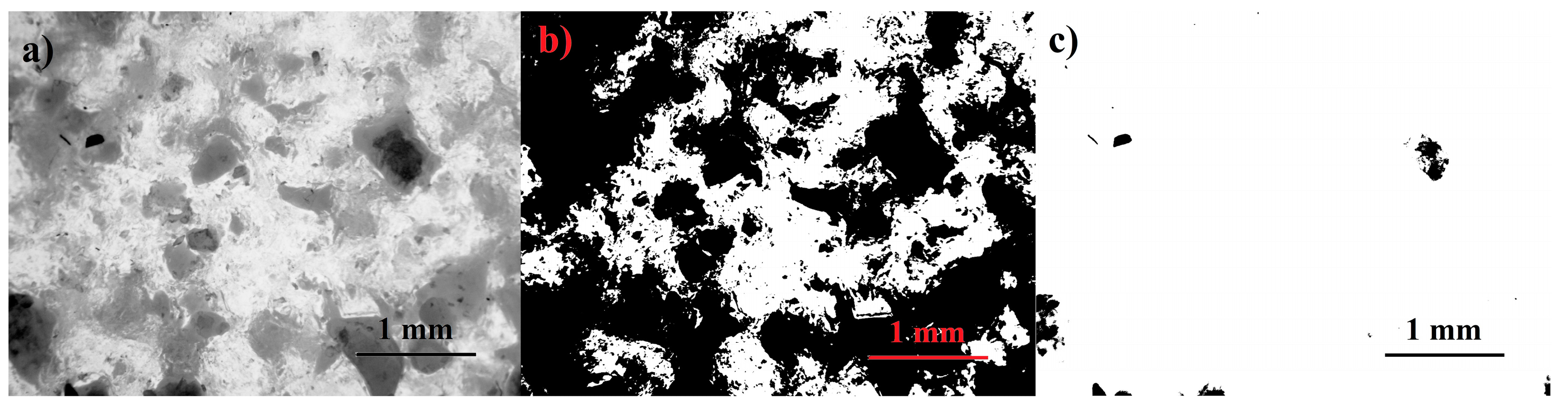
2.5.2. Global Threshold Method
3. Results and Discussion
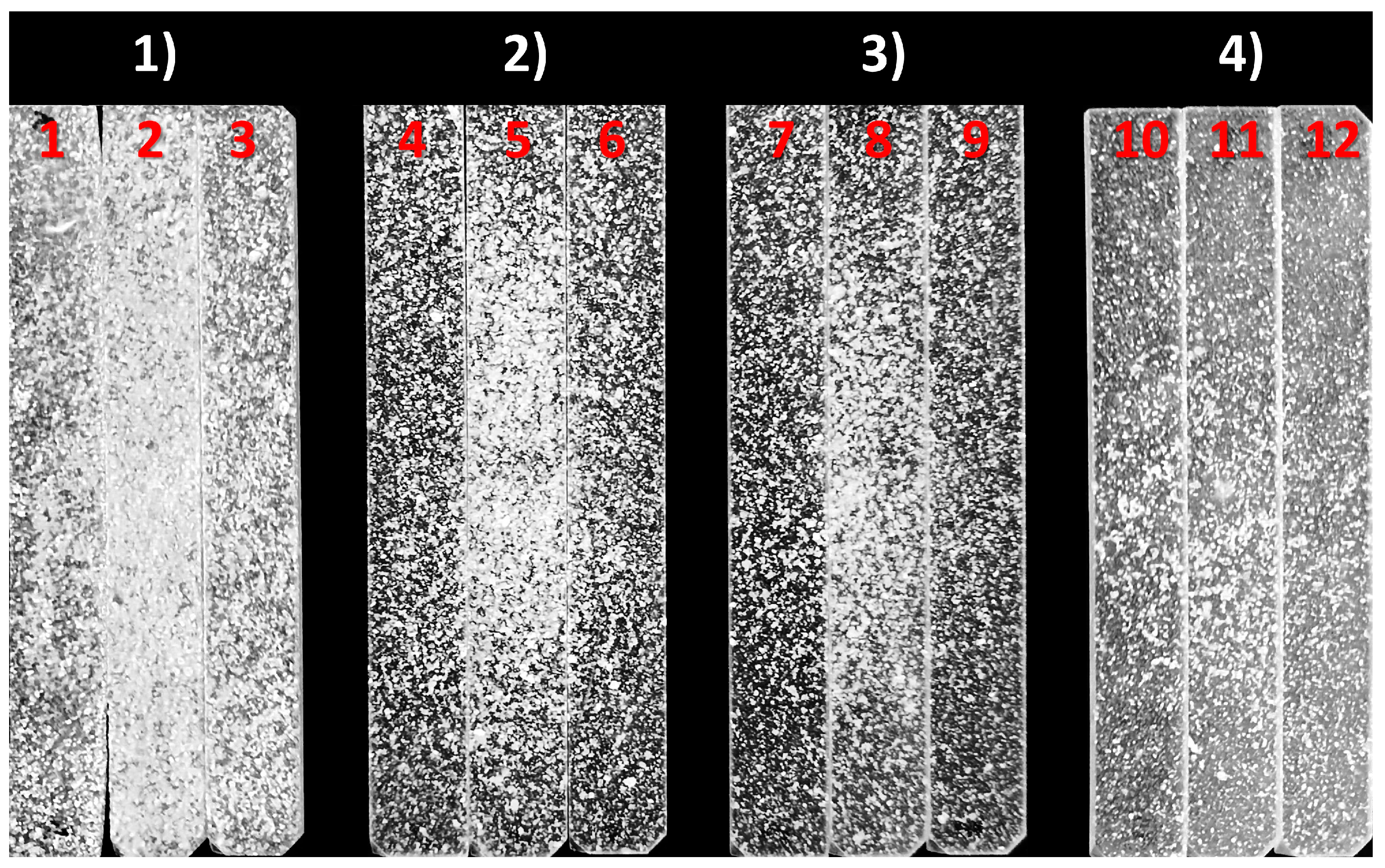
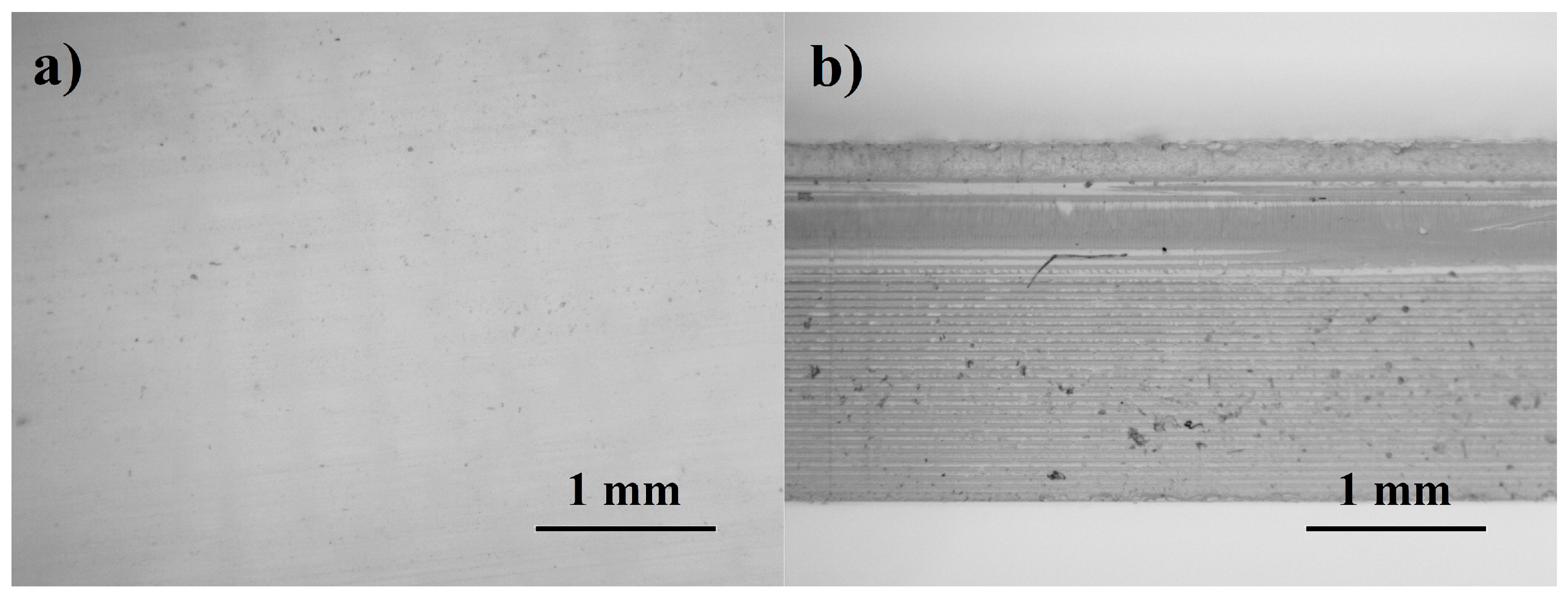
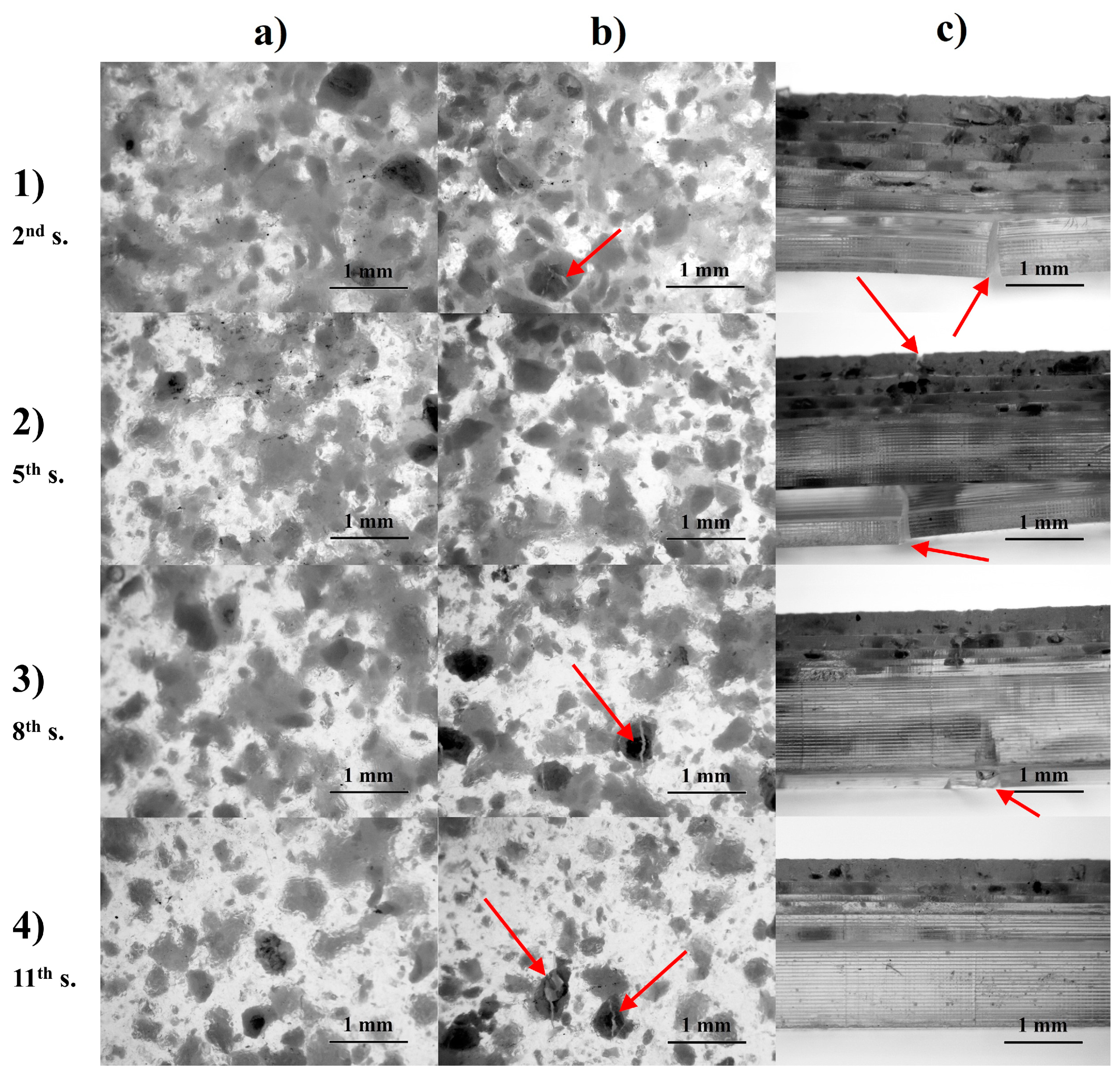
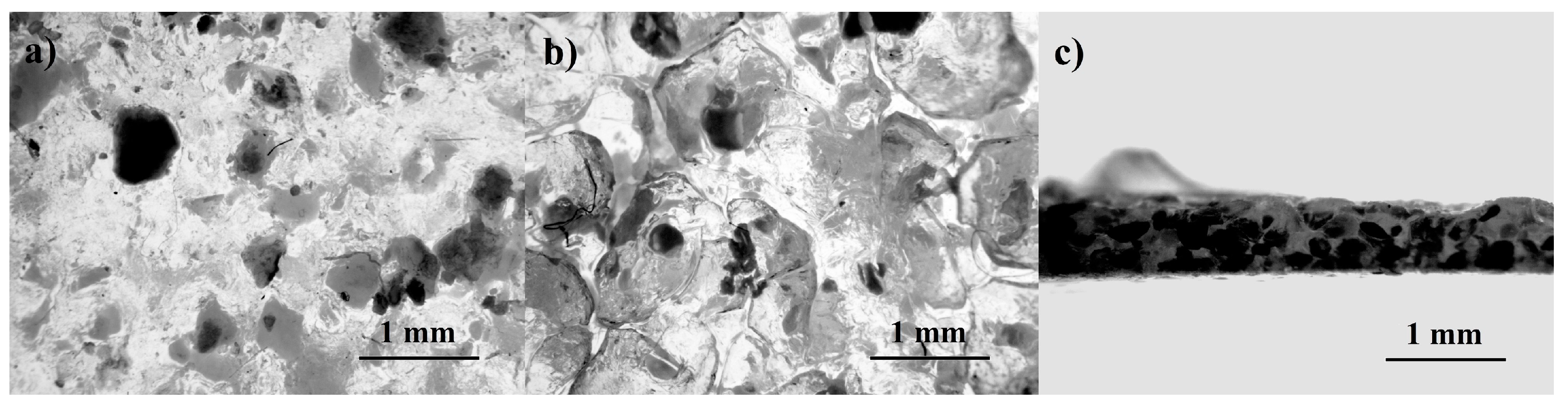
3.1. Evaluation of Manufacturing Process and Samples
3.2. Mechanical Testing
3.3. Caffeine Release
3.3.1. Gravimetric Analysis
3.3.2. Global Threshold Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Aita, I.; Ponsar, H.; Quodbach, J. A critical review on 3D-printed dosage forms. Curr. Pharm. Des. 2018, 24, 4957–4978. [Google Scholar] [CrossRef] [PubMed]
- Herrada-Manchón, H.; Rodríguez-González, D.; Alejandro Fernández, M.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective Laser Sintering 3D Printing—An Overview of the Technology and Pharmaceutical Applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Yu, D.G.; Yang, X.L.; Huang, W.D.; Liu, J.; Wang, Y.G.; Xu, H. Tablets with Material Gradients Fabricated by Three-Dimensional Printing. J. Pharm. Sci. 2007, 96, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Maines, E.M.; Porwal, M.K.; Ellison, C.J.; Reineke, T.M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021, 23, 6863–6897. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basti, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Arnett, P.; Bagde, A.; Azim, N.; Kouagou, E.; Singh, M.; Rajaraman, S. DLP 3D Printed “Intelligent” Microneedle Array (iμNA) for Stimuli Responsive Release of Drugs and Its in Vitro and ex Vivo Characterization. J. Microelectromechanical Syst. 2020, 29, 685–691. [Google Scholar] [CrossRef]
- Lim, S.H.; Kathuria, H.; Amir, M.H.B.; Zhang, X.; Duong, H.T.T.; Chi-Lui Ho, P.; Kang, L. High resolution photopolymer for 3D printing of personalised microneedle for transdermal delivery of anti-wrinkle small peptide. J. Control. Release 2021, 329, 907–918. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Godfrin, P.D.; Myerson, A.S.; Trout, B.L.; Doyle, P.S. Core–Shell Composite Hydrogels for Controlled Nanocrystal Formation and Release of Hydrophobic Active Pharmaceutical Ingredients. Adv. Healthc. Mater. 2016, 5, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Himawan, A.; Anjani, Q.K.; Detamornrat, U.; Vora, L.K.; Permana, A.D.; Ghanma, R.; Naser, Y.; Rahmawanty, D.; Scott, C.J.; Donnelly, R.F. Multifunctional low temperature-cured PVA/PVP/citric acid-based hydrogel forming microarray patches: Physicochemical characteristics and hydrophilic drug interaction. Eur. Polym. J. 2023, 186, 111836. [Google Scholar] [CrossRef]
- Huang, L.; Himawan, E.; Belhadj, S.; Oswaldo Pérez García, R.; Durand, F.P.; Schipper, N.; Buzgo, M.; Simaite, A.; Marigo, V. Efficient Delivery of Hydrophilic Small Molecules to Retinal Cell Lines Using Gel Core-Containing Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 74. [Google Scholar] [CrossRef]
- Roy, A.; Ray, P.G.; Bose, A.; Dhara, S.; Pal, S. pH-Responsive Copolymeric Network Gel Using Methacrylated β-Cyclodextrin for Controlled Codelivery of Hydrophilic and Hydrophobic Drugs. ACS Appl. Bio Mater. 2022, 5, 3530–3543. [Google Scholar] [CrossRef]
- Chandran, R.; Tohit, M.R.E.; Stanslas, J.; Salim, N.; Mazlelaa, T.M.T. Investigation and Optimization of Hydrogel Microneedles for Transdermal Delivery of Caffeine. Tissue Eng. Part C Methods 2022, 10, 545–556. [Google Scholar] [CrossRef]
- Safety Data Sheet for Aqua Clear Resin by Phrozen. Available online: https://drive.google.com/file/d/1NR46rNJ1z3yM8TRqyZ5TOedubqhtr4II/view (accessed on 27 February 2023).
- Safety Data Sheet for Caffeine. Available online: https://www.sigmaaldrich.com/PL/en/product/sial/c0750 (accessed on 27 February 2023).
- Ye, Q.; Spencer, P.; Wang, Y.; Misra, A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J. Biomed. Mater. Res. Part A 2007, 80, 342–350. [Google Scholar] [CrossRef]
- Bochnia, J. A Study of the Mechanical Properties of Naturally Aged Photopolymers Printed Using the PJM Technology. Materials 2023, 16, 400. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Xu, W.; Yu, A.B.; Paul, D.R. Quantification of the Interface Interactions in Polymer Nanocomposites. Mater. Sci. Forum 2010, 654–656, 2608–2611. [Google Scholar] [CrossRef]
- Hu, K.; Kong, W.; Fu, X.; Zhou, C.; Lei, L. Resistivity optimization and properties of silver nanoparticles-filled alcohol-soluble conductive coating based on acrylic resin. High Perform. Polym. 2015, 27, 930–938. [Google Scholar] [CrossRef]
- Padmasini, N.; Umamaheswari, R.; Sikkandar, M.Y. Chapter 10-State-of-the-Art of Level-Set Methods in Segmentation and Registration of Spectral Domain Optical Coherence Tomographic Retinal Images. In Soft Computing Based Medical Image Analysis, 1st ed.; Dey, N., Ashour, A.S., Shi, F., Balas, V.E., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 163–181. [Google Scholar] [CrossRef]
- Davies, E.R. The Role of thresholding. In Computer Vision, 5th ed.; Davies, E.R., Ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Rashidi, B.; Rashidi, B. Implementation of a High Speed Technique for Character Segmentation of License Plate Based on Thresholding Algorithm. Int. J. Image Graph. Signal Process. 2012, 4, 9–18. [Google Scholar] [CrossRef]
- Chaubey, A.K. Comparison of the local and global thresholding methods in image segmentation. J. Res. Rev. 2016, 2, 1–4. [Google Scholar]

| Parameter | Value |
|---|---|
| Density | 1.11 g/cm3 |
| Melting point | Below 25 °C—liquid state at operating temperature |
| Solubility in alcohol | + |
| Parameter | Value |
|---|---|
| Density | 1.23 g/cm3 [21] |
| Melting point | 234–236.5 °C [21] |
| Maximum solubility in alcohol | 0.02 g/mL in 75 °C |
| Parameter | Value |
|---|---|
| Layer height | 0.05 mm |
| Bottom exposure time | 35 s |
| Transition layer count (TL) | 6 |
| Bottom layer count | 6 |
| Exposure time | 10.5 s |
| Transition type | linear |
| Rest time before lift | 0 s |
| Rest time after lift | 0 s |
| Rest time after retract | 4 s |
| Bottom lift distance | 6 mm |
| Lifting distance | 6 mm |
| Bottom lift speed | 150 mm/min |
| Bottom retract speed | 150 mm/min |
| Bottom layer compensation | a = 0 mm, b = −0.04 mm |
| Series | Sample | σm (MPa) | E (MPa) | εB (%) |
|---|---|---|---|---|
| (0) | 0.1–0.9 | 18.2 ± 1.9 | 834 ± 101 | 16.1 ± 4.0 |
| (1) | 1 | 12.6 | 1100 | 2.1 |
| 2 | 7.9 | 503 | 5.6 | |
| 3 | 8.5 | 438 | 9.6 | |
| (2) | 4 | 10.7 | 507 | 10.7 |
| 5 | 10.3 | 530 | 10.3 | |
| 6 | 9.9 | 480 | 10.0 | |
| (3) | 7 | 13.9 | 703 | 9.6 |
| 8 | 12.9 | 682 | 8.8 | |
| 9 | 12.3 | 612 | 11.9 | |
| (4) | 10 | 18.6 | 958 | 9.00 |
| 11 | 15.6 | 831 | 7.0 | |
| 12 | 16.3 | 857 | 7.0 |
| Series | Sample | m0 (g) | m1 (g) | m2 (g) | m3 (g) | CR (%) |
|---|---|---|---|---|---|---|
| 1 | 0.57 | 0.53 | 0.58 | 0.48 | 10.0 | |
| (1) | 2 | 1.90 | 1.88 | 2.02 | 1.76 | 5.9 |
| 3 | 1.88 | 1.86 | 2.01 | 1.76 | 5.1 | |
| 4 | 1.96 | 1.94 | 2.12 | 1.87 | 3.5 | |
| (2) | 5 | 1.97 | 1.95 | 2.13 | 1.87 | 3.8 |
| 6 | 1.95 | 1.92 | 2.10 | 1.86 | 3.6 | |
| 7 | 1.97 | 1.95 | 2.13 | 1.90 | 2.2 | |
| (3) | 8 | 1.98 | 1.96 | 2.13 | 1.91 | 2.3 |
| 9 | 1.96 | 1.94 | 2.12 | 1.89 | 2.4 | |
| 10 | 1.98 | 1.96 | 2.14 | 1.94 | 0.8 | |
| (4) | 11 | 1.98 | 1.96 | 2.13 | 1.93 | 1.2 |
| 12 | 1.96 | 1.94 | 2.11 | 1.92 | 1.1 |
| Series | Sample | Surface Caffeine— IKS (%) | Internal Caffeine— IKI (%) | All Caffeine—IK = IKS + IKI (%) | mK = m1 × IK (g) | CTCR (%) |
|---|---|---|---|---|---|---|
| 1 | 1.2 | 63.0 | 64.2 | 0.34 | 15.6 | |
| (1) | 2 | 0.6 | 89.6 | 90.2 | 1.69 | 6.5 |
| 3 | 0.1 | 80.4 | 80.5 | 1.50 | 6.3 | |
| 4 | 0.1 | 64.0 | 64.1 | 1.24 | 5.4 | |
| (2) | 5 | 0.5 | 69.8 | 70.3 | 1.37 | 5.4 |
| 6 | 0.2 | 58.5 | 58.8 | 1.13 | 6.1 | |
| 7 | 0.0 | 57.4 | 57.4 | 1.12 | 3.8 | |
| (3) | 8 | 0.0 | 71.4 | 71.4 | 1.40 | 3.2 |
| 9 | 0.2 | 56.8 | 57.0 | 1.10 | 4.1 | |
| 10 | 0.4 | 57.0 | 57.4 | 1.12 | 1.5 | |
| (4) | 11 | 0.2 | 60.1 | 60.3 | 1.18 | 2.1 |
| 12 | 0.1 | 58.0 | 58.1 | 1.13 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, D.; Wichniarek, R.; Kuczko, W. Caffeine–Acrylic Resin DLP-Manufactured Composite as a Modern Biomaterial. Designs 2023, 7, 49. https://doi.org/10.3390/designs7020049
Tomczak D, Wichniarek R, Kuczko W. Caffeine–Acrylic Resin DLP-Manufactured Composite as a Modern Biomaterial. Designs. 2023; 7(2):49. https://doi.org/10.3390/designs7020049
Chicago/Turabian StyleTomczak, Dorota, Radosław Wichniarek, and Wiesław Kuczko. 2023. "Caffeine–Acrylic Resin DLP-Manufactured Composite as a Modern Biomaterial" Designs 7, no. 2: 49. https://doi.org/10.3390/designs7020049
APA StyleTomczak, D., Wichniarek, R., & Kuczko, W. (2023). Caffeine–Acrylic Resin DLP-Manufactured Composite as a Modern Biomaterial. Designs, 7(2), 49. https://doi.org/10.3390/designs7020049







