The Effect of Oxygenated Turpentine Oil Additive in Diesel Fuel on the Performance and Emission Characteristics in One-Cylinder DI Engines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Setup
3. Results and Discussion
3.1. Physiochemical Properties
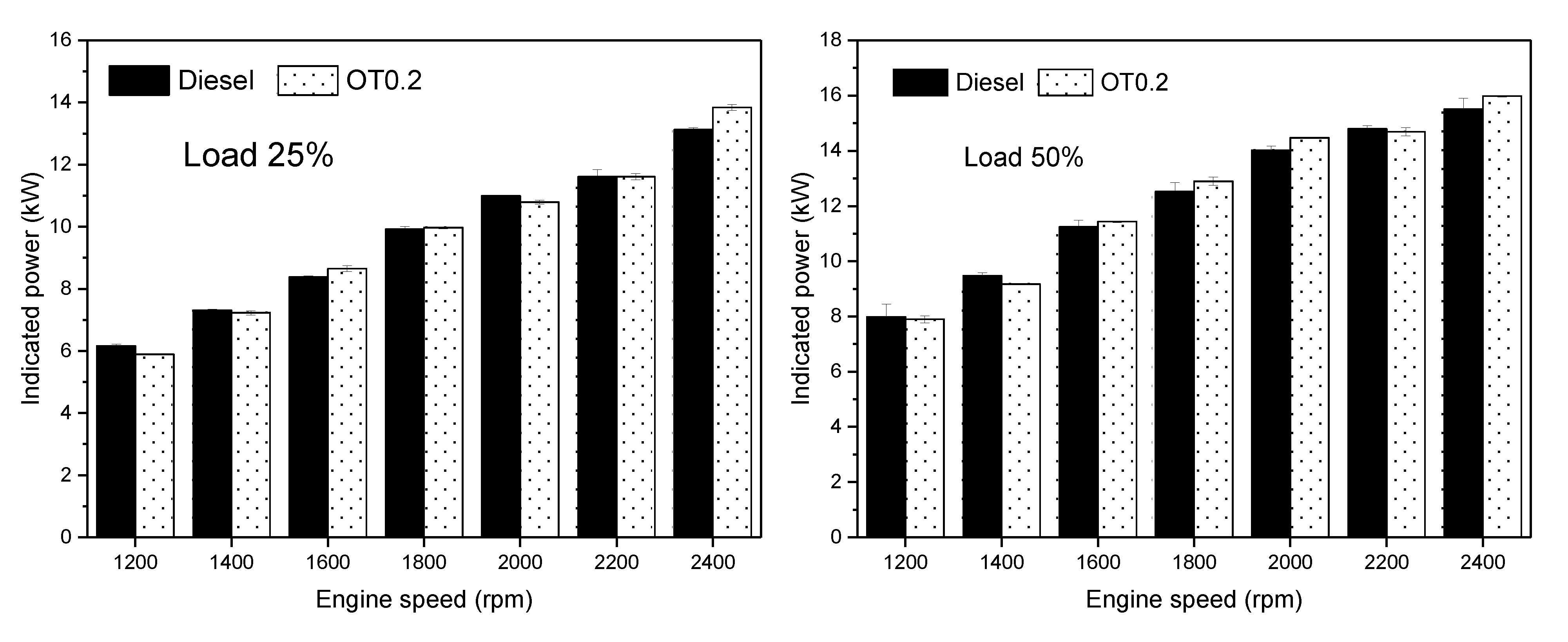
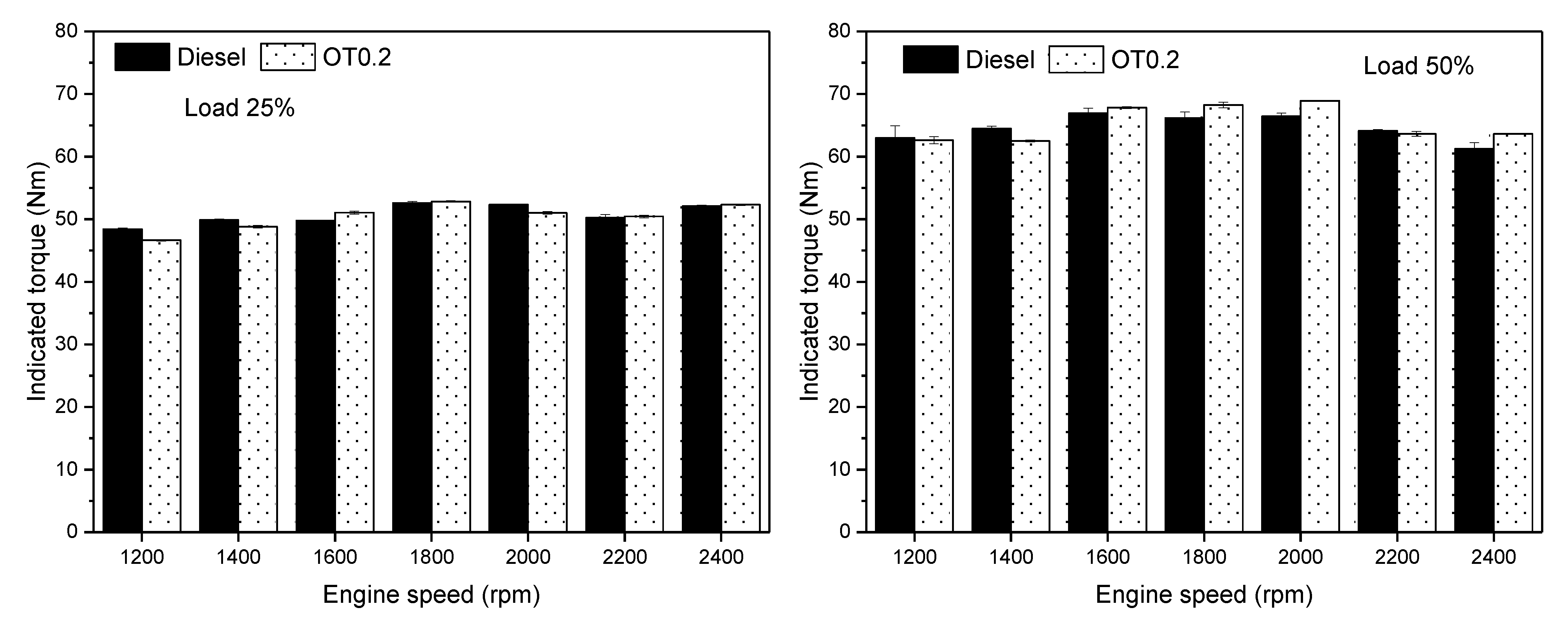
3.2. Engine Performance
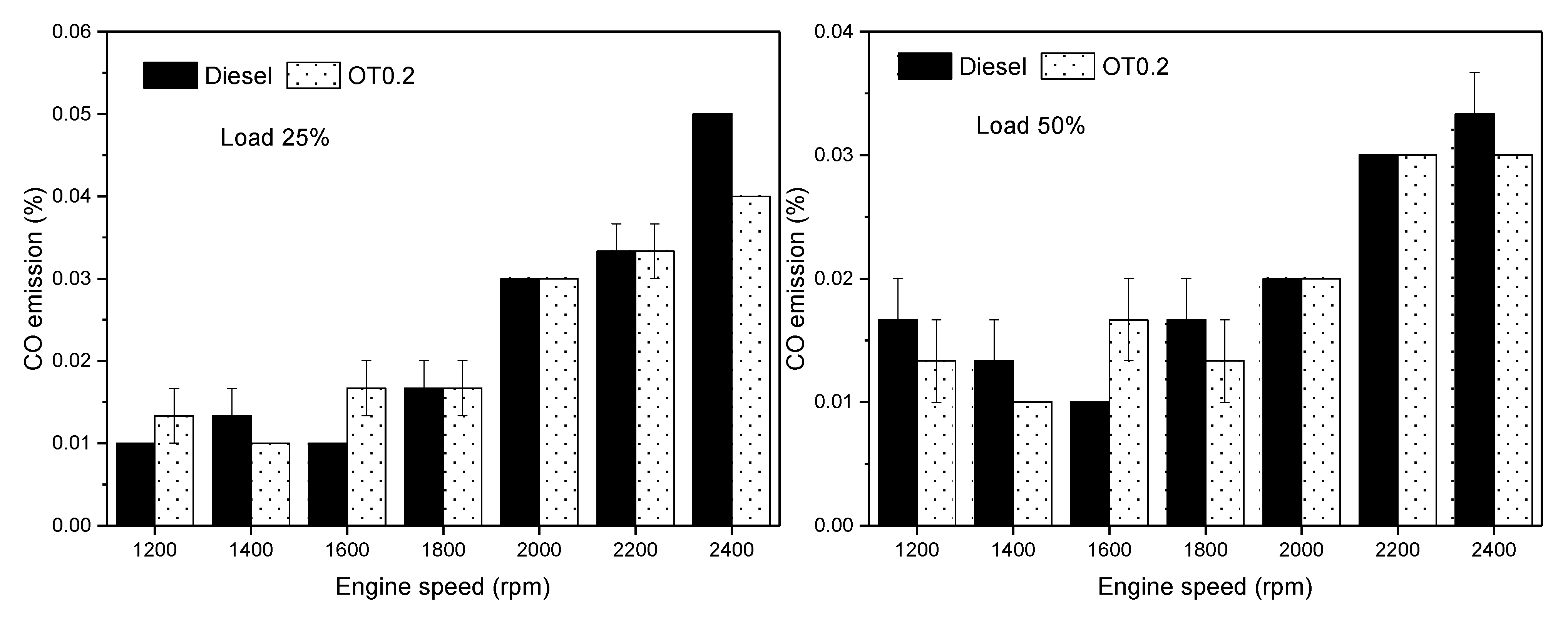
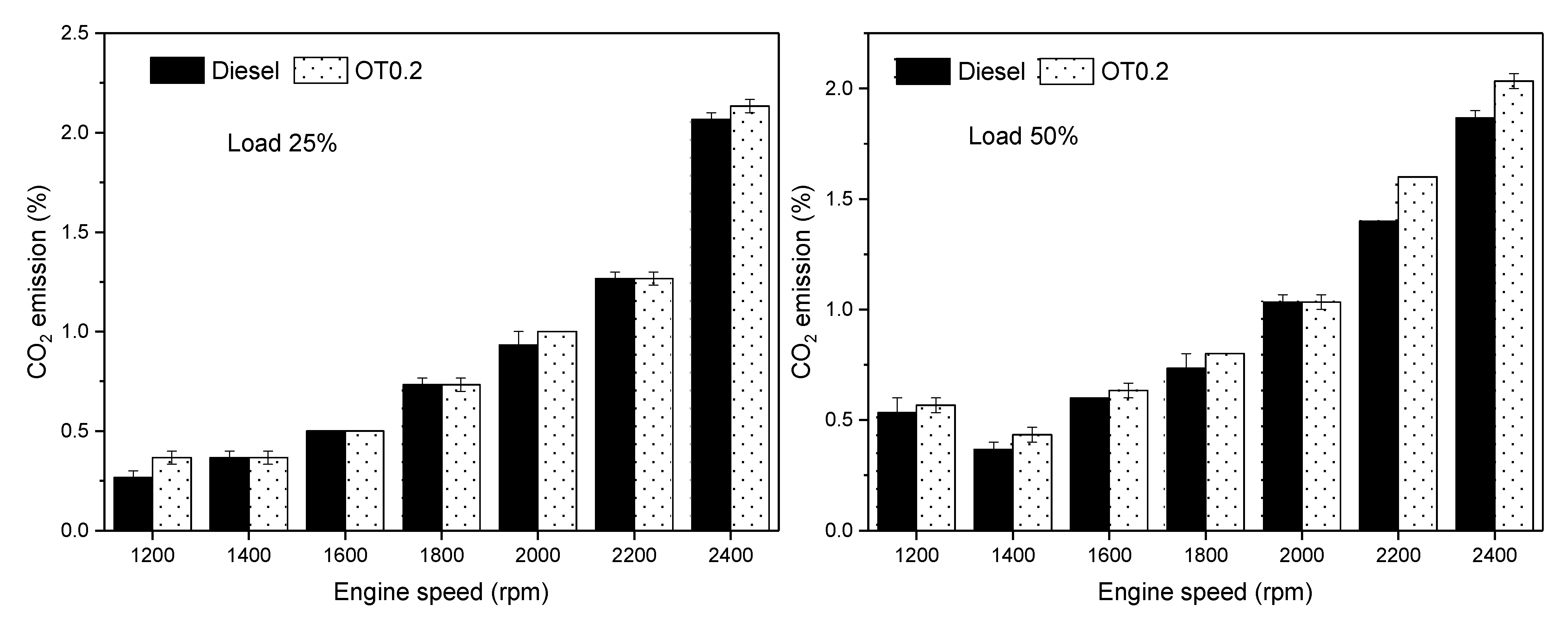
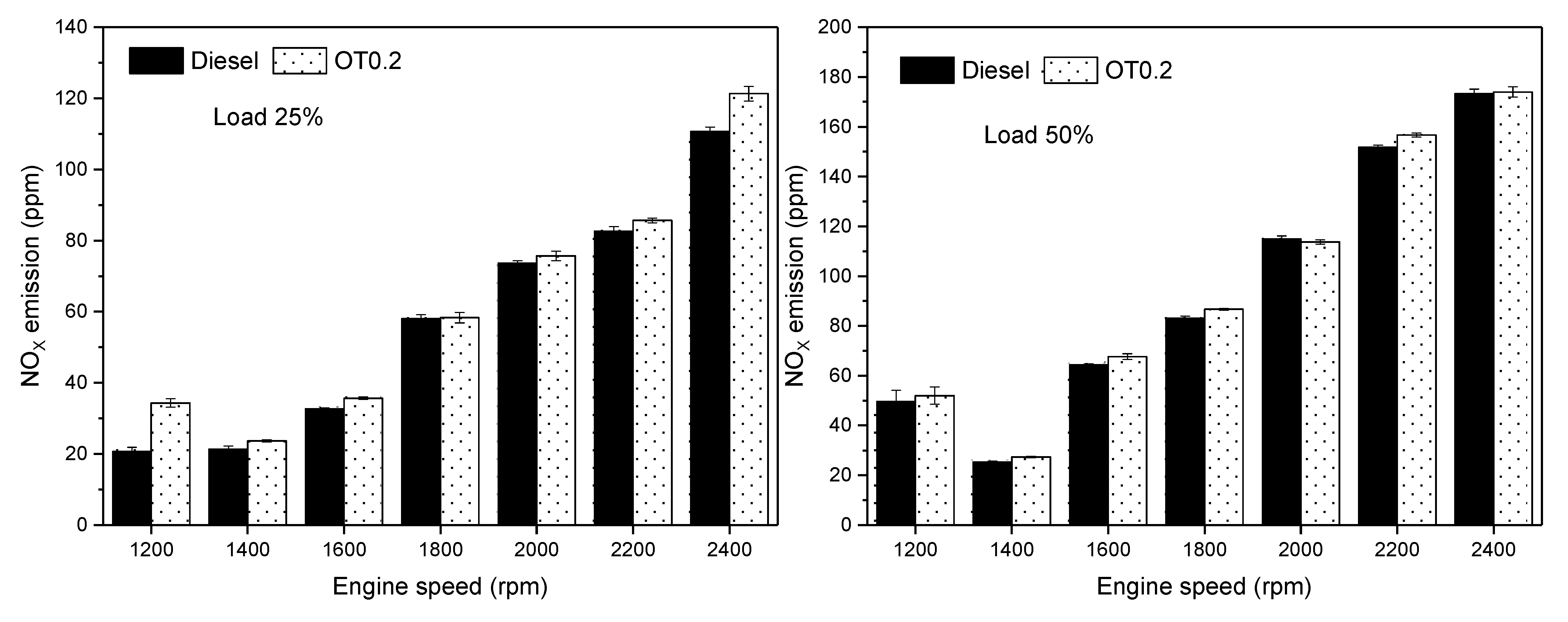
3.3. Gas Emissions
4. Conclusions
- The engine power shows slight increments, 0.7–1.1%, whereas the engine torque was slightly decreased using oxygenated turpentine oil-diesel fuel compared to diesel fuel in most conditions.
- The fuel flow rate was lower for OT0.2 compared to diesel in most conditions for low load. The enhancement rate of fuel flow while using an additive is between 5 and 9.09 percent.
- CO emission shows a slight increment when OT0.2 was used, 1.2% on average compared to diesel.
- CO2 emission increases with OT0.2 usage in diesel fuel up to 37.5%.
- NOX emission decreased by about 0.3–66% in addition to oxygenated turpentine in diesel compared to diesel fuel.
- The load applied to the engine could be increased to a high-level load;
- For wider understanding of the effect of oxygenated turpentine to the performance and emission, a larger volume of additive could be tested;
- The application of the additive could be tested in higher power and different types of engines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| OT0.2 | Diesel fuel + 0.2% of oxygenated turpentine oil |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| NOX | Nitrogen oxides |
| HC | Hydro carbons |
| DI | Direct injection |
References
- Kaimal, V.K.; Vijayabalan, P. A study on synthesis of energy fuel from waste plastic and assessment of its potential as an alternative fuel for diesel engines. Waste Manag. 2016, 51, 91–96. [Google Scholar] [CrossRef]
- Jayed, M.; Masjuki, H.; Kalam, M.; Mahlia, T.; Husnawan, M.; Liaquat, A. Prospects of dedicated biodiesel engine vehicles in Malaysia and Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 220–235. [Google Scholar] [CrossRef]
- Pilusa, T.J. The use of modified tyre derived fuel for compression ignition engines. Waste Manag. 2017, 60, 451–459. [Google Scholar] [CrossRef]
- Faussone, G.C. Transportation fuel from plastic: Two cases of study. Waste Manag. 2018, 73, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.; Park, H.; Bae, C. Intake air strategy for low HC and CO emissions in dual-fuel (CNG-diesel) premixed charge compression ignition engine. Appl. Energy 2018, 225, 1068–1077. [Google Scholar] [CrossRef]
- Ospina, G.; Selim, M.Y.; Al Omari, S.A.; Ali, M.I.H.; Hussien, A.M. Engine roughness and exhaust emissions of a diesel engine fueled with three biofuels. Renew. Energy 2018, 134, 1465–1472. [Google Scholar] [CrossRef]
- Amin, N.A.S.; Talebian-Kiakalaieh, A. Reduction of CO2 emission by INCAM model in Malaysia biomass power plants during the year 2016. Waste Manag. 2018, 73, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Møller, J.; Mønster, J.; Scheutz, C. Quantification of greenhouse gas emissions from a biological waste treatment facility. Waste Manag. 2017, 67, 375–384. [Google Scholar] [CrossRef] [PubMed]
- How, H.; Masjuki, H.; Kalam, M.; Teoh, Y.; Chuah, H. Effect of Calophyllum Inophyllum biodiesel-diesel blends on combustion, performance, exhaust particulate matter and gaseous emissions in a multi-cylinder diesel engine. Fuel 2018, 227, 154–164. [Google Scholar] [CrossRef]
- Hupponen, M.; Grönman, K.; Horttanainen, M. Areas on which to focus when seeking to reduce the greenhouse gas emissions of commercial waste management. A case study of a hypermarket, Finland. Waste Manag. 2018, 76, 1–18. [Google Scholar] [CrossRef]
- Röder, M.; Thornley, P. Waste wood as bioenergy feedstock. Climate change impacts and related emission uncertainties from waste wood based energy systems in the UK. Waste Manag. 2018, 74, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Huang, R.; Liao, J.; Ouyang, T.; Zheng, Z.; Lv, D.; Huang, H. Effect of EGR dilution on combustion, performance and emission characteristics of a diesel engine fueled with n-pentanol and 2-ethylhexyl nitrate additive. Energy Convers. Manag. 2018, 176, 246–255. [Google Scholar] [CrossRef]
- Allen, E.; Browne, J.; Hynes, S.; Murphy, J.D. The potential of algae blooms to produce renewable gaseous fuel. Waste Manag. 2013, 33, 2425–2433. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, L.; Fan, L.; Cao, L.; Zhou, Y.; Zhao, Y.; Liu, Y.; Ruan, R. Catalytic co-pyrolysis of waste vegetable oil and high density polyethylene for hydrocarbon fuel production. Waste Manag. 2017, 61, 276–282. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.A.V.; Ferreira, B.L.G.; Marques, L.G.d.; Murta, A.L.S.; de Freitas, M.A.V. Comparative study of NOx emissions of biodiesel-diesel blends from soybean, palm and waste frying oils using methyl and ethyl transesterification routes. Fuel 2017, 194, 144–156. [Google Scholar] [CrossRef]
- El Sheikh, K.; Khan, M.J.H.; Hamid, M.D.; Shrestha, S.; Ali, B.S.; Ryabov, G.A.; Dolgushin, L.A.; Hussain, M.A.; Bukharkina, T.V.; Golerova, E.A. Advances in reduction of NOx and N2O emission formation in an oxy-fired fluidized bed boiler. Chin. J. Chem. Eng. 2018, 27, 426–443. [Google Scholar] [CrossRef]
- Hu, N.; Tan, J.; Wang, X.; Zhang, X.; Yu, P. Volatile organic compound emissions from an engine fueled with an ethanol-biodiesel-diesel blend. J. Energy Inst. 2017, 90, 101–109. [Google Scholar] [CrossRef]
- Nayyar, A.; Sharma, D.; Soni, S.L.; Bhardwaj, B.; Augustine, M. Modeling and experimental investigation for performance and emissions on a diesel engine using bio-oxygenated ternary fuel blends. Energy 2019, 168, 136–150. [Google Scholar] [CrossRef]
- Manigandan, S.; Gunasekar, P.; Devipriya, J.; Nithya, S. Emission and injection characteristics of corn biodiesel blends in diesel engine. Fuel 2019, 235, 723–735. [Google Scholar] [CrossRef]
- Mueller, C.J.; Pitz, W.J.; Pickett, L.M.; Martin, G.C.; Siebers, D.L.; Westbrook, C.K. Effects of Oxygenates on Soot Processes in DI Diesel Engines: Experiments and Numerical Simulations; SAE Technical Paper 0148-7191; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Awad, O.I.; Mamat, R.; Ali, O.M.; Yusri, I.M.; Abdullah, A.A.; Yusop, A.F.; Noor, M.M. The effect of adding fusel oil to diesel on the performance and the emissions characteristics in a single cylinder CI engine. J. Energy Inst. 2017, 90, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Couth, R.; Trois, C. Carbon emissions reduction strategies in Africa from improved waste management: A review. Waste Manag. 2010, 30, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, D.; Sathiyagnanam, A.P.; Rana, D.; Saravanan, S.; Kumar, B.R.; Sethuramasamyraja, B. Effective utilization of waste plastic oil in a direct injection diesel engine using high carbon alcohols as oxygenated additives for cleaner emissions. Energy Convers. Manag. 2018, 166, 81–97. [Google Scholar] [CrossRef]
- Chong, C.T.; Ng, J.-H.; Ahmad, S.; Rajoo, S. Oxygenated palm biodiesel: Ignition, combustion and emissions quantification in a light-duty diesel engine. Energy Convers. Manag. 2015, 101, 317–325. [Google Scholar] [CrossRef]
- Ahmed, S.; Krumpelt, M. Hydrogen from hydrocarbon fuels for fuel cells. Int. J. Hydrog. Energy 2001, 26, 291–301. [Google Scholar] [CrossRef]
- Szybist, J.P.; Song, J.; Alam, M.; Boehman, A.L. Biodiesel combustion, emissions and emission control. Fuel Process. Technol. 2007, 88, 679–691. [Google Scholar] [CrossRef]
- Sastrohamidjojo, H. Kimia Minyak Atsiri; Universitas Gadjah Mada: Yogyakarta, Indonesia, 2004. [Google Scholar]
- Dubey, P.; Gupta, R. Influences of dual bio-fuel (Jatropha biodiesel and turpentine oil) on single cylinder variable compression ratio diesel engine. Renew. Energy 2018, 115, 1294–1302. [Google Scholar] [CrossRef]
- Dubey, P.; Gupta, R. Effects of dual bio-fuel (Jatropha biodiesel and turpentine oil) on a single cylinder naturally aspirated diesel engine without EGR. Appl. Therm. Eng. 2017, 115, 1137–1147. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Mahalakshmi, N.V. Performance and emission characteristics of a turpentine–diesel dual fuel engine. Energy 2007, 32, 1202–1209. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Anand, B.P.; Saravanan, C.; Srinivasan, C.A. Performance and exhaust emission of turpentine oil powered direct injection diesel engine. Renew. Energy 2010, 35, 1179–1184. [Google Scholar] [CrossRef]
- Anandavelu, K.; Alagumurthi, N.; Saravanan, C. Performance and emission evaluation of low heat rejection direct injection diesel engine fueled by diesel–turpentine oil blends. In Proceedings of the ASME 2010 International Mechanical Engineering Congress and Exposition, Vancouver, BC, Canada, 12–18 November 2010; pp. 1581–1587. [Google Scholar]
- Polonowski, C.J.; Mathur, V.K.; Naber, J.; Blough, J.R. Accelerometer Based Sensing of Combustion in a High Speed HPCR Diesel Engine; SAE Technical Paper 0148-7191; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Akbarian, E.; Najafi, B. A novel fuel containing glycerol triacetate additive, biodiesel and diesel blends to improve dual-fuelled diesel engines performance and exhaust emissions. Fuel 2019, 236, 666–676. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Zhang, Z.; Chen, J.; Pham, M.; Zhao, X.; Peng, Q.; Zhang, B.; Yin, Z. Performance and emission evaluation of a marine diesel engine fueled by water biodiesel-diesel emulsion blends with a fuel additive of a cerium oxide nanoparticle. Energy Convers. Manag. 2018, 169, 194–205. [Google Scholar]
- Butkus, A.; Pukalskas, S.; Bogdanovičius, Z. The influence of turpentine additive on the ecological parameters of diesel engines. Transport 2007, 22, 80–82. [Google Scholar] [CrossRef] [Green Version]
- Kadarohman, A.; Khoerunisa, F.H.; Astuti, R.M. Potency of clove oil and turpentine oil as a diesel fuel bioadditives and their performance on one cylinder engine. In Proceedings of the International Seminar on Chemistry, Jatinangor, Indonesia, 30–31 October 2008; pp. 30–31. [Google Scholar]
- Godiño, J.A.V.; Aguilar, F.J.J.-E.; García, M.T. Simulation of HCCI combustion in air-cooled off-road engines fuelled with diesel and biodiesel. J. Energy Inst. 2018, 91, 549–562. [Google Scholar] [CrossRef]
- Bednarski, M.; Orliński, P.; Wojs, M.K.; Sikora, M. Evaluation of methods for determining the combustion ignition delay in a diesel engine powered by liquid biofuel. J. Energy Inst. 2018, 92, 1107–1114. [Google Scholar] [CrossRef]
- Kannan, M.; Karthikeyan, R.; Deepanraj, B.; Baskaran, R. Feasibility and performance study of turpentine fueled DI diesel engine operated under HCCI combustion mode. J. Mech. Sci. Technol. 2014, 28, 729–737. [Google Scholar] [CrossRef]
- Berndt, T.; Böge, O.; Stratmann, F. Gas-phase ozonolysis of α-pinene: Gaseous products and particle formation. Atmos. Environ. 2003, 37, 3933–3945. [Google Scholar] [CrossRef]
- Kadarohman, A.; Rohman, I.; Kusrini, R.; Astuti, R.M. Combustion characteristics of diesel fuel on one cylinder diesel engine using clove oil, eugenol, and eugenyl acetate as fuel bio-additives. Fuel 2012, 98, 73–79. [Google Scholar] [CrossRef]
- Song, J.; Cheenkachorn, K.; Wang, J.; Perez, J.; Boehman, A.L.; Young, P.J.; Waller, F.J. Effect of oxygenated fuel on combustion and emissions in a light-duty turbo diesel engine. Energy Fuels 2002, 16, 294–301. [Google Scholar] [CrossRef]
- Choi, C.-Y.; Reitz, R.D. An experimental study on the effects of oxygenated fuel blends and multiple injection strategies on DI diesel engine emissions. Fuel 1999, 78, 1303–1317. [Google Scholar] [CrossRef]
- Fuel, D. Diesel Fuel And Exhaust Emissions; WHO Library Cataloguing: Geneva, Switzerland, 1996. [Google Scholar]
- Jonsson, Å.M.; Hallquist, M.; Ljungström, E. Impact of humidity on the ozone initiated oxidation of limonene, Δ3-carene, and α-pinene. Environ. Sci. Technol. 2006, 40, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chauhan, S.; Goel, V. Assessment of diesel engine combustion, performance and emission characteristics fuelled with dual fuel blends. Renew. Energy 2018, 125, 501–510. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Nallusamy, N.; Rajkumar, S. A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew. Sustain. Energy Rev. 2017, 79, 1134–1159. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Kalam, M.A.; Badruddin, I.; Banapurmath, N.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Vigneswaran, R.; Annamalai, K.; Dhinesh, B.; Krishnamoorthy, R. Experimental investigation of unmodified diesel engine performance, combustion and emission with multipurpose additive along with water-in-diesel emulsion fuel. Energy Convers. Manag. 2018, 172, 370–380. [Google Scholar] [CrossRef]
- Sahoo, R.R.; Jain, A. Experimental analysis of nanofuel additives with magnetic fuel conditioning for diesel engine performance and emissions. Fuel 2019, 236, 365–372. [Google Scholar] [CrossRef]
- Och, S.H.; Moura, L.M.; Mariani, V.C.; Coelho, L.d.S.; Velasquez, J.A.; Domingues, E. Volumetric efficiency optimization of a single-cylinder DI diesel engine using differential evolution algorithm. Appl. Therm. Eng. 2016, 108, 660–669. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; Teng, W.; Zhou, C.; Huang, R.; Liu, H.; Pan, M. Influence of n-butanol-diesel-PODE3-4 fuels coupled pilot injection strategy on combustion and emission characteristics of diesel engine. Fuel 2019, 236, 313–324. [Google Scholar] [CrossRef]
- Örs, I.; Sarıkoç, S.; Atabani, A.; Ünalan, S.; Akansu, S. The effects on performance, combustion and emission characteristics of DICI engine fuelled with TiO2 nanoparticles addition in diesel/biodiesel/n-butanol blends. Fuel 2018, 234, 177–188. [Google Scholar] [CrossRef]
- Yadav, A.K.; Dewangan, A.; Mallick, A. Effect of n-Butanol and Diethyl Ether on Performance and Emission Characteristics of a Diesel Engine Fueled with Diesel–Pongamia Biodiesel Blend. J. Energy Eng. 2018, 144, 4018062. [Google Scholar] [CrossRef]
- Basha, J.S. Impact of Carbon Nanotubes and Di-Ethyl Ether as additives with biodiesel emulsion fuels in a diesel engine–An experimental investigation. J. Energy Inst. 2016, 91, 289–303. [Google Scholar] [CrossRef]
- Sharma, G.; Lal, H. Effects of ethanol-gasoline blends on engine performance and exhaust emissions in a spark ignition. Int. J. Emerg. Technol. 2015, 6, 184. [Google Scholar]
- Bilgin, A.; Durgun, O.; Sahin, Z. The effects of diesel-ethanol blends on diesel engine performance. Energy Sources 2002, 24, 431–440. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Pyrc, M.; Gruca, M.; Kočiško, M. Study on co-combustion of diesel fuel with oxygenated alcohols in a compression ignition dual-fuel engine. Fuel 2018, 221, 329–345. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Ali, O.M.; Mamat, R.; Abdullah, A.A. Effect of emulsification and blending on the oxygenation and substitution of diesel fuel for compression ignition engine. Renew. Sustain. Energy Rev. 2017, 75, 1281–1294. [Google Scholar] [CrossRef] [Green Version]
- Redel-Macías, M.; Pinzi, S.; Leiva-Candia, D.; López, I.; Dorado, M. Ternary blends of diesel fuel oxygenated with ethanol and castor oil for diesel engines. Energy Procedia 2017, 142, 855–860. [Google Scholar] [CrossRef]
- Park, W.; Park, S.; Reitz, R.D.; Kurtz, E. The effect of oxygenated fuel properties on diesel spray combustion and soot formation. Combust. Flame 2017, 180, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Algayyim, S.J.M.; Wandel, A.P.; Yusaf, T.; Al-Lwayzy, S.; Hamawand, I. Impact of butanol-acetone mixture as a fuel additive on diesel engine performance and emissions. Fuel 2018, 227, 118–126. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Structured Catalysts and Reactors; CRC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Naha, S.; Aggarwal, S.K. Fuel effects on NOx emissions in partially premixed flames. Combust. Flame 2004, 139, 90–105. [Google Scholar] [CrossRef]
- Winer, W.O.; Bergles, A.E.; Klutke, G.A.; Wang, K.K.; Finnie, I.; Welty, J.R.; Bryant, M.D.; Yang, H.T.; Mow, V.C.; Leckie, F.A. Nanoindentation; Mechanical Engineering Series; Springer: New York, NY, USA, 2011. [Google Scholar]
- Balaji, G.; Cheralathan, M. Study of antioxidant effect on oxidation stability and emissions in a methyl ester of neem oil fuelled DI diesel engine. J. Energy Inst. 2014, 87, 188–195. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Q.; Teng, W.; Pan, M.; Liu, C.; Wang, Q. Improvement of combustion performance and emissions in diesel engines by fueling n-butanol/diesel/PODE3–4 mixtures. Appl. Energy 2018, 227, 38–48. [Google Scholar] [CrossRef]

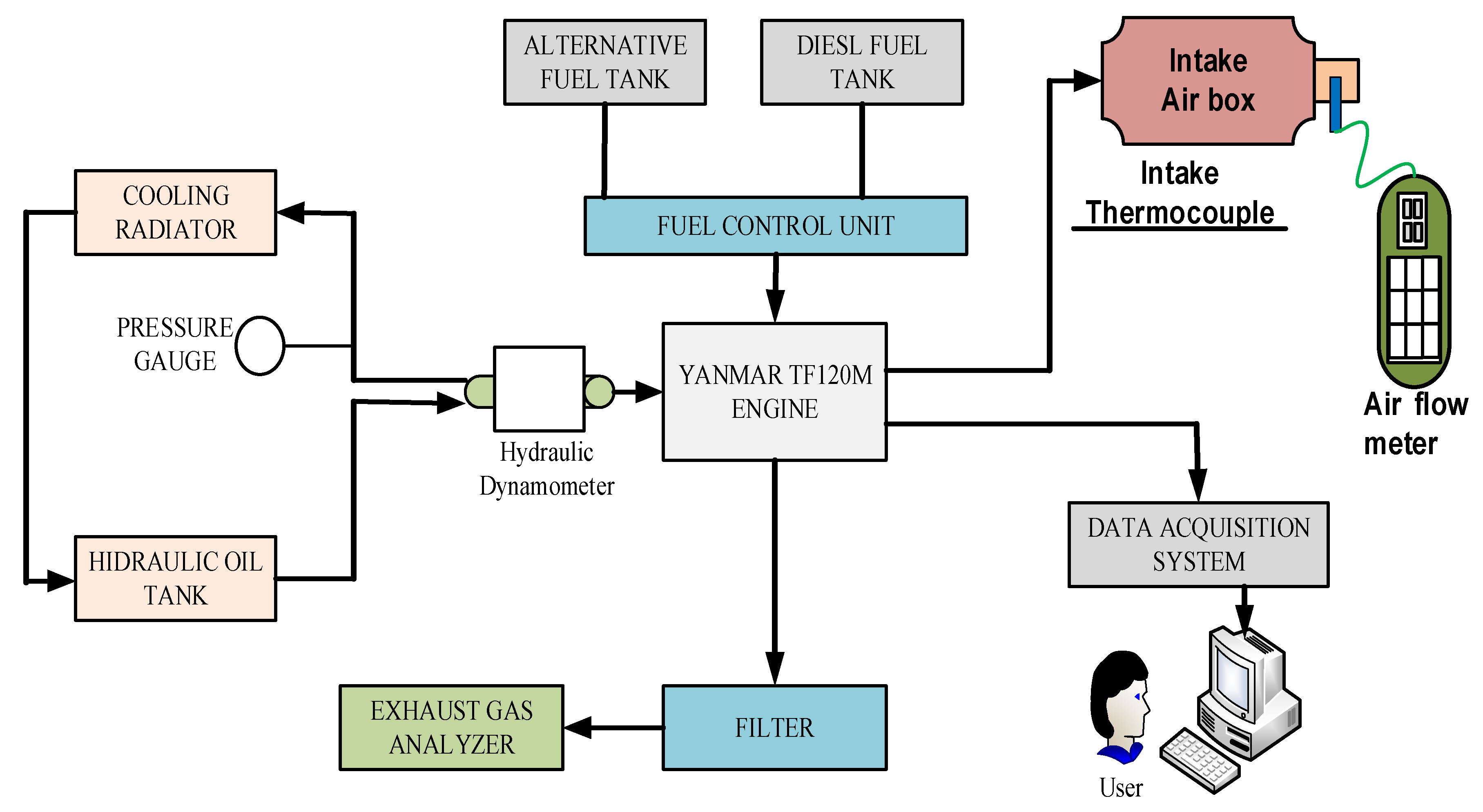

| Description | Specification |
|---|---|
| Engine model | YANMAR TF120M |
| Engine year | 2016 |
| Engine type | Horizontal, 4-cycle, 4 stroke, diesel engine |
| Number of cylinders | 1 |
| Continuous power output (kW) | 7.82 kW at 2400 rpm |
| Rated power output (kW) | 8.94 kW at 2400 rpm |
| Bore x Stroke (mm) | 92 × 96 |
| Displacement (L) | 0.638 |
| Injection timing | 17° BTDC |
| Compression ratio | 17.7 |
| Combustion system | Direct injection |
| Aspiration | Natural aspiration |
| Cooling system | Water-cooled |
| Starting system | Manual (Hand) Starting |
| Parameters | Diesel Fuel | OT0.2 | ASTM D975 Limit | |
|---|---|---|---|---|
| Min | Max | |||
| Specific Gravity at 25 °C (g/mL) | 0.8452 | 0.8549 | - | - |
| Specific Gravity at 15.55 °C (g/mL) | 0.8522 | - | 0.848 | 0.87 |
| API Gravity | 34.5408 | - | - | - |
| Anilin point (°F) | 156.2 | 159.2 | 129.6 | - |
| Index Diesel | 53.9527 | 51.3883 | - | - |
| Viscosities (cSt) | 3.7215 | 4.4625 | 1.3 | 4.5 |
| Flash Point (°C) | 61.89 | - | 60 | 80 |
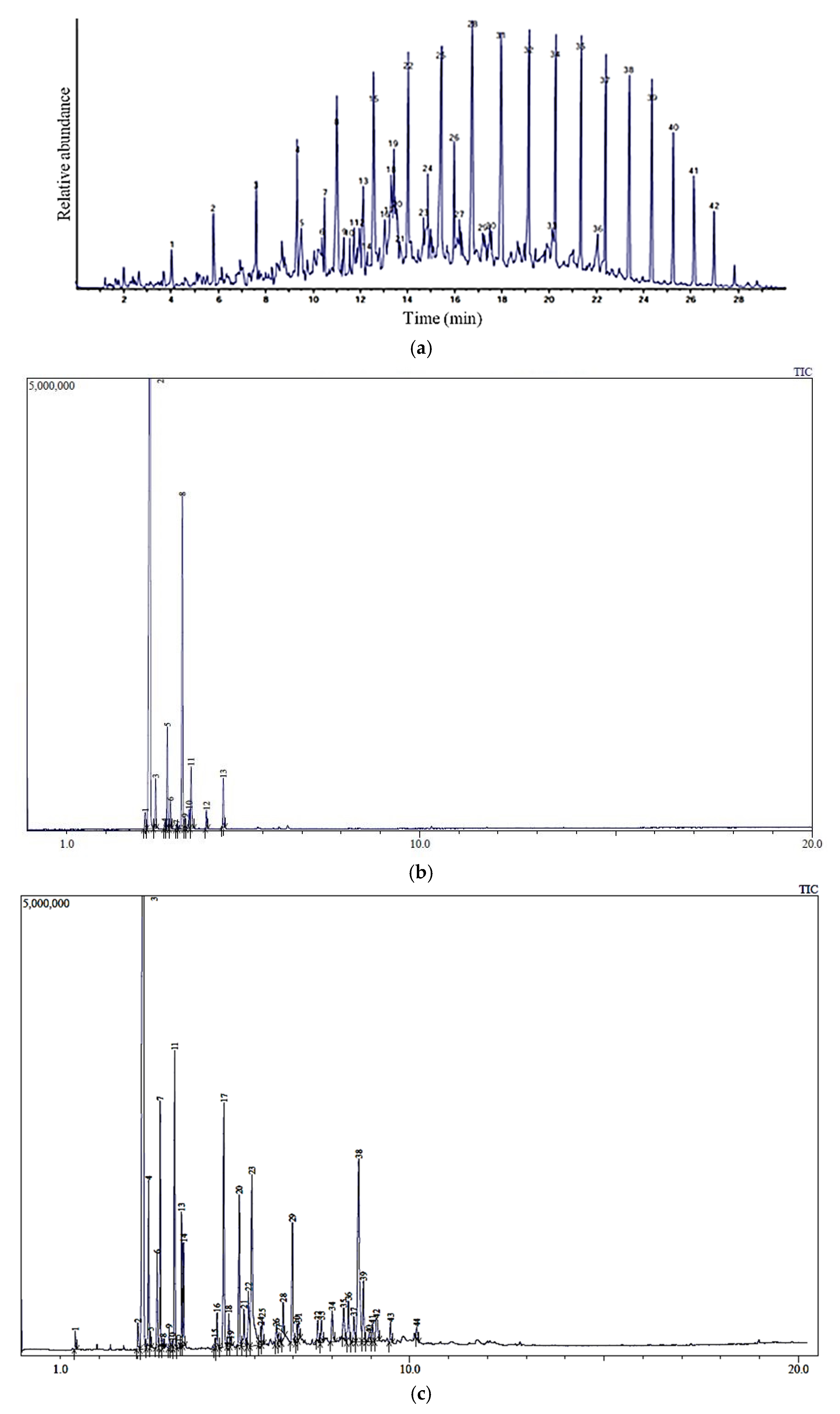
| Peak No | Molecular Formula | Name | Retention Time (Min) | Conc. (%) | Structure |
|---|---|---|---|---|---|
| 22 | C15H32 | Pentadecane | 14.050 | 5.27 | 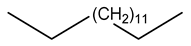 |
| 26 | C19H40 | 2,6,10,14-tetramethylpentadecane (pristane) | 15.993 | 2.44 |  |
| 28 | C16H34 | Hexadecane (n-cetane) | 16.769 | 10.67 | 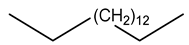 |
| 31 | C18H38 | n-octadecane | 17.980 | 7.47 | 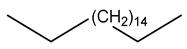 |
| 32 | C19H40 | n-nonadecane | 19.151 | 5.37 | 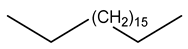 |
| 34 | C21H44 | n-heneicosane | 20.284 | 4.84 |  |
| 35 | C22H46 | n-docosane | 21.365 | 4.81 |  |
| 37 | C23H48 | n-tricosane | 22.404 | 4.07 |  |
| Peak No | Molecular Formula | Name | Retention Time (Min) | Conc. (%) | Structure |
|---|---|---|---|---|---|
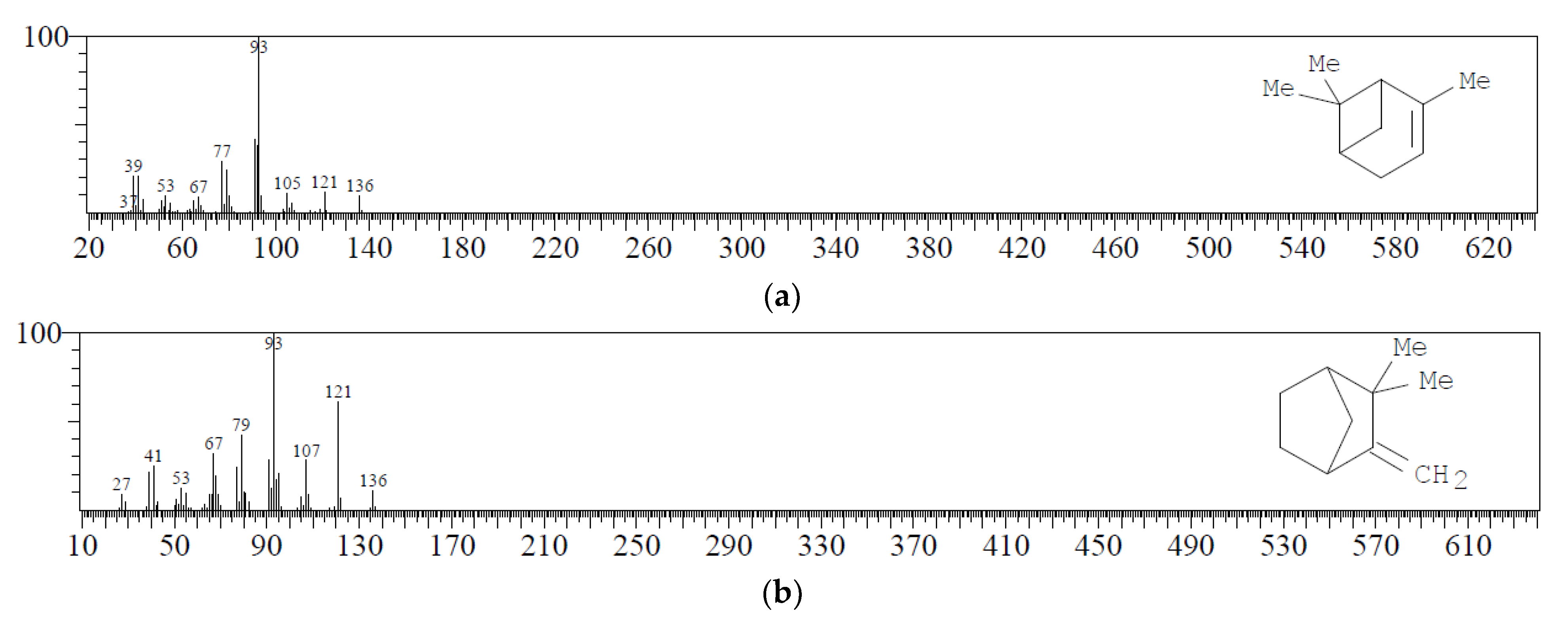
| 2 | C10H16 | Alpha-pinene | 3.127 | 61.81 | 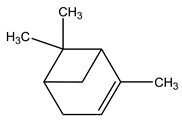 |
| 3 | C10H16 | Camphene | 3.267 | 2.25 | 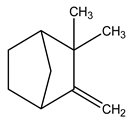 |
| 5 | C10H16 | Beta-pinene | 3.568 | 4.80 | 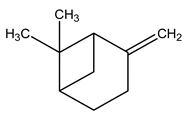 |
| 8 | C10H16 | Delta 3 Carene | 3.950 | 19.70 | 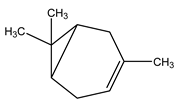 |
| 11 | C10H16 | dl-Limonene | 4.172 | 3.58 | 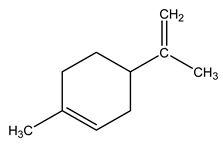 |
| 13 | C10H16 | Alpha-terpinolene | 4.993 | 2.49 | 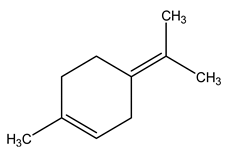 |
| Peak No | Molecular Formula | Name | Retention Time (Min) | Conc. (%) | Structure |
|---|---|---|---|---|---|
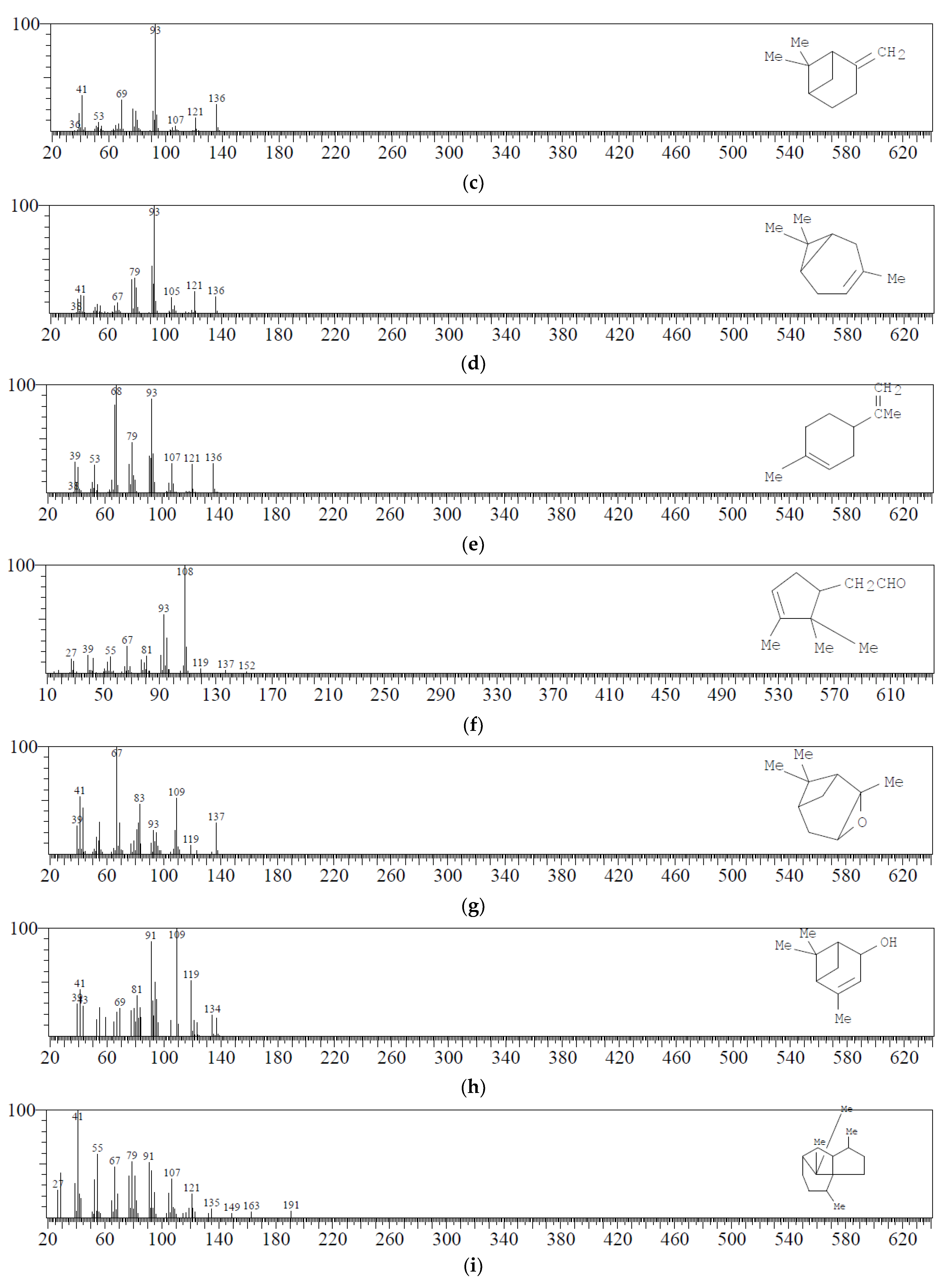
| 3 | C10H16 | Alpha-pinene | 3.143 | 32.68 | 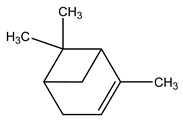 |
| 4 | C10H16 | Camphene | 3.272 | 2.94 | 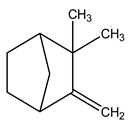 |
| 7 | C10H16 | Beta-pinene | 3.571 | 4.44 | 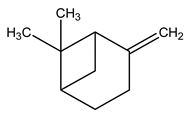 |
| 11 | C10H16 | Delta 3 Carene | 3.944 | 5.77 | 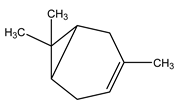 |
| 14 | C10H16 | dl-Limonene | 4.173 | 1.93 | 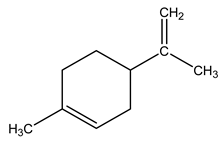 |
| 17 | C10H16O | Alpha-pinene oxide | 5.213 | 6.15 | 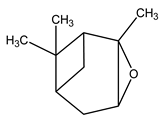 |
| 20 | C10H16O | Alpha-campholene aldehyde | 5.604 | 3.59 | 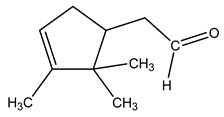 |
| 23 | C10H16O | Trans-verbenol | 5.932 | 6.66 | 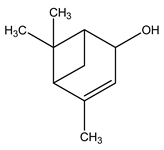 |
| 29 | C10H14O | Verbenone | 6.974 | 3.11 |  |
| 38 | C15H26 | Patchoulane | 8.684 | 8.29 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadarohman, A.; Khoerunnisa, F.; Sapee, S.; Eko Sardjono, R.; Izzudin, I.; Hendrawan; Mamat, R.; Yusop, A.F.; Erdiwansyah; Yusaf, T. The Effect of Oxygenated Turpentine Oil Additive in Diesel Fuel on the Performance and Emission Characteristics in One-Cylinder DI Engines. Designs 2021, 5, 73. https://doi.org/10.3390/designs5040073
Kadarohman A, Khoerunnisa F, Sapee S, Eko Sardjono R, Izzudin I, Hendrawan, Mamat R, Yusop AF, Erdiwansyah, Yusaf T. The Effect of Oxygenated Turpentine Oil Additive in Diesel Fuel on the Performance and Emission Characteristics in One-Cylinder DI Engines. Designs. 2021; 5(4):73. https://doi.org/10.3390/designs5040073
Chicago/Turabian StyleKadarohman, Asep, Fitri Khoerunnisa, Syazwana Sapee, Ratnaningsih Eko Sardjono, Izuan Izzudin, Hendrawan, Rizalman Mamat, Ahmad Fitri Yusop, Erdiwansyah, and Talal Yusaf. 2021. "The Effect of Oxygenated Turpentine Oil Additive in Diesel Fuel on the Performance and Emission Characteristics in One-Cylinder DI Engines" Designs 5, no. 4: 73. https://doi.org/10.3390/designs5040073
APA StyleKadarohman, A., Khoerunnisa, F., Sapee, S., Eko Sardjono, R., Izzudin, I., Hendrawan, Mamat, R., Yusop, A. F., Erdiwansyah, & Yusaf, T. (2021). The Effect of Oxygenated Turpentine Oil Additive in Diesel Fuel on the Performance and Emission Characteristics in One-Cylinder DI Engines. Designs, 5(4), 73. https://doi.org/10.3390/designs5040073








