A Simulated Visual Field Defect Impairs Temporal Processing: An Effect Not Modulated by Emotional Faces
Abstract
1. Introduction
- Compared to normal vision, does an artificial scotoma impair temporal processing as measured by the two-flash fusion paradigm?
- If temporal processing is impaired under scotoma conditions, does this impairment manifest uniformly across all interstimulus intervals, or are there specific temporal windows where the effect is most pronounced?
- Do emotional faces (angry, happy, neutral) modulate temporal processing in the two-flash fusion task, and does this modulation differ between normal vision and artificial scotoma conditions?
- Are reaction times affected by the presence of an artificial scotoma, emotional content, or their interaction?
2. Materials and Methods
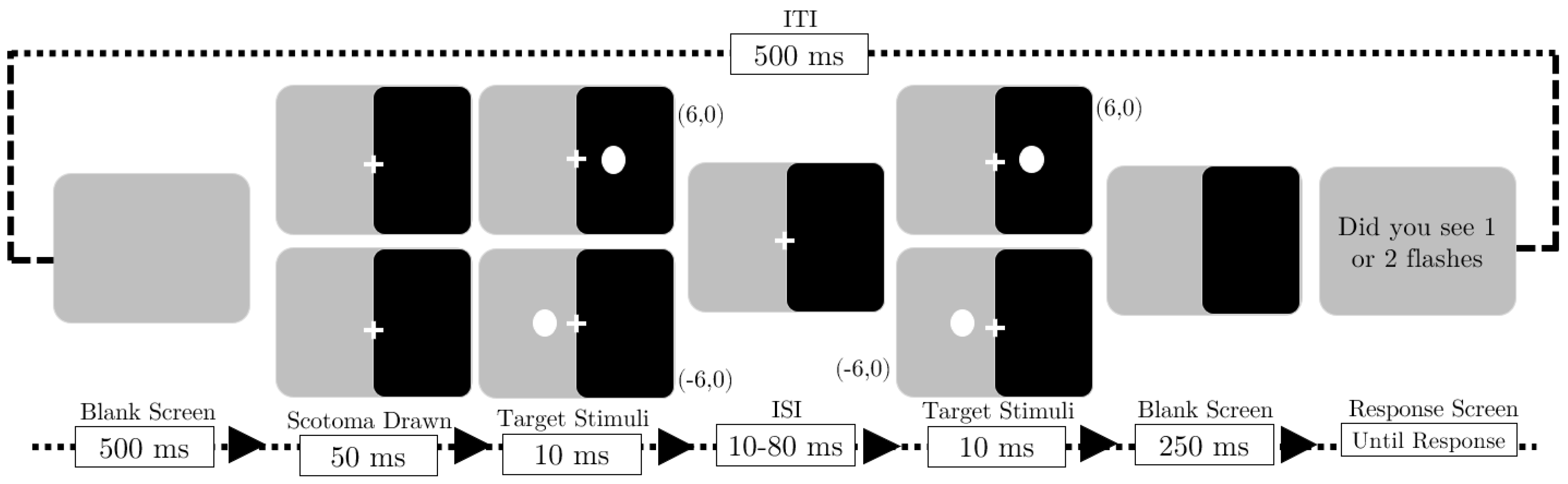
2.1. Stimuli and Apparatus
2.2. Experiment 1: Two-Flash Fusion with Scotoma
2.2.1. Participants
2.2.2. Experimental Design
2.2.3. Procedure
2.3. Experiment 2: Influence of Emotional Faces on Two-Flash Fusion Under Artificial Scotoma Conditions
2.3.1. Participants
2.3.2. Experimental Design
2.3.3. Procedure
2.4. Data Analysis
3. Results
3.1. Experiment 1: Two-Flash Fusion Detection with Artificial Scotoma
3.1.1. Accuracy Analysis
3.1.2. Threshold Analysis
3.1.3. Reaction Time Analysis
3.2. Experiment 2: Effect of Emotional Faces on Two-Flash Fusion
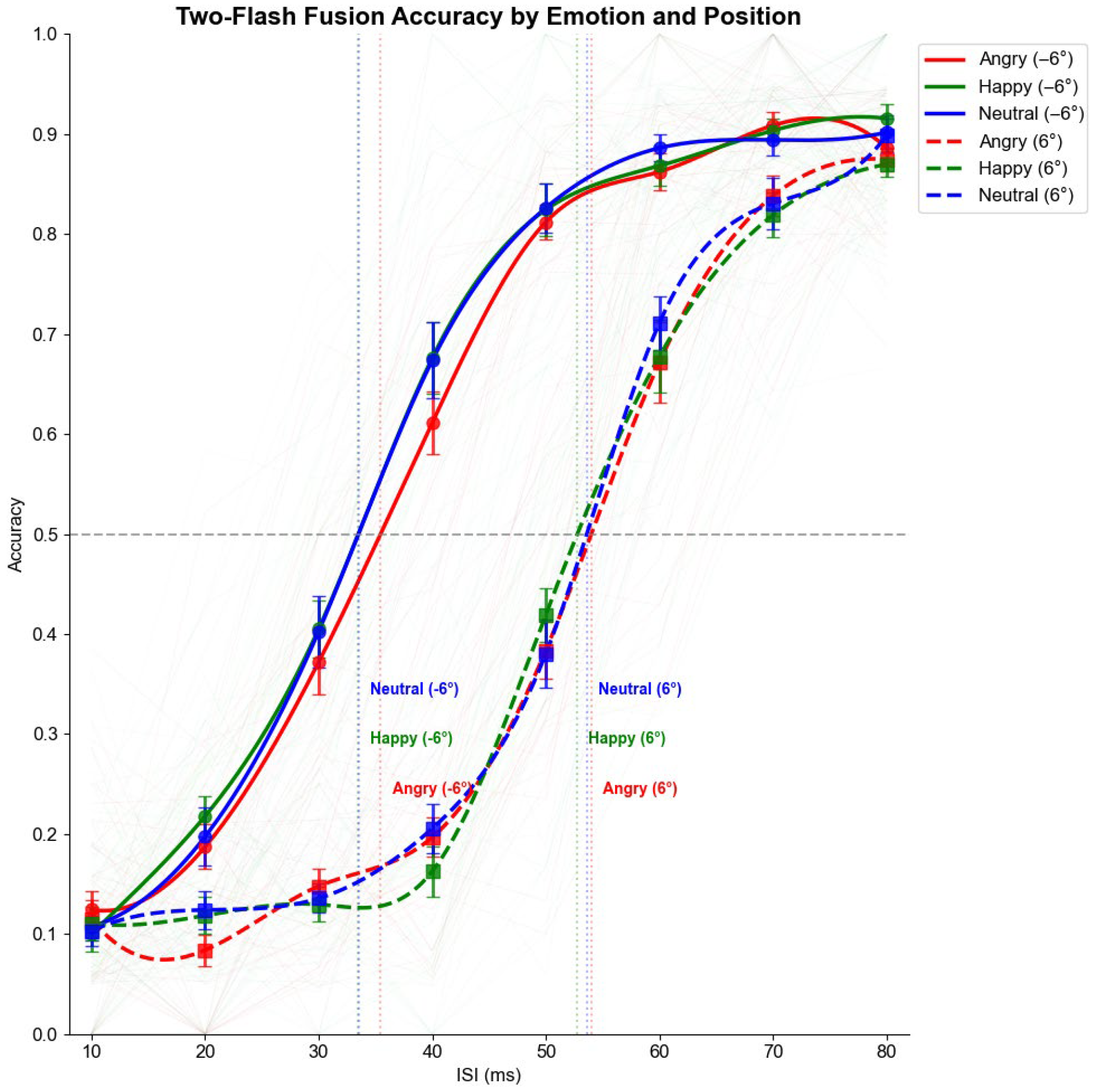
3.2.1. Accuracy Analysis
3.2.2. Threshold Analysis
3.2.3. Reaction Time Analysis
3.3. Comparison Between Experiments and Conclusions
4. Discussion
Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Centanino, V.; Fortunato, G.; Bueti, D. The Neural Link between Stimulus Duration and Spatial Location in the Human Visual Hierarchy. Nat. Commun. 2024, 15, 10720. [Google Scholar] [CrossRef]
- Deodato, M.; Melcher, D. Continuous Temporal Integration in the Human Visual System. J. Vis. 2024, 24, 5. [Google Scholar] [CrossRef]
- Martin, A.B.; Yang, X.; Saalmann, Y.; Wang, L.; Shestyuk, A.; Lin, J.J.; Parvizi, J.; Knight, R.; Kastner, S. Temporal Dynamics and Response Modulation across the Human Visual System in a Spatial Attention Task: An ECoG Study. J. Neurosci. 2018, 39, 333–352. [Google Scholar] [CrossRef]
- Párraga, C.; Troscianko, T.; Tolhurst, D. The Human Visual System Is Optimised for Processing the Spatial Information in Natural Visual Images. Curr. Biol. 2000, 10, 35–38. [Google Scholar] [CrossRef]
- Petras, K.; ten Oever, S.; Dalal, S.; Goffaux, V. Information Redundancy across Spatial Scales Modulates Early Visual Cortical Processing. Neuroimage 2021, 244, 118613. [Google Scholar] [CrossRef] [PubMed]
- Akyürek, E.G.; Wolff, M.J. Extended Temporal Integration in Rapid Serial Visual Presentation: Attentional Control at Lag 1 and Beyond. Acta Psychol. 2016, 168, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Samaha, J.; Postle, B.R. The Speed of Alpha-Band Oscillations Predicts the Temporal Resolution of Visual Perception. Curr. Biol. 2015, 25, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Letang, J.M.; Rebuffel, V.; Bouthemy, P. Motion Detection Based on a Temporal Multiscale Approach. In 1992 11th IAPR International Conference on Pattern Recognition; IEEE Computer Society Press: The Hague, The Netherlands, 1992; pp. 65–68. [Google Scholar]
- Holloway, S.R.; Náñez Sr, J.E.; Seitz, A.R. Word-Decoding as a Function of Temporal Processing in the Visual System. PLoS ONE 2013, 8, e84010. [Google Scholar] [CrossRef]
- Hood, M.; Conlon, E. Visual and Auditory Temporal Processing and Early Reading Development. Dyslexia 2004, 10, 234–252. [Google Scholar] [CrossRef]
- Heidari-Gorji, H.; Ebrahimpour, R.; Zabbah, S. A Temporal Hierarchical Feedforward Model Explains Both the Time and the Accuracy of Object Recognition. Sci. Rep. 2021, 11, 5640. [Google Scholar] [CrossRef]
- McKeeff, T.J.; Remus, D.A.; Tong, F. Temporal Limitations in Object Processing Across the Human Ventral Visual Pathway. J. Neurophysiol. 2007, 98, 382–393. [Google Scholar] [CrossRef]
- Deodato, M.; Melcher, D. Correlations between Visual Temporal Resolution and Individual Alpha Peak Frequency: Evidence That Internal and Measurement Noise Drive Null Findings. J. Cogn. Neurosci. 2024, 36, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Melcher, D.; Lapomarda, G.; Deodato, M. Seeing Fast and Slow: Systematic State and Trait Variations in Visual Temporal Acuity. J. Vis. 2023, 23, 5222. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Levy, L. Transient Spatial Attention Degrades Temporal Resolution. Psychol. Sci. 2003, 14, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Eisen-Enosh, A.; Farah, N.; Burgansky-Eliash, Z.; Polat, U.; Mandel, Y. Evaluation of Critical Flicker-Fusion Frequency Measurement Methods for the Investigation of Visual Temporal Resolution. Sci. Rep. 2017, 7, 15621. [Google Scholar] [CrossRef]
- Umeton, D.; Read, J.C.A.; Rowe, C. Unravelling the Illusion of Flicker Fusion. Biol. Lett. 2017, 13, 20160831. [Google Scholar] [CrossRef]
- Mewborn, C.; Renzi, L.M.; Hammond, B.R.; Miller, L.S. Critical Flicker Fusion Predicts Executive Function in Younger and Older Adults. Arch. Clin. Neuropsychol. 2015, 30, 605–610. [Google Scholar] [CrossRef]
- Pearson, L.A.; Tong, J.E. Two-Flash Fusion Threshold: The Influence of Age, Psychophysical Method, Instructions, Viewing Conditions, Sex and Subject Variability. Br. J Psychol. 1968, 59, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Dosher, B.; Lu, Z.-L. Visual Perceptual Learning and Models. Annu. Rev. Vis. Sci. 2017, 3, 343–363. [Google Scholar] [CrossRef]
- Salvioni, P.; Murray, M.M.; Kalmbach, L.; Bueti, D. How the Visual Brain Encodes and Keeps Track of Time. J. Neurosci. 2013, 33, 12423–12429. [Google Scholar] [CrossRef]
- Safran, A. Scotomes: Le point de vue du patient et le point de vue du medecin. Cela n’a rien à voir. Klin. Monatsblätter Augenheilkd. 1997, 210, 316–318. [Google Scholar] [CrossRef]
- Bola, M.; Gall, C.; Sabel, B.A. The Second Face of Blindness: Processing Speed Deficits in the Intact Visual Field after Pre- and Post-Chiasmatic Lesions. PLoS ONE 2013, 8, e63700. [Google Scholar] [CrossRef]
- Fletcher, D.C.; Schuchard, R.A.; Renninger, L.W. Patient Awareness of Binocular Central Scotoma in Age-Related Macular Degeneration. Optom. Vis. Sci. 2012, 89, 1395–1398. [Google Scholar] [CrossRef]
- Schuchard, R.A. Adaptation to Macular Scotomas in Persons With Low Vision. Am. J. Occup. Ther. 1995, 49, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Bola, M.; Gall, C.; Sabel, B.A. “Sightblind”: Perceptual Deficits in the “Intact” Visual Field. Front. Neurol. 2013, 4, 50254. [Google Scholar] [CrossRef]
- Khodami, M.A.; Altieri, E.; Battaglini, L. Hemianopia Rehabilitation: From Lab to Life, the Missing Piece. Preprints 2023. [Google Scholar] [CrossRef]
- Pollock, A.; Hazelton, C.; Rowe, F.J.; Jonuscheit, S.; Kernohan, A.; Angilley, J.; Henderson, C.A.; Langhorne, P.; Campbell, P. Interventions for Visual Field Defects in People with Stroke. Cochrane Database Syst. Rev. 2019, 5, CD008388. [Google Scholar] [CrossRef]
- Cavézian, C.; Gaudry, I.; Perez, C.; Coubard, O.; Doucet, G.; Peyrin, C.; Marendaz, C.; Obadia, M.; Gout, O.; Chokron, S. Specific Impairments in Visual Processing Following Lesion Side in Hemianopic Patients. Cortex 2010, 46, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.F.; Pointer, J.S. Spatial and Temporal Contrast Sensitivity in Hemianopia: A Comparative Study of the Sighted and Blind Hemifields. Brain 1989, 112, 871–894. [Google Scholar] [CrossRef]
- Leo, F.; Bolognini, N.; Passamonti, C.; Stein, B.E.; Ladavas, E. Cross-Modal Localization in Hemianopia: New Insights on Multisensory Integration. Brain 2008, 131, 855–865. [Google Scholar] [CrossRef]
- Poggel, D.A.; Treutwein, B.; Strasburger, H. Time Will Tell: Deficits of Temporal Information Processing in Patients with Visual Field Loss. Brain Res. 2011, 1368, 196–207. [Google Scholar] [CrossRef]
- Covington, N.V.; Duff, M.C. Heterogeneity Is a Hallmark of Traumatic Brain Injury, Not a Limitation: A New Perspective on Study Design in Rehabilitation Research. Am. J. Speech Lang. Pathol. 2021, 30, 974–985. [Google Scholar] [CrossRef]
- Piras, F.; Piras, F.; Ciullo, V.; Danese, E.; Caltagirone, C.; Spalletta, G. Time Dysperception Perspective for Acquired Brain Injury. Front. Neurol. 2014, 4, 217. [Google Scholar] [CrossRef]
- Ağaoğlu, M.N.; Fung, W.; Chung, S.T.L. Oculomotor Responses of the Visual System to an Artificial Central Scotoma May Not Represent Genuine Visuomotor Adaptation. J. Vis. 2022, 22, 17. [Google Scholar] [CrossRef]
- De Weerd, P.; Desimone, R.; Ungerleider, L.G. Perceptual Filling-in: A Parametric Study. Vis. Res. 1998, 38, 2721–2734. [Google Scholar] [CrossRef] [PubMed]
- Weerd, P.D. Perceptual Filling-in: More than the Eye Can See. Prog. Brain Res. 2006, 154, 227–245. [Google Scholar] [PubMed]
- Weil, R.S.; Watkins, S.; Rees, G. Neural Correlates of Perceptual Completion of an Artificial Scotoma in Human Visual Cortex Measured Using Functional MRI. NeuroImage 2008, 42, 1519–1528. [Google Scholar] [CrossRef]
- Cornelissen, F.; Bruin, K.J.; Kooijman, A. The Influence of Artificial Scotomas on Eye Movements during Visual Search. Optom. Vis. Sci. 2005, 82, 27–35. [Google Scholar]
- McIlreavy, L.; Fiser, J.; Bex, P.J. Impact of Simulated Central Scotomas on Visual Search in Natural Scenes. Optom. Vis. Sci. 2012, 89, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, P. Affective and Motivational Control of Vision. Curr. Opin. Neurol. 2015, 28, 29–35. [Google Scholar] [CrossRef]
- Vuilleumier, P.; Driver, J. Modulation of Visual Processing by Attention and Emotion: Windows on Causal Interactions Between Human Brain Regions. Phil. Trans. R. Soc. B 2007, 362, 837–855. [Google Scholar] [CrossRef]
- Schindler, S.; Bublatzky, F. Attention and Emotion: An Integrative Review of Emotional Face Processing as a Function of Attention. Cortex 2020, 130, 362–386. [Google Scholar] [CrossRef]
- Vuilleumier, P. Facial Expression and Selective Attention. Curr. Opin. Psychiatry 2002, 15, 291–300. [Google Scholar] [CrossRef]
- Bernstein, M.; Yovel, G. Two Neural Pathways of Face Processing: A Critical Evaluation of Current Models. Neurosci. Biobehav. Rev. 2015, 55, 536–546. [Google Scholar] [CrossRef]
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. The Distributed Human Neural System for Face Perception. Trends Cogn. Sci. 2000, 4, 223–233. [Google Scholar] [CrossRef]
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. Human Neural Systems for Face Recognition and Social Communication. Biol. Psychiatry 2002, 51, 59–67. [Google Scholar] [CrossRef]
- Haxby, J.V.; Gobbini, M.I. Distributed Neural Systems for Face Perception; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Foley, E.; Rippon, G.; Thai, N.J.; Longe, O.; Senior, C. Dynamic Facial Expressions Evoke Distinct Activation in the Face Perception Network: A Connectivity Analysis Study. J. Cogn. Neurosci. 2012, 24, 507–520. [Google Scholar] [CrossRef]
- Battaglini, L.; Khodami, M.A.; Capizzi, M.; Mioni, G. The Effect of the Symbolic Meaning of Speed on Implicit Timing. Timing Time Percept. 2024, 1–14. [Google Scholar] [CrossRef]
- Ciesielski, B.G.; Armstrong, T.; Zald, D.H.; Olatunji, B.O. Emotion Modulation of Visual Attention: Categorical and Temporal Characteristics. PLoS ONE 2010, 5, e13860. [Google Scholar] [CrossRef]
- Droit-Volet, S.; Meck, W.H. How Emotions Colour Our Perception of Time. Trends Cogn. Sci. 2007, 11, 504–513. [Google Scholar] [CrossRef]
- Peirce, J.W. PsychoPy—Psychophysics Software in Python. J. Neurosci. Methods 2007, 162, 8–13. [Google Scholar] [CrossRef]
- Peirce, J.W. Generating Stimuli for Neuroscience Using PsychoPy. Front. Neuroinform. 2008, 2, 10. [Google Scholar] [CrossRef]
- Takeshima, Y. Emotional Information Affects Fission Illusion Induced by Audio-Visual Interactions. Sci. Rep. 2020, 10, 998. [Google Scholar] [CrossRef]
- Deodato, M.; Melcher, D. Aperiodic EEG Predicts Variability of Visual Temporal Processing. J. Neurosci. 2024, 44, e2308232024. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Neri, P.; Levi, D.M. Spatial Resolution for Feature Binding Is Impaired in Peripheral and Amblyopic Vision. J. Neurophysiol. 2006, 96, 142–153. [Google Scholar] [CrossRef]
- Zlody, R.L. The Relationship between Critical Flicker Frequency (CFF) and Several Intellectual Measures. Am. J. Psychol. 1965, 78, 596–602. [Google Scholar] [CrossRef]
- Holcombe, A.O. Seeing Slow and Seeing Fast: Two Limits on Perception. Trends Cogn. Sci. 2009, 13, 216–221. [Google Scholar] [CrossRef]
- Mishra, M.V.; Ray, S.B.; Srinivasan, N. Effect of Emotions on Temporal Attention. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 236, pp. 287–309. ISBN 978-0-12-813450-4. [Google Scholar]
- Jacques, C.; Caharel, S. The Time Course of Categorical Perception of Facial Expressions. Neuropsychologia 2022, 177, 108424. [Google Scholar] [CrossRef]
- Eimer, M.; Holmes, A. Event-Related Brain Potential Correlates of Emotional Face Processing. Neuropsychologia 2007, 45, 15–31. [Google Scholar] [CrossRef]
- Rigoulot, S.; D’Hondt, F.; Defoort-Dhellemmes, S.; Despretz, P.; Honoré, J.; Sequeira, H. Fearful Faces Impact in Peripheral Vision: Behavioral and Neural Evidence. Neuropsychologia 2011, 49, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Benson, N.; Kay, K.N.; Winawer, J. Compressive Temporal Summation in Human Visual Cortex. J. Neurosci. 2017, 38, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Schofield, T.M.; Leff, A.P. Rehabilitation of Hemianopia. Curr. Opin. Neurol. 2009, 22, 36. [Google Scholar] [CrossRef]

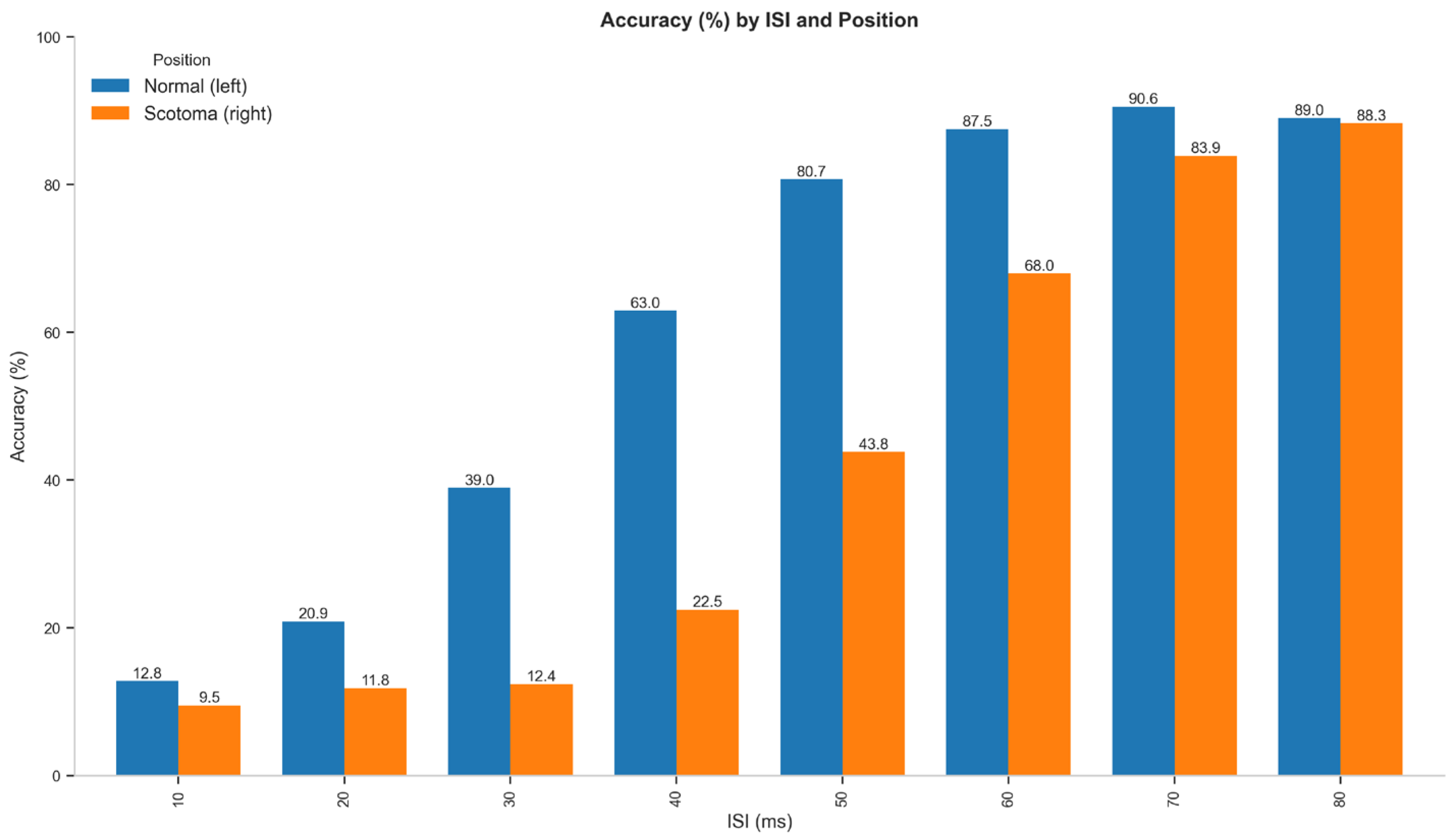


| ISI (ms) | Normal (Left) | Scotoma (Right) | Difference |
|---|---|---|---|
| 10 | 12.8 | 9.5 | 3.3 |
| 20 | 20.9 | 11.8 | 9.1 |
| 30 | 39.0 | 12.4 | 26.6 |
| 40 | 63.0 | 22.5 | 40.5 |
| 50 | 80.7 | 43.8 | 36.9 |
| 60 | 87.5 | 68.0 | 19.5 |
| 70 | 90.6 | 83.9 | 6.7 |
| 80 | 89.0 | 88.3 | 0.7 |
| Position | Threshold (ms) | 95% CI | Slope |
|---|---|---|---|
| Normal (left) | 34.78 | 32.42–37.14 | 0.096 |
| Scotoma (right) | 52.29 | 50.00–54.59 | 0.092 |
| Position | Angry | Happy | Neutral | Average |
|---|---|---|---|---|
| Normal (left) | 59.4 | 62.0 | 60.8 | 60.7 |
| Scotoma (right) | 41.7 | 41.5 | 41.8 | 41.7 |
| Difference | 17.7 | 20.5 | 19.0 | 19.0 |
| Position | Emotion | Threshold (ms) | 95% CI | Slope |
|---|---|---|---|---|
| Normal (left) | Angry | 35.58 | 33.04–38.10 | 0.100 |
| Happy | 33.80 | 31.47–36.12 | 0.101 | |
| Neutral | 33.95 | 31.40–36.51 | 0.116 | |
| Scotoma (right) | Angry | 53.51 | 50.72–56.31 | 0.105 |
| Happy | 53.50 | 50.40–56.61 | 0.109 | |
| Neutral | 52.86 | 49.84–55.88 | 0.111 |
| Position | Angry | Happy | Neutral | Average |
|---|---|---|---|---|
| Normal (left) | 580.0 | 578.0 | 578.8 | 579.0 |
| Scotoma (right) | 616.1 | 615.0 | 616.0 | 615.7 |
| Difference | 36.1 | 37.0 | 37.2 | 36.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodami, M.A.; Battaglini, L. A Simulated Visual Field Defect Impairs Temporal Processing: An Effect Not Modulated by Emotional Faces. Vision 2025, 9, 79. https://doi.org/10.3390/vision9030079
Khodami MA, Battaglini L. A Simulated Visual Field Defect Impairs Temporal Processing: An Effect Not Modulated by Emotional Faces. Vision. 2025; 9(3):79. https://doi.org/10.3390/vision9030079
Chicago/Turabian StyleKhodami, Mohammad Ahsan, and Luca Battaglini. 2025. "A Simulated Visual Field Defect Impairs Temporal Processing: An Effect Not Modulated by Emotional Faces" Vision 9, no. 3: 79. https://doi.org/10.3390/vision9030079
APA StyleKhodami, M. A., & Battaglini, L. (2025). A Simulated Visual Field Defect Impairs Temporal Processing: An Effect Not Modulated by Emotional Faces. Vision, 9(3), 79. https://doi.org/10.3390/vision9030079






