Too Bright to Focus? Influence of Brightness Illusions and Ambient Light Levels on the Dynamics of Ocular Accommodation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup and Apparatus
- (i)
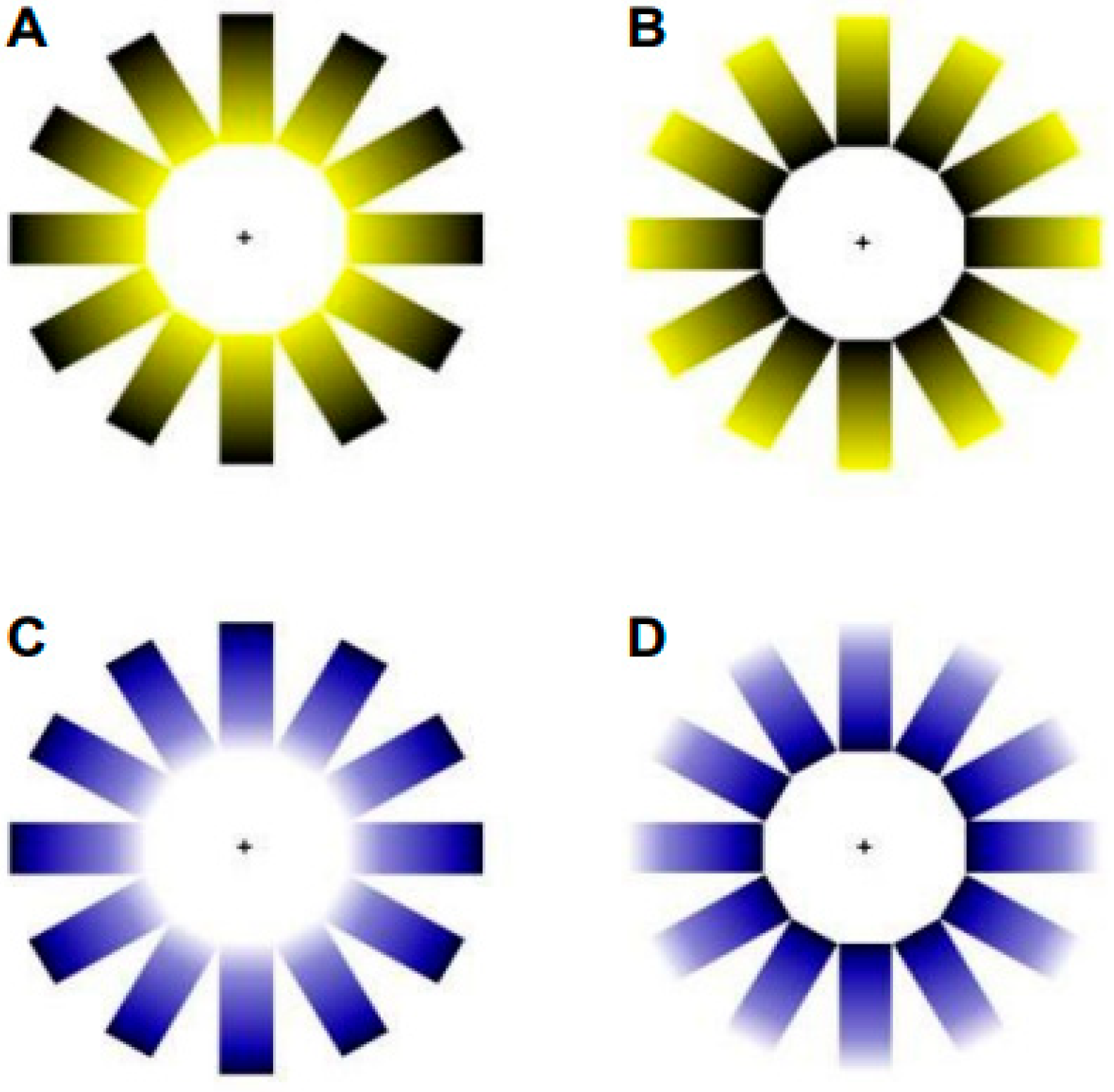
- Illusion of brightness and color. The brightness illusion was induced using an adapted version of the Asahi brightness illusion [5]. Two levels of brightness were presented (with and without the brightness illusion) and two stimulus colors (blue and yellow), resulting in a total of four stimuli (see Figure 1).
- (ii)
- Contrast luminance. Room luminance was adjusted relative to the screen brightness to modify the contrast ratio, resulting in two experimental conditions: a low contrast ratio, when the room was illuminated (photopic ambient condition); and a high contrast ratio, when the room lights were turned off, leaving only ambient light emitted from the screen (mesopic ambient condition) (see Figure 2). According to the guidelines of the Illuminating Engineering Society of North America (IESNA) and the National Board for Industrial and Technical Development (NUTEK), a moderate contrast ratio between the ambient environment and the light source (in this case, the screen) was defined as falling within the range of 3:1 to 10:1. A contrast ratio below 3:1 was classified as a low luminance contrast condition, whereas a ratio above 10:1 was classified as a high luminance contrast condition. Illuminance for the source and the background was obtained using a calibrated luxmeter (model PCE-L335), positioned perpendicularly at both the monitor and the background wall. The illuminance of the source was 430 lx and 40 lx for the photopic and mesopic ambient conditions, respectively. The background illuminance was 295 lx under the photopic ambient condition and 0.05 lx under the mesopic ambient condition. To convert illuminance to luminance, the following formula was applied: L = E × R/π, where E represents the measured illuminance and R the reflectance factor. A reflectance value of 0.03 was used for the source (matte, non-glossy screen), and 0.8 for the background (matte white wall). Finally, the contrast ratio was calculated as Lsource/Lbackground [14,15]. Accordingly, a contrast ratio of 0.05:1 (low contrast) was obtained for the photopic ambient condition, and 30:1 (high contrast) for the mesopic ambient condition.
2.3. Procedure
(Residual refractive error at far distance − Accommodative response).
2.4. Statistical Analysis
3. Results
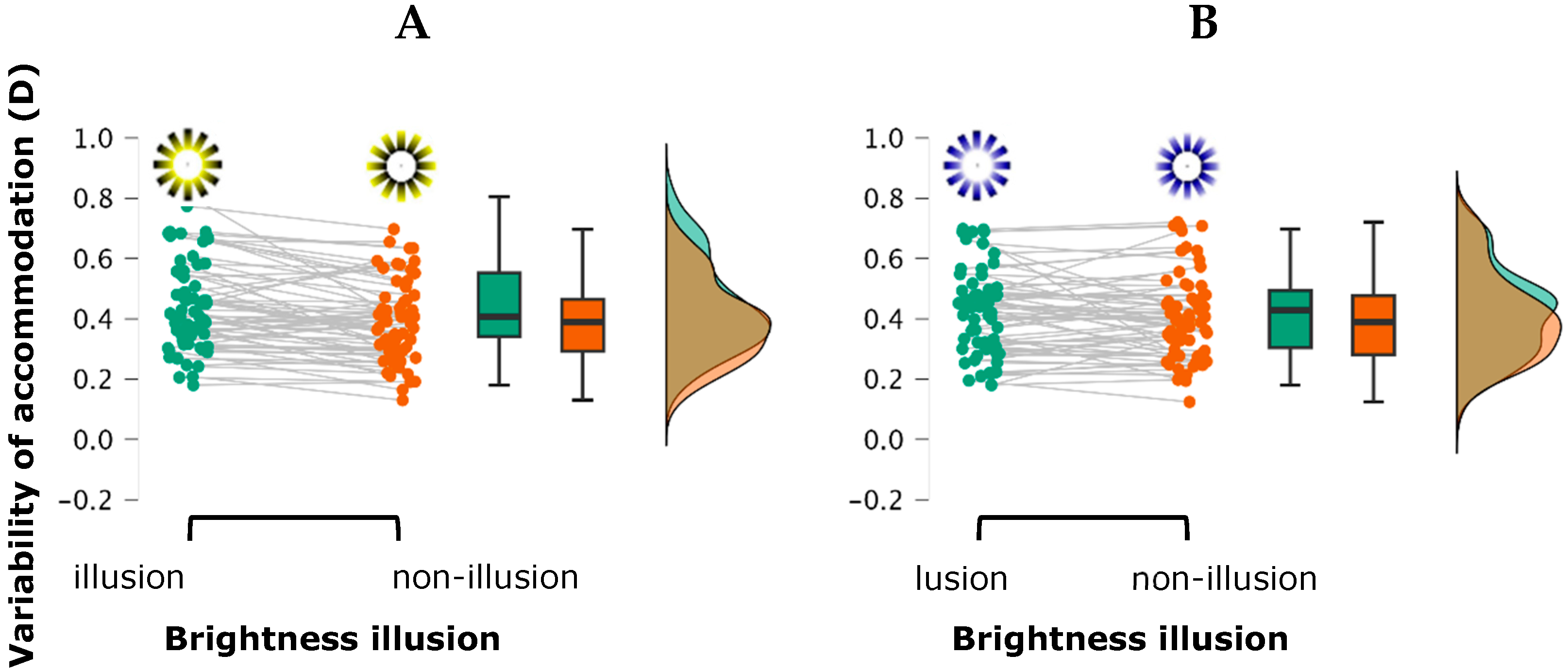
3.1. Accommodative Response: Lag and Variability of Accommodation
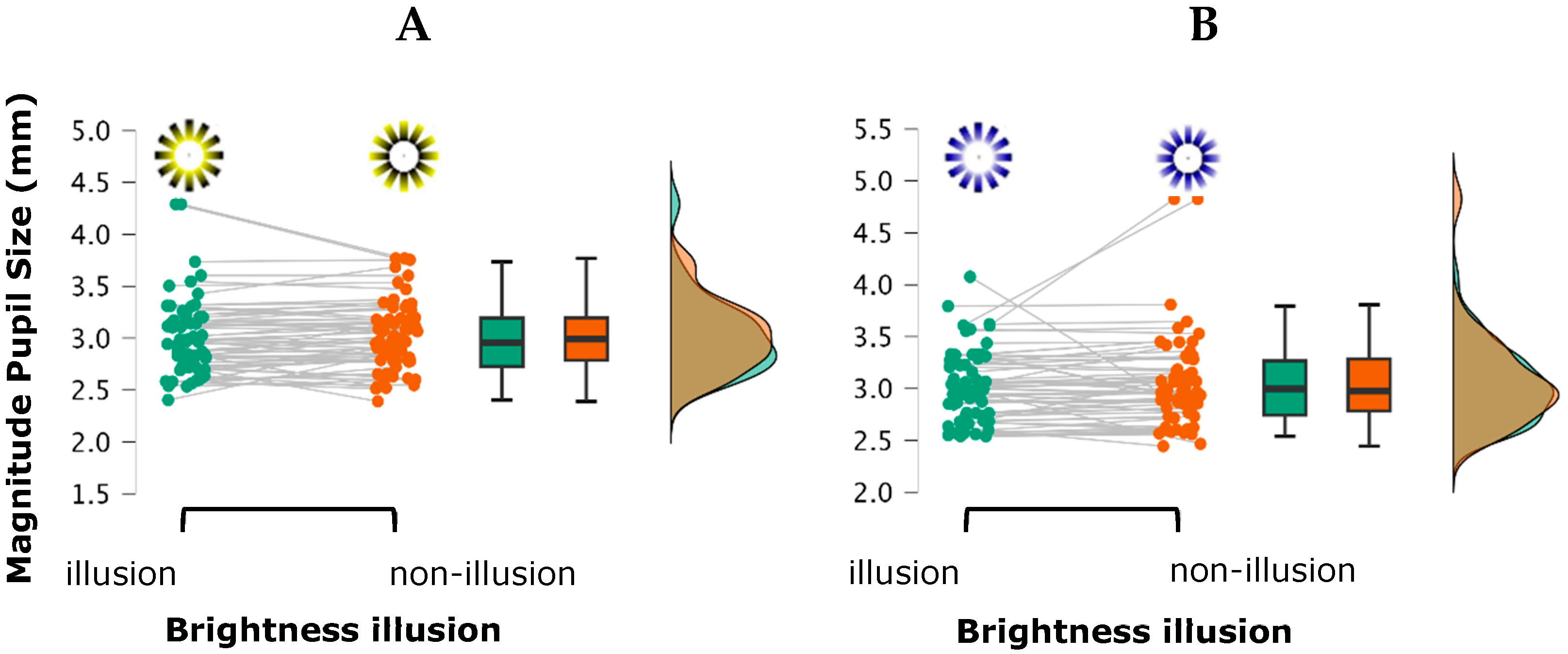
3.2. Pupillary Responses: Magnitude and Variability of Pupil Size
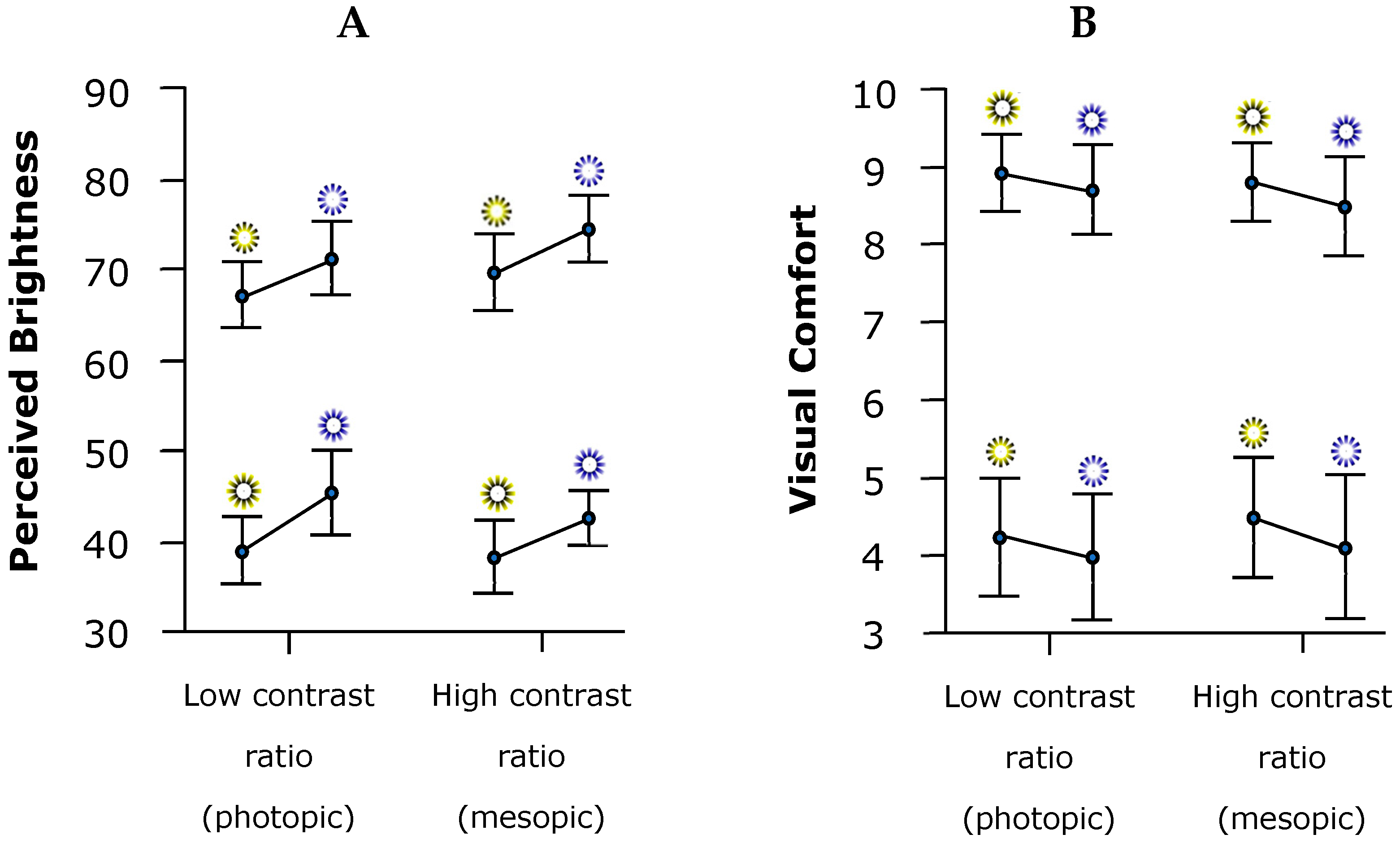
3.3. Perceived Brightness (PB) and Visual Comfort (VC)
4. Discussion
4.1. Accommodative Responses
4.2. Pupillary Responses
4.3. Perceived Brightness (PB) and Visual Comfort (VC)
4.4. Implications of the Study
4.5. Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szczęśniak, M.; Sikorska, E.; Rajca, M.; Koper, M.; Kopacz, W.; Sikorski, P.; Maciejewicz, P.; Kasarełło, K. The etiology, diagnostics and treatment of the spasm of the near reflex-a narrative review. Eur. J. Ophthalmol. 2024, 34, 1655–1666. [Google Scholar] [CrossRef]
- Kohn, M.; Clynes, M. Color dynamics of the pupil. Ann. N. Y. Acad. Sci. 1969, 156, 931–950. [Google Scholar] [CrossRef]
- Ellis, C.J. The pupillary light reflex in normal subjects. Br. J. Ophthalmol. 1981, 65, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Barbur, J.L.; Harlow, A.J.; Sahraie, A. Pupillary responses to stimulus structure, colour and movement. Ophthalmic Physiol. Opt. 1992, 12, 137–141. [Google Scholar] [CrossRef]
- Laeng, B.; Endestad, T. Bright illusions reduce the eye’s pupil. Proc. Natl. Acad. Sci. USA 2012, 109, 2162–2167. [Google Scholar] [CrossRef]
- Suzuki, Y.; Minami, T.; Laeng, B.; Nakauchi, S. Colorful glares: Effects of colors on brightness illusions measured with pupillometry. Acta Psychol. 2019, 198, 102882. [Google Scholar] [CrossRef]
- Wolfe, J.M.; Owens, D.A. Is accommodation colorblind? Focusing chromatic contours. Perception 1981, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, R.; Redondo, B.; Molina, R.; Martínez-Domingo, M.Á.; Hernández-Andrés, J.; Vera, J. Short-term effects of text-background color combinations on the dynamics of the accommodative response. Vis. Res. 2020, 166, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.E.; Smith, R.; Hayes, J. Visual effects of the luminance surrounding a computer display. Ergonomics 2005, 48, 1114–1128. [Google Scholar] [CrossRef]
- Shieh, K.K. Effects of reflection and polarity on LCD viewing distance. Int. J. Ind. Ergon. 2000, 25, 275–282. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lin, P.H.; Hwang, S.L.; Jeng, S.C.; Liao, C.C. Investigation of legibility and visual fatigue for simulated flexible electronic paper under various surface treatments and ambient illumination conditions. Appl. Ergon. 2009, 40, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Redondo, B.; Serramito, M.; Vera, J.; Alguacil-Espejo, M.; Rubio-Martínez, M.; Molina, R.; Jiménez, R. Diurnal Variation in Accommodation, Binocular Vergence, and Pupil Size. Optom. Vis. Sci. 2023, 100, 847–854. [Google Scholar] [CrossRef]
- Vera, J.; Redondo, B.; Ocaso, E.; Martínez-Guillorme, S.; Molina, R.; Jiménez, R. Manipulating expectancies in optometry practice: Ocular accommodation and stereoacuity are sensitive to placebo and nocebo effects. Ophthalmic Physiol. Opt. 2022, 42, 1390–1398. [Google Scholar] [CrossRef]
- Rogers, S.; Spiker, V.; Cicinelli, J. Luminance and luminance contrast requirements for legibility of self-luminous displays in aircraft cockpits. Appl. Ergon. 1986, 17, 271–277. [Google Scholar] [CrossRef]
- Sund, P. Perceived contrast on displays with different luminance ranges. Med. Phys. 2022, 49, 2270–2278. [Google Scholar] [CrossRef]
- Hoddes, E.; Zarcone, V.; Smythe, H.; Phillips, R.; Dement, W.C. Quantification of sleepiness: A new approach. Psychophysiology 1973, 10, 431–436. [Google Scholar] [CrossRef]
- Redondo, B.; Vera, J.; Molina, R.; Davies, L.N.; Jiménez, R. Accommodative dynamics and attention: The influence of manipulating attentional capacity on accommodative lag and variability. Ophthalmic Physiol. Opt. 2020, 40, 510–518. [Google Scholar] [CrossRef]
- Redondo, B.; Vera, J.; Molina, R.; García, J.A.; Catena, A.; Muñoz-Hoyos, A.; Jimenez, R. Accommodation and pupil dynamics as potential objective predictors of behavioural performance in children with attention-deficit/hyperactivity disorder. Vis. Res. 2020, 175, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.S.; Winn, B.; Gilmartin, B. Effect of target luminance on microfluctuations of accommodation. Ophthalmic Physiol. Opt. 1993, 13, 258–265. [Google Scholar] [CrossRef]
- Edgar, G.K.; Reeves, C.A. Visual accommodation and virtual images: Do attentional factors mediate the interacting effects of perceived distance, mental workload, and stimulus presentation modality? Hum. Factors 1997, 39, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Poltavski, D.V.; Biberdorf, D.; Petros, T.V. Accommodative response and cortical activity during sustained attention. Vis. Res. 2012, 63, 1–8. [Google Scholar] [CrossRef]
- Dresp-Langley, B.; Reeves, A. Simultaneous brightness and apparent depth from true colors on grey: Chevreul revisited. Seeing Perceiving 2012, 25, 597–618. [Google Scholar] [CrossRef]
- Grossberg, S. How visual illusions illuminate complementary brain processes: Illusory depth from brightness and apparent motion of illusory contours. Front. Hum. Neurosci. 2014, 8, 854. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, Y.; Komine, K.; Sawahata, Y.; Morita, T. Undetectable changes in image resolution of luminance-contrast gradients affect depth perception. Front. Psychol. 2016, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Masuda, T.; Igarashi, Y.; Omori, K.; Aizawa, Y.; Masuda, N. Depth inversion with a 3D structure influences brightness perception. PLoS ONE 2019, 14, e0224192. [Google Scholar] [CrossRef]
- Chin, B.; Banks, M.; Nankivil, D.; Roorda, A.; Cooper, E. Contributed Session II: Bringing color into focus: Dynamic accommodation responses to polychromatic stimuli. J. Vis. 2023, 23, 76. [Google Scholar] [CrossRef]
- Zou, T.; Aissati, S.; Marcos, S. Poster Session II: The impact of eye’s longitudinal chromatic aberration on visual acuity and accommodation response. J. Vis. 2023, 23, 62. [Google Scholar] [CrossRef]
- Chen, A.H.; Ahmad, A.; Kearney, S.; Strang, N. The influence of age, refractive error, visual demand and lighting conditions on accommodative ability in Malay children and adults. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1997–2004. [Google Scholar] [CrossRef]
- Barkan, Y.; Spitzer, H. Neuronal mechanism for compensation of longitudinal chromatic aberration-derived algorithm. Front. Bioeng. Biotechnol. 2018, 6, 12. [Google Scholar] [CrossRef]
- Winter, S.; Sabesan, R.; Tiruveedhula, P.; Privitera, C.; Unsbo, P.; Lundström, L.; Roorda, A. Transverse chromatic aberration across the visual field of the human eye. J. Vis. 2016, 16, 9. [Google Scholar] [CrossRef]
- Mathôt, S.; Van Der Stigchel, S. New light on the mind’s eye: The pupillary light response as active vision. Curr. Dir. Psychol. Sci. 2015, 24, 374–378. [Google Scholar] [CrossRef]
- Sperandio, I.; Bond, N.; Binda, P. Pupil size as a gateway into conscious interpretation of brightness. Front. Neurol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Sandoval Salinas, C.; Hermans, S.; Sandoval, J.; Smet, K.A.; Hanselaer, P.; Colombo, E. Relationship between pupillary size, brightness, and photoreceptor responses for unrelated self-luminous stimuli at low photopic light levels. Color Res. Appl. 2020, 45, 977–991. [Google Scholar] [CrossRef]
- Oster, J.; Huang, J.; White, B.J.; Radach, R.; Itti, L.; Munoz, D.P.; Wang, C.A. Pupillary responses to differences in luminance, color and set size. Exp. Brain Res. 2022, 240, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Rukmini, A.; Milea, D.; Gooley, J. Chromatic pupillometry methods for assessing photoreceptor health in retinal and optic nerve diseases. Front. Neurol. 2019, 10, 76. [Google Scholar] [CrossRef]
- Zauner, J.; Plischke, H.; Strasburger, H. Spectral dependency of the human pupillary light reflex. Influences of pre-adaptation and chronotype. PLoS ONE 2022, 17, e0253030. [Google Scholar] [CrossRef]
- Gibertoni, G.; Di Pinto, V.; Cattini, S.; Tramarin, F.; Geiser, M.; Rovati, L. A simple Maxwellian optical system to investigate the photoreceptors contribution to pupillary light reflex. In Proceedings of the Ophthalmic Technologies XXXII, San Francisco, CA, USA, 20–24 February 2022. [Google Scholar]
- Shapiro, A.; Lu, Z.L. Relative brightness in natural images can be accounted for by removing blurry content. Psychol. Sci. 2011, 22, 1452–1459. [Google Scholar] [CrossRef]
- Zavagno, D.; Daneyko, O.; Liu, Z. The influence of physical illumination on lightness perception in simultaneous contrast displays. Iperception 2018, 9, 2041669518787212. [Google Scholar] [CrossRef]
- Tian, P.; Xu, G.; Han, C.; Zheng, X.; Zhang, K.; Du, C.; Wei, F.; Zhang, S. Effects of paradigm color and screen brightness on visual fatigue in light environment of night based on eye tracker and EEG acquisition equipment. Sensors 2022, 22, 4082. [Google Scholar] [CrossRef]
- Tian, P.; Xu, G.; Han, C.; Zheng, X.; Zhang, K.; Du, C.; Zhang, X.; Wei, F.; Ma, Y.; Zhang, S.; et al. Research on an Indoor Light Environment Comfort Evaluation Index Based on Electroencephalogram and Pupil Signals. Electronics 2024, 13, 3411. [Google Scholar] [CrossRef]
- Bullough, J.D.; Brons, J.A.; Qi, R.; Rea, M.S. Predicting discomfort glare from outdoor lighting installations. Light Res. Technol. 2008, 40, 225–242. [Google Scholar] [CrossRef]
- Benedetto, S.; Carbone, A.; Drai-Zerbib, V.; Pedrotti, M.; Baccino, T. Effects of luminance and illuminance on visual fatigue and arousal during digital reading. Comput. Hum. Behav. 2014, 41, 112–119. [Google Scholar] [CrossRef]
- Kaur, K.; Gurnani, B.; Nayak, S.; Deori, N.; Kaur, S.; Jethani, J.; Mishra, D. Digital eye strain-a comprehensive review. Ophthalmol. Ther. 2022, 11, 1655–1680. [Google Scholar] [CrossRef] [PubMed]
- Takao, S.; Clifford, C.W.; Watanabe, K. Ebbinghaus illusion depends more on the retinal than perceived size of surrounding stimuli. Vis. Res. 2019, 154, 80–84. [Google Scholar] [CrossRef] [PubMed]


| Contrast Luminance | Color | Bright Illusion | Lag of Accommodation (D) | Variability of Accommodation (D) | Magnitude of Pupil Size (mm) | Variability of Pupil Size (mm) |
|---|---|---|---|---|---|---|
| Low contrast (Photopic condition) | Yellow | Bright | 0.68 ± 0.39 | 0.41 ± 0.14 | 3.04 ± 0.44 | 0.79 ± 0.41 |
| Non-bright | 0.70 ± 0.36 | 0.36 ± 0.13 | 3.04 ± 0.35 | 0.82 ± 0.37 | ||
| Blue | Bright | 0.66 ± 0.37 | 0.40 ± 0.14 | 3.05 ± 0.38 | 0.83 ± 0.44 | |
| Non-bright | 0.64 ± 0.41 | 0.40 ± 0.14 | 3.11 ± 0.54 | 0.87 ± 0.44 | ||
| High contrast (Mesopic condition) | Yellow | Bright | 0.64 ± 0.43 | 0.47 ± 0.16 | 2.94 ± 0.30 | 0.82 ± 0.39 |
| Non-bright | 0.64 ± 0.39 | 0.42 ± 0.14 | 2.98 ± 0.33 | 0.81 ± 0.33 | ||
| Blue | Bright | 0.65 ± 0.37 | 0.44 ± 0.14 | 2.98 ± 0.32 | 0.86 ± 0.38 | |
| Non-bright | 0.66 ± 0.43 | 0.41 ± 0.15 | 2.99 ± 0.35 | 0.87 ± 0.42 |
| Contrast Luminance | Color | Bright Illusion | PB (0–2100) | VC (0–10) |
|---|---|---|---|---|
| Low contrast (Photopic condition) | Yellow | Bright | 67.17 ± 10.89 | 4.26 ± 2.24 |
| Non-bright | 38.94 ± 11.16 | 8.90 ± 1.47 | ||
| Blue | Bright | 71.97 ± 12.26 | 3.97 ± 2.38 | |
| Non-bright | 45.50 ± 13.71 | 8.67 ± 1.71 | ||
| High contrast (Mesopic condition) | Yellow | Bright | 69.81 ± 12.69 | 4.43 ± 2.29 |
| Non-bright | 38.22 ± 12.21 | 8.81 ± 1.53 | ||
| Blue | Bright | 74.81 ± 11.08 | 4.06 ± 2.76 | |
| Non-bright | 42.58 ± 9.09 | 8.49 ± 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodán, A.; Fernández-López, A.; Vera, J.; Montoro, P.R.; Redondo, B.; Prieto, A. Too Bright to Focus? Influence of Brightness Illusions and Ambient Light Levels on the Dynamics of Ocular Accommodation. Vision 2025, 9, 81. https://doi.org/10.3390/vision9040081
Rodán A, Fernández-López A, Vera J, Montoro PR, Redondo B, Prieto A. Too Bright to Focus? Influence of Brightness Illusions and Ambient Light Levels on the Dynamics of Ocular Accommodation. Vision. 2025; 9(4):81. https://doi.org/10.3390/vision9040081
Chicago/Turabian StyleRodán, Antonio, Angélica Fernández-López, Jesús Vera, Pedro R. Montoro, Beatriz Redondo, and Antonio Prieto. 2025. "Too Bright to Focus? Influence of Brightness Illusions and Ambient Light Levels on the Dynamics of Ocular Accommodation" Vision 9, no. 4: 81. https://doi.org/10.3390/vision9040081
APA StyleRodán, A., Fernández-López, A., Vera, J., Montoro, P. R., Redondo, B., & Prieto, A. (2025). Too Bright to Focus? Influence of Brightness Illusions and Ambient Light Levels on the Dynamics of Ocular Accommodation. Vision, 9(4), 81. https://doi.org/10.3390/vision9040081






