Development of a Neural Network to Predict Optimal IOP Reduction in Glaucoma Management
Abstract
1. Introduction
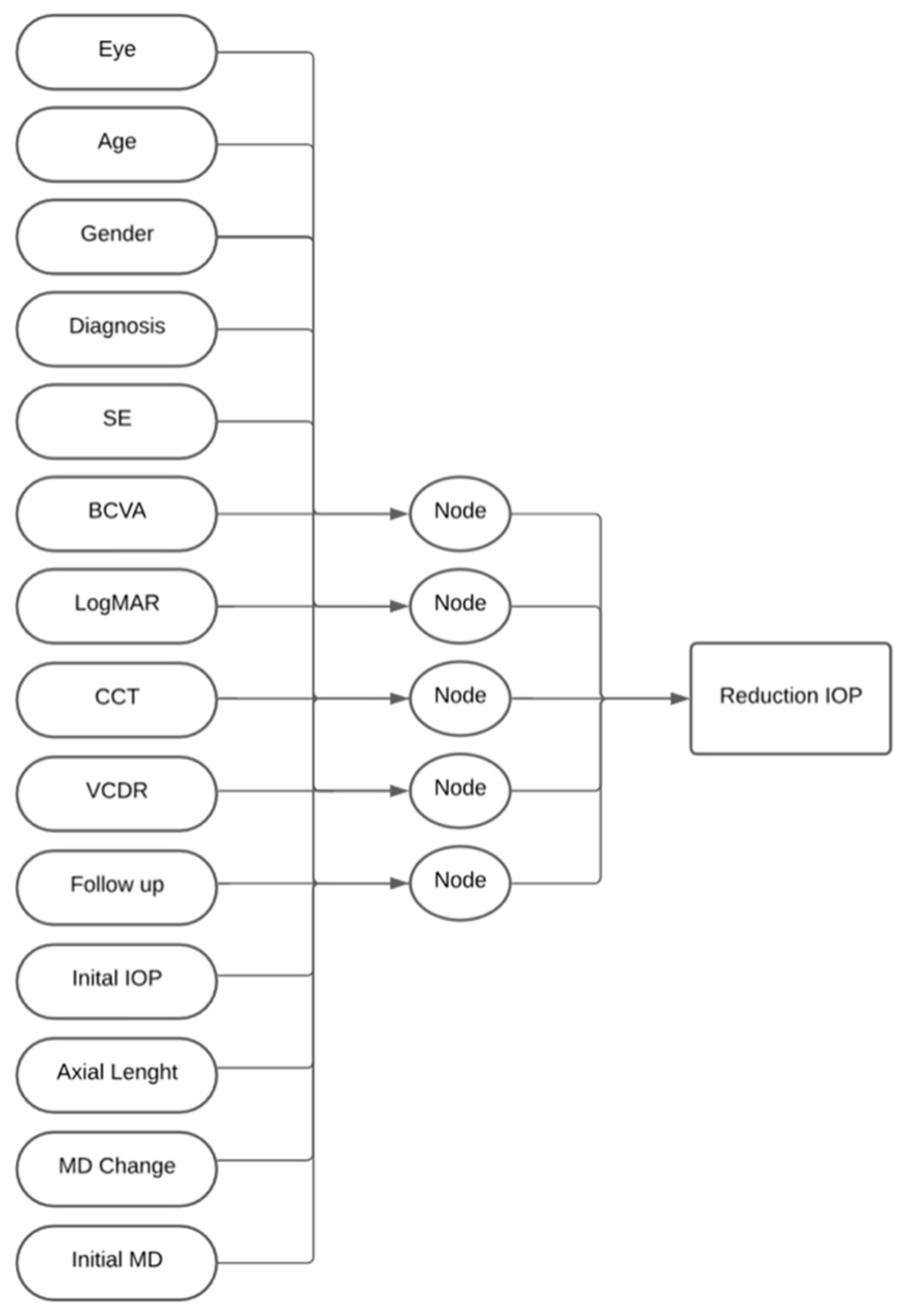
2. Materials and Methods
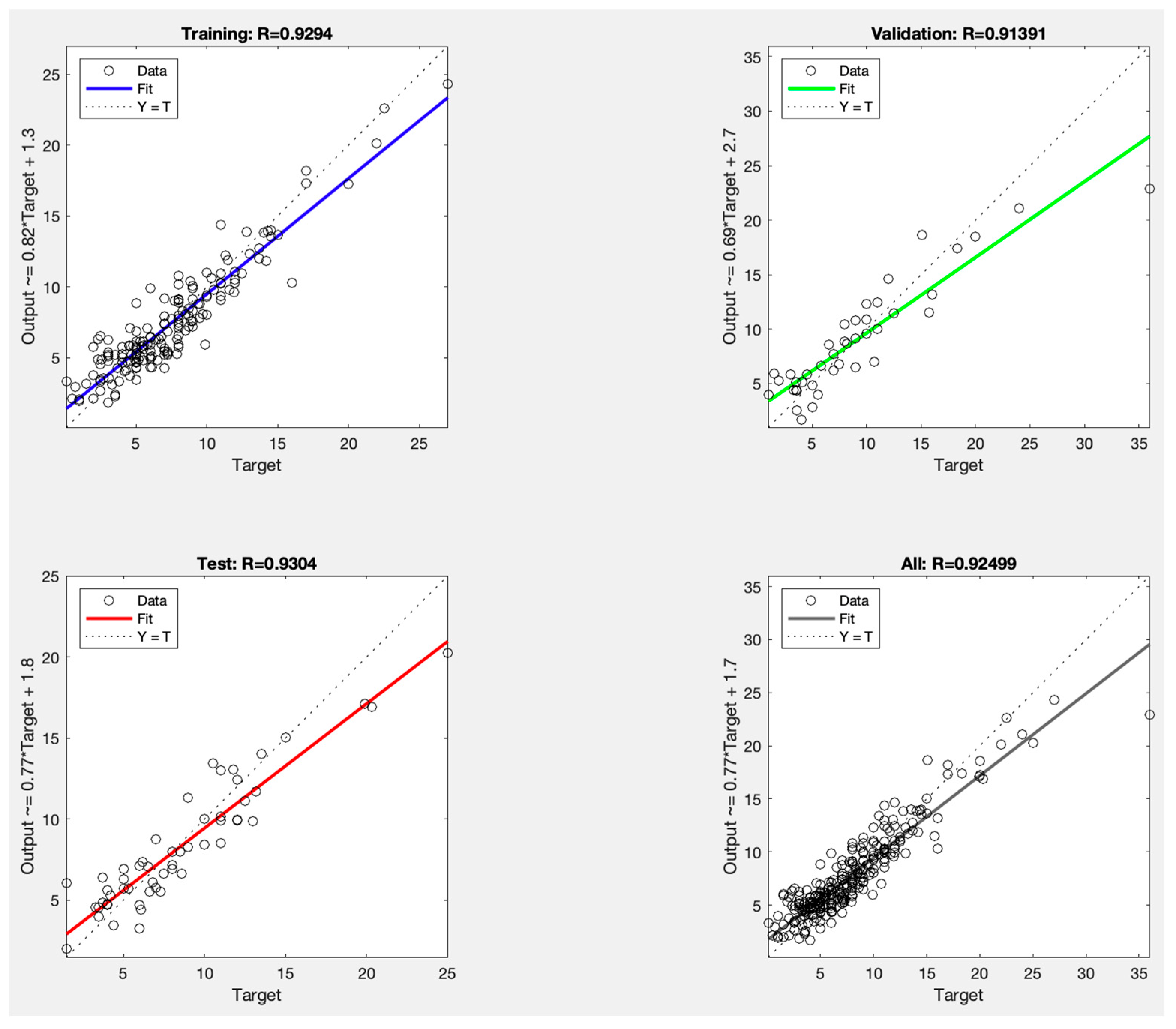
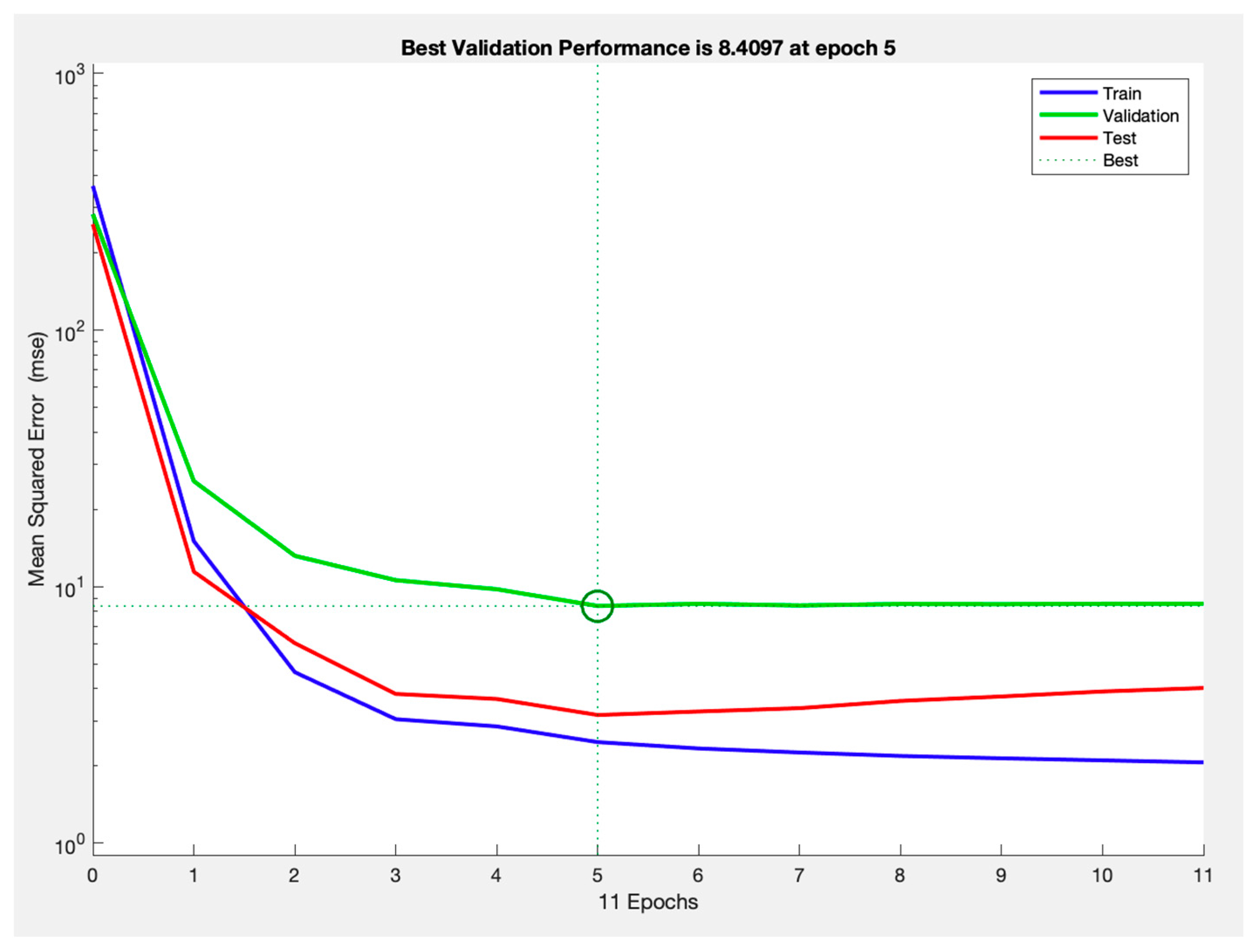
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCVA | Best-Corrected Visual Acuity |
| CAV1/CAV2 | Caveolin 1/Caveolin 2 |
| CCT | Central Corneal Thickness |
| CNTGS | Collaborative Normal-Tension Glaucoma Study |
| CYP1B1 | Cytochrome P450 Family 1 Subfamily B Member 1 |
| EMGT | Early Manifest Glaucoma Trial |
| IOP | Intraocular Pressure |
| LogMAR | Logarithm of the Minimum Angle of Resolution |
| LOFO | Leave-One-Feature-Out |
| LTBP2 | Latent Transforming Growth Factor Beta Binding Protein 2 |
| MD | Mean Deviation (on Visual Field) |
| MYOC | Myocilin |
| NTG | Normal-Tension Glaucoma |
| OPTN | Optineurin |
| POAG | Primary Open-Angle Glaucoma |
| RMSE | Root Mean Squared Error |
| SE | Spherical Equivalence |
| VF | Visual Field |
| VCDR | Vertical Cup-to-Disk Ratio |
| ZING | Zero IOP-Induced Nerve Ganglionopathy |
References
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hussein, M. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Normal Tension Glaucoma, S. Collaborative normal tension glaucoma study. Curr. Opin. Ophthalmol. 2003, 14, 86–90. [Google Scholar] [CrossRef]
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105 (Suppl. 1), 1–169. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L. Primary Open-Angle Glaucoma Preferred Practice Pattern(R). Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef]
- Anderson, D.R.; Drance, S.M.; Schulzer, M.; Collaborative Normal-Tension Glaucoma Study, G. Natural history of normal-tension glaucoma. Ophthalmology 2001, 108, 247–253. [Google Scholar] [CrossRef]
- Prum, B.E., Jr.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern® Guidelines. Ophthalmology 2016, 123, P41–P111. [Google Scholar] [CrossRef] [PubMed]
- Trivli, A.; Koliarakis, I.; Terzidou, C.; Siganos, C.S.; Dalianis, G.; Detorakis, E.T.; Goulielmos, G.N.; Spandidos, D.A. Normal-tension glaucoma: Pathogenesis and genetics. Exp. Ther. Med. 2019, 17, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Jammal, A.A.; Thompson, A.C. From Machine to Machine: An OCT-Trained Deep Learning Algorithm for Objective Quantification of Glaucomatous Damage in Fundus Photographs. Ophthalmology 2019, 126, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, R.; Murata, H.; Hirasawa, K.; Fujino, Y.; Matsuura, M.; Miki, A.; Kanamoto, T.; Ikeda, Y.; Mori, K.; Iwase, A.; et al. Using Deep Learning and Transfer Learning to Accurately Diagnose Early-Onset Glaucoma From Macular Optical Coherence Tomography Images. Am. J. Ophthalmol. 2019, 198, 136–145. [Google Scholar] [CrossRef]
- Acharya, U.R.; Acharya, U.R.; Ng, E.Y.; Eugene, L.W.; Noronha, K.; Min, L.C.; Nayak, K.; Bhandary, S.V. Decision support system for the glaucoma using Gabor transformation. Biomed. Signal Process. Control. 2015, 15, 18–26. [Google Scholar] [CrossRef]
- Anton, N.; Doroftei, B.; Curteanu, S.; Catãlin, L.; Ilie, O.-D.; Târcoveanu, F.; Bogdănici, C.M. Comprehensive Review on the Use of Artificial Intelligence in Ophthalmology and Future Research Directions. Diagnostics 2022, 13, 100. [Google Scholar] [CrossRef]
- Qiu, C.; Qian, S.; Sun, X.; Zhou, C.; Meng, F. Axial Myopia Is Associated with Visual Field Prognosis of Primary Open-Angle Glaucoma. PLoS ONE 2015, 10, e0133189. [Google Scholar] [CrossRef]
- Hemmati-Sarapardeh, A.; Larestani, A.; Nait Amar, M.; Hajirezaie, S. Training and optimization algorithms. In Applications of Artificial Intelligence Techniques in the Petroleum Industry; Gulf Professional Publishing: Houston, TX, USA, 2020; pp. 51–78. [Google Scholar]
- Molnar, C. Interpretable Machine Learning: A Guide for Making Black Box Models Explainable; Illustrations; Leanpub: Victoria, BC, Canada, 2019; 314p. [Google Scholar]
- Power, L.; Guha, K. Feature Importance and Explainability in Quantum Machine Learning. arXiv 2024, arXiv:2405.08917. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. Why Should I Trust You? In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016.
- Bengtsson, B.; Heijl, A. Diurnal IOP fluctuation: Not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 513–518. [Google Scholar] [CrossRef]
- Crabb, D.P.; Garway-Heath, D.F. Intervals between visual field tests when monitoring the glaucomatous patient: Wait-and-see approach. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2770–2776. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chen, R.I.; Lin, S.C. Myopia and glaucoma: Sorting out the difference. Curr. Opin. Ophthalmol. 2015, 26, 90–95. [Google Scholar] [CrossRef]
- Kwon, J.; Sung, K.R.; Park, J.M. Myopic glaucomatous eyes with or without optic disc shape alteration: A longitudinal study. Br. J. Ophthalmol. 2017, 101, 1618–1622. [Google Scholar] [CrossRef]
- Abrams, L.S.; Scott, I.U.; Spaeth, G.L.; Quigley, H.A.; Varma, R. Agreement among optometrists, ophthalmologists, and residents in evaluating the optic disc for glaucoma. Ophthalmology 1994, 101, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.; Ong, D.N.; Kong, Y.X.; O’NEill, E.C.; Pandav, S.S.; Coote, M.A.; Crowston, J.G. Glaucomatous optic neuropathy evaluation (GONE) project: The effect of monoscopic versus stereoscopic viewing conditions on optic nerve evaluation. Am. J. Ophthalmol. 2014, 157, 936–944. [Google Scholar] [CrossRef]
- Jampel, H.D.; Friedman, D.; Quigley, H.; Vitale, S.; Miller, R.; Knezevich, F.; Ding, Y. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am. J. Ophthalmol. 2009, 147, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Swanson, W.H.; Garway-Heath, D.F. ‘Structure-function relationship’ in glaucoma: Past thinking and current concepts. Clin. Exp. Ophthalmol. 2012, 40, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Ramspek, C.L.; Jager, K.J.; Dekker, F.W.; Zoccali, C.; van Diepen, M. External validation of prognostic models: What, why, how, when and where? Clin. Kidney J. 2021, 14, 49–58. [Google Scholar] [CrossRef]
- Varma, R.; Steinmann, W.C.; Scott, I.U. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology 1992, 99, 215–221. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segrè, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Wiggs, J.L. Genetic etiologies of glaucoma. Arch. Ophthalmol. 2007, 125, 30–37. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Osman, E.A.; Mousa, A.; Wheeler, J.; Whigham, B.; Allingham, R.R.; Hauser, M.A.; Al-Obeidan, S.A. Screening of CYP1B1 and LTBP2 genes in Saudi families with primary congenital glaucoma: Genotype-phenotype correlation. Mol. Vis. 2011, 17, 2911–2919. [Google Scholar] [PubMed]
- Passan, S.; Goyal, S.; Bhat, M.A.; Singh, D.; Vanita, V. Association of TNF-alpha gene alterations (c.-238G>A, c.-308G>A, c.-857C>T, c.-863C>A) with primary glaucoma in north Indian cohort. Gene 2019, 709, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Hui, F.; Quintero, H.; El Hajji, S.; Bell, K.; Di Polo, A.; Williams, P.A. Neuroprotection in glaucoma: Mechanisms beyond intraocular pressure lowering. Mol. Asp. Med. 2023, 92, 101193. [Google Scholar] [CrossRef]
- Hofer, I.S.; Burns, M.; Kendale, S.; Wanderer, J.P. Realistically Integrating Machine Learning Into Clinical Practice: A Road Map of Opportunities, Challenges, and a Potential Future. Anesth. Analg. 2020, 130, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
| RMSE Average | r | |
|---|---|---|
| Training | 1.90 ± 0.29 | 0.92 ± 0.02 |
| Validation | 2.18 ± 0.34 | 0.88 ± 0.02 |
| Test | 2.11 ± 0.30 | 0.88 ± 0.04 |
| RMSE | r | |
|---|---|---|
| Training | 1.57 | 0.93 |
| Validation | 2.90 | 0.91 |
| Test | 1.77 | 0.93 |
| RMSE | RMSE Increase | p-Value | |
|---|---|---|---|
| Initial IOP | 4.56 ± 0.96 | 2.45 | <0.001 |
| Axial length | 2.74 ± 0.52 | 0.63 | <0.01 |
| CCT | 2.65 ± 0.34 | 0.54 | <0.001 |
| Glaucoma Diagnosis | 2.63 ± 0.67 | 0.52 | 0.04 |
| VCDR | 2.59 ± 0.49 | 0.48 | 0.01 |
| SE | 2.58 ± 0.47 | 0.47 | <0.01 |
| MD Data | 2.43 ± 0.38 | 0.32 | 0.04 |
| Eye laterality | 2.38 ± 0.41 | 0.27 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remtulla, R.; Rahman, S.; Saheb, H. Development of a Neural Network to Predict Optimal IOP Reduction in Glaucoma Management. Vision 2025, 9, 87. https://doi.org/10.3390/vision9040087
Remtulla R, Rahman S, Saheb H. Development of a Neural Network to Predict Optimal IOP Reduction in Glaucoma Management. Vision. 2025; 9(4):87. https://doi.org/10.3390/vision9040087
Chicago/Turabian StyleRemtulla, Raheem, Sidrat Rahman, and Hady Saheb. 2025. "Development of a Neural Network to Predict Optimal IOP Reduction in Glaucoma Management" Vision 9, no. 4: 87. https://doi.org/10.3390/vision9040087
APA StyleRemtulla, R., Rahman, S., & Saheb, H. (2025). Development of a Neural Network to Predict Optimal IOP Reduction in Glaucoma Management. Vision, 9(4), 87. https://doi.org/10.3390/vision9040087





