Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review of Ophthalmic Management and Treatment
Abstract
1. Introduction
2. Methods
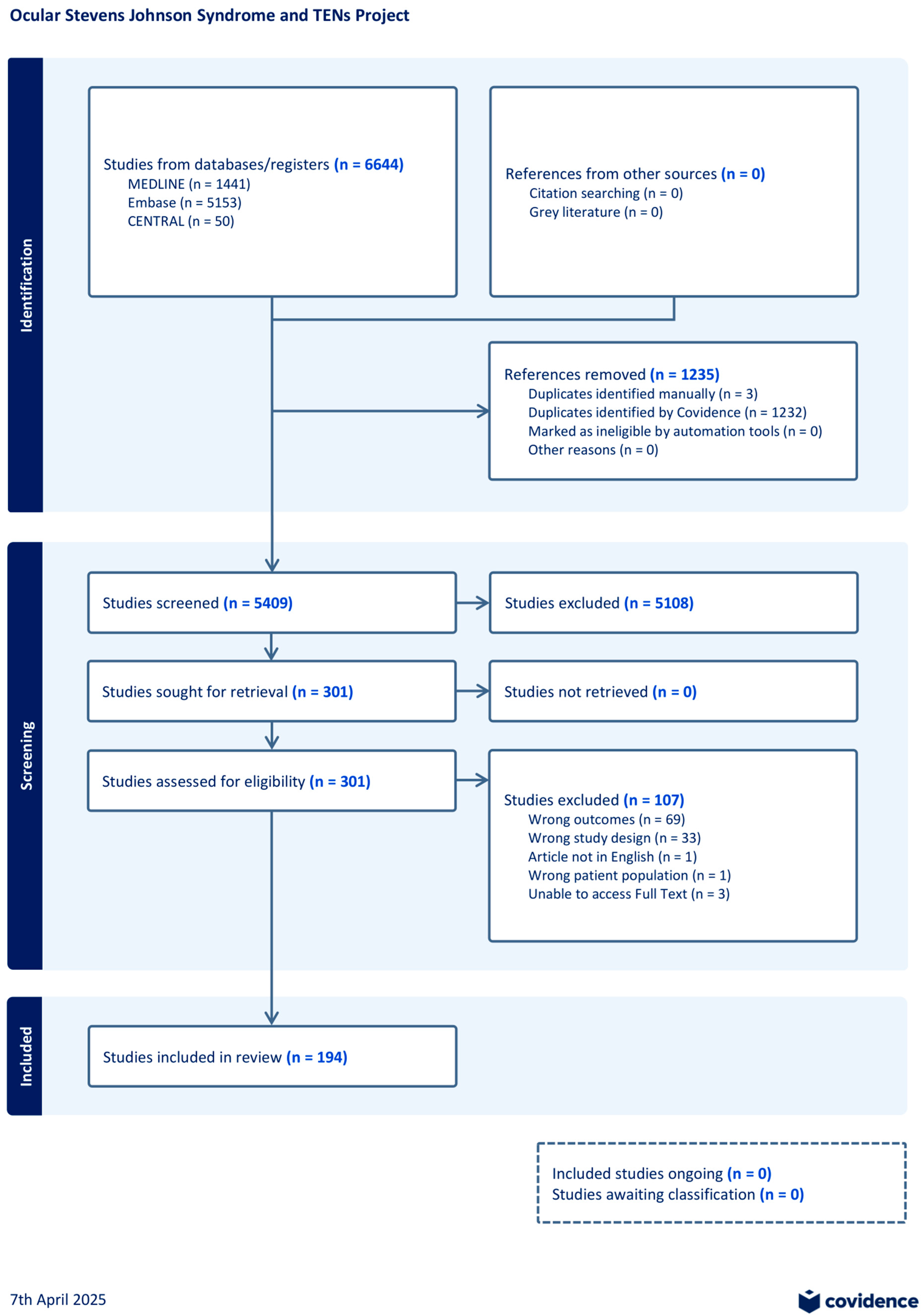
3. Results
- Acute Treatment
- Chronic Treatment
- Emerging Treatments
- Quality Assessment
4. Discussion
- Acute Treatment
- Chronic Treatment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araki, Y.; Sotozono, C.; Inatomi, T.; Ueta, M.; Yokoi, N.; Ueda, E.; Kishimoto, S.; Kinoshita, S. Successful treatment of Stevens-Johnson syndrome with steroid pulse therapy at disease onset. Am. J. Ophthalmol. 2009, 147, 1004–1011.e1. [Google Scholar] [CrossRef]
- Stevens, A.M. A New Eruptive Fever Associated with Stomatitis and Ophthalmia. Am. J. Dis. Child. 1922, 24, 526. [Google Scholar] [CrossRef]
- Chan, H.L.; Stern, R.S.; Arndt, K.A.; Langlois, J.; Jick, S.S.; Jick, H.; Walker, A.M. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch. Dermatol. 1990, 126, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Prabhasawat, P.; Barton, K.; Gray, T.; Meller, D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch. Ophthalmol. 1998, 116, 431–441. [Google Scholar] [CrossRef]
- Tugal-Tutkun, I.; Akova, Y.A.; Foster, C.S. Penetrating keratoplasty in cicatrizing conjunctival diseases. Ophthalmology 1995, 102, 576–585. [Google Scholar] [CrossRef]
- De Rojas, M.V.; Dart, J.K.; Saw, V.P. The natural history of Stevens Johnson syndrome: Patterns of chronic ocular disease and the role of systemic immunosuppressive therapy. Br. J. Ophthalmol. 2007, 91, 1048–1053. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Rashad, R.; Chodosh, J.; Saeed, H.N. Long-Term Effect of a Treatment Protocol for Acute Ocular Involvement in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Am. J. Ophthalmol. 2019, 208, 331–341. [Google Scholar] [CrossRef]
- Fu, Y.; Gregory, D.G.; Sippel, K.C.; Bouchard, C.S.; Tseng, S.C. The ophthalmologist’s role in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis. Ocul. Surf. 2010, 8, 193–203. [Google Scholar] [CrossRef]
- Dodiuk-Gad, R.P.; Chung, W.H.; Valeyrie-Allanore, L.; Shear, N.H. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am. J. Clin. Dermatol. 2015, 16, 475–493. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Yang, J.Y.; Su, S.C.; Huang, S.P.; Wei, C.Y.; Chin, S.W.; Chiou, C.C.; Chu, S.C.; Ho, H.C.; et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat. Med. 2008, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Nassif, A.; Bensussan, A.; Dorothee, G.; Mami-Chouaib, F.; Bachot, N.; Bagot, M.; Boumsell, L.; Roujeau, J.C. Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysis. J. Investig. Dermatol. 2002, 118, 728–733. [Google Scholar] [CrossRef]
- Viard, I.; Wehrli, P.; Bullani, R.; Schneider, P.; Holler, N.; Salomon, D.; Hunziker, T.; Saurat, J.H.; Tschopp, J.; French, L.E. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998, 282, 490–493. [Google Scholar] [CrossRef]
- Nassif, A.; Moslehi, H.; Le Gouvello, S.; Bagot, M.; Lyonnet, L.; Michel, L.; Boumsell, L.; Bensussan, A.; Roujeau, J.C. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J. Investig. Dermatol. 2004, 123, 850–855. [Google Scholar] [CrossRef]
- Posadas, S.J.; Padial, A.; Torres, M.J.; Mayorga, C.; Leyva, L.; Sanchez, E.; Alvarez, J.; Romano, A.; Juarez, C.; Blanca, M. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J. Allergy Clin. Immunol. 2002, 109, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Caproni, M.; Torchia, D.; Schincaglia, E.; Volpi, W.; Frezzolini, A.; Schena, D.; Marzano, A.; Quaglino, P.; De Simone, C.; Parodi, A.; et al. Expression of cytokines and chemokine receptors in the cutaneous lesions of erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis. Br. J. Dermatol. 2006, 155, 722–728. [Google Scholar] [CrossRef]
- Quinn, A.M.; Brown, K.; Bonish, B.K.; Curry, J.; Gordon, K.B.; Sinacore, J.; Gamelli, R.; Nickoloff, B.J. Uncovering histologic criteria with prognostic significance in toxic epidermal necrolysis. Arch. Dermatol. 2005, 141, 683–687. [Google Scholar] [CrossRef]
- Lerch, M.; Mainetti, C.; Terziroli Beretta-Piccoli, B.; Harr, T. Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clin. Rev. Allergy Immunol. 2018, 54, 147–176. [Google Scholar] [CrossRef]
- Sotozono, C.; Ang, L.P.; Koizumi, N.; Higashihara, H.; Ueta, M.; Inatomi, T.; Yokoi, N.; Kaido, M.; Dogru, M.; Shimazaki, J.; et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology 2007, 114, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Kohanim, S.; Palioura, S.; Saeed, H.N.; Akpek, E.K.; Amescua, G.; Basu, S.; Blomquist, P.H.; Bouchard, C.S.; Dart, J.K.; Gai, X.; et al. Acute and Chronic Ophthalmic Involvement in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis—A Comprehensive Review and Guide to Therapy. II. Ophthalmic Disease. Ocul. Surf. 2016, 14, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Nuvoli, E.; Blasetti, F.; Posadinu, M.A.; Boscia, F. Plasmapheresis, Intravenous Immunoglobulins, and Autologous Serum Eyedrops in the Acute Eye Complications of Toxic Epidermal Necrolysis. Eur. J. Ophthalmol. 2017, 27, 658–663. [Google Scholar] [CrossRef]
- Saeed, H.N.; Chodosh, J. Ocular manifestations of Stevens-Johnson syndrome and their management. Curr. Opin. Ophthalmol. 2016, 27, 522–529. [Google Scholar] [CrossRef]
- Sharma, N.; Thenarasun, S.A.; Kaur, M.; Pushker, N.; Khanna, N.; Agarwal, T.; Vajpayee, R.B. Adjuvant Role of Amniotic Membrane Transplantation in Acute Ocular Stevens-Johnson Syndrome: A Randomized Control Trial. Ophthalmology 2016, 123, 484–491. [Google Scholar] [CrossRef]
- Dua, H.S.; Azuara-Blanco, A. Allo-limbal transplantation in patients with limbal stem cell deficiency. Br. J. Ophthalmol. 1999, 83, 414–419. [Google Scholar] [CrossRef]
- Rao, S.K.; Rajagopal, R.; Sitalakshmi, G.; Padmanabhan, P. Limbal allografting from related live donors for corneal surface reconstruction. Ophthalmology 1999, 106, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Shimazaki, J. Surgical treatment of children blinded by Stevens-Johnson syndrome. Am. J. Ophthalmol. 1999, 128, 573–581. [Google Scholar] [CrossRef]
- Honavar, S.G.; Bansal, A.K.; Sangwan, V.S.; Rao, G.N. Amniotic membrane transplantation for ocular surface reconstruction in Stevens-Johnson syndrome. Ophthalmology 2000, 107, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Romero-Rangel, T.; Stavrou, P.; Cotter, J.; Rosenthal, P.; Baltatzis, S.; Foster, C.S. Gas-permeable scleral contact lens therapy in ocular surface disease. Am. J. Ophthalmol. 2000, 130, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.; Cotter, J.M.; Baum, J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am. J. Ophthalmol. 2000, 130, 33–41. [Google Scholar] [CrossRef]
- Shimazaki, J.; Shimmura, S.; Fujishima, H.; Tsubota, K. Association of preoperative tear function with surgical outcome in severe Stevens-Johnson syndrome. Ophthalmology 2000, 107, 1518–1523. [Google Scholar] [CrossRef]
- Daya, S.M.; Ilari, F.A. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology 2001, 108, 126–133; discussion 33. [Google Scholar] [CrossRef]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Sotozono, C.; Kinoshita, S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 2001, 108, 1569–1574. [Google Scholar] [CrossRef]
- Tappin, M.J.; Pullum, K.W.; Buckley, R.J. Scleral contact lenses for overnight wear in the management of ocular surface disorders. Eye 2001, 15 Pt 2, 168–172. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Foulks, G.N.; John, M.E.; Cheng, K.; Hu, D. Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology 2002, 109, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Samson, C.M.; Nduaguba, C.; Baltatzis, S.; Foster, C.S. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology 2002, 109, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Aiba, M.; Goto, E.; Kato, N.; Shimmura, S.; Tsubota, K. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology 2002, 109, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Meller, D.; Prabhasawat, P.; John, T.; Espana, E.M.; Steuhl, K.-P.; Tseng, S.C. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology 2002, 109, 694–703. [Google Scholar] [CrossRef]
- Tsubota, K.; Shimmura, S.; Shinozaki, N.; Holland, E.J.; Shimazaki, J. Clinical application of living-related conjunctival-limbal allograft. Am. J. Ophthalmol. 2002, 133, 134–135. [Google Scholar] [CrossRef]
- Geerling, G.; Liu, C.S.; Dart, J.K.; Sieg, P.; Herold, J.; Collin, J.R. Sight and comfort: Complex procedures in end-stage Stevens-Johnson syndrome. Eye 2003, 17, 89–91. [Google Scholar] [CrossRef]
- Gomes, J.A.; Santos, M.S.; Ventura, A.S.; Donato, W.B.; Cunha, M.C.; Höfling-Lima, A.L. Amniotic membrane with living related corneal limbal/conjunctival allograft for ocular surface reconstruction in Stevens-Johnson syndrome. Arch Ophthalmol. 2003, 121, 1369–1374. [Google Scholar] [CrossRef][Green Version]
- Park, E.H.; Korn, T.S.; Vasani, S.N.; Kikkawa, D.O. Autologous allogeneic amniotic membrane grafting in Stevens-Johnson syndrome. Ophthalmic Plast. Reconstr. Surg. 2003, 19, 250–251. [Google Scholar] [CrossRef]
- Solomon, A.; Espana, E.M.; Tseng, S.C.G. Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology 2003, 110, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.; Panigrahi, D.; Barazi, M.; Abelson, M.; Butrus, S. A case of rofecoxib-associated stevens-johnson syndrome with corneal and conjunctival changes. Cornea 2004, 23, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Kaido, M.; Goto, E.; Dogru, M.; Tsubota, K. Punctal occlusion in the management of chronic Stevens-Johnson syndrome. Ophthalmology 2004, 111, 895–900. [Google Scholar] [CrossRef]
- Lam, N.S.; Yang, Y.H.; Wang, L.C.; Lin, Y.T.; Chiang, B.L. Clinical characteristics of childhood erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in Taiwanese children. J. Microbiol. Immunol. Infect.=Wei Mian Yu Gan Ran Za Zhi 2004, 37, 366–370. [Google Scholar]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef]
- Fukuda, M.; Nakao, A.; Hamada, S.; Liu, C.; Shimomura, Y. A case of severe Stevens-Johnson syndrome successfully treated by osteo-odonto-keratoprosthesis surgery. Jpn J. Ophthalmol. 2005, 49, 423–424. [Google Scholar] [CrossRef]
- Yip, L.W.; Thong, B.Y.; Tan, A.W.; Khin, L.W.; Chng, H.H.; Heng, W.J. High-dose intravenous immunoglobulin in the treatment of toxic epidermal necrolysis: A study of ocular benefits. Eye 2005, 19, 846–853. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yoshita, T.; Sugiyama, K.; Miyashita, K.; Niida, Y.; Koizumi, S.; Tseng, S.C. Amniotic membrane transplantation in acute phase of toxic epidermal necrolysis with severe corneal involvement. Ophthalmology 2006, 113, 126–132. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Ang, L.P.K.; Koizumi, N.; Yokoi, N.; Kinoshita, S. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology 2006, 113, 1765–1772. [Google Scholar] [CrossRef]
- Ang, L.P.; Sotozono, C.; Koizumi, N.; Suzuki, T.; Inatomi, T.; Kinoshita, S. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens-Johnson syndrome. Am. J. Ophthalmol. 2007, 143, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Huang, F.C.; Tseng, S.H.; Hsu, C.K.; Ho, C.L.; Sheu, H.M. Erythema multiforme, Stevens-Johnson Syndrome, and toxic epidermal necrolysis: Acute ocular manifestations, causes, and management. Cornea 2007, 26, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Cackett, P.; Mulvihill, A.; Fleck, B. Amniotic membrane grafting for conjunctival and lid surface disease in the acute phase of toxic epidermal necrolysis. J. AAPOS 2007, 11, 612–613. [Google Scholar] [CrossRef]
- Yip, L.W.; Thong, B.Y.; Lim, J.; Tan, A.W.; Wong, H.B.; Handa, S.; Heng, W.J. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: An Asian series. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Shome, D.; Natarajan, S. Nevirapine-induced Stevens-Johnson syndrome in an HIV patient. Cornea 2008, 27, 366–367. [Google Scholar] [CrossRef]
- Sayegh, R.R.; Ang, L.P.; Foster, C.S.; Dohlman, C.H. The Boston keratoprosthesis in Stevens-Johnson syndrome. Am. J. Ophthalmol. 2008, 145, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Uy, H.S.; Chan, P.S.; Ang, R.E. Topical bevacizumab and ocular surface neovascularization in patients with stevens-johnson syndrome. Cornea 2008, 27, 70–73. [Google Scholar] [CrossRef]
- Sotozono, C.; Ueta, M.; Koizumi, N.; Inatomi, T.; Shirakata, Y.; Ikezawa, Z.; Hashimoto, K.; Kinoshita, S. Diagnosis and Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis with Ocular Complications. Ophthalmology 2009, 116, 685–690. [Google Scholar] [CrossRef]
- Tougeron-Brousseau, B.; Delcampe, A.; Gueudry, J.; Vera, L.; Doan, S.; Hoang-Xuan, T.; Muraine, M. Vision-Related Function After Scleral Lens Fitting in Ocular Complications of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Am. J. Ophthalmol. 2009, 148, 852–859.e2. [Google Scholar] [CrossRef]
- Iyer, G.; Pillai, V.S.; Srinivasan, B.; Guruswami, S.; Padmanabhan, P. Mucous membrane grafting for lid margin keratinization in Stevens–Johnson syndrome: Results. Cornea 2010, 29, 146–151. [Google Scholar] [CrossRef]
- Marinho, D.R.; Burmann, T.G.; Kwitko, S. Labial salivary gland transplantation for severe dry eye due to chemical burns and Stevens-Johnson syndrome. Ophthalmic Plast Reconstr Surg 2010, 26, 182–184. [Google Scholar] [CrossRef]
- Shammas, M.C.; Lai, E.C.; Sarkar, J.S.; Yang, J.; Starr, C.E.; Sippel, K.C. Management of Acute Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Utilizing Amniotic Membrane and Topical Corticosteroids. Am. J. Ophthalmol. 2010, 149, 203–213.e2. [Google Scholar] [CrossRef] [PubMed]
- Shay, E.; Khadem, J.J.; Tseng, S.C.G. Efficacy and limitation of sutureless amniotic membrane transplantation for acute toxic epidermal necrolysis. Cornea 2010, 29, 359–361. [Google Scholar] [CrossRef]
- Das, J.K.; Medhi, J.; Chakravarty, R.; Soibam, R. Mucous membrane grafting for the post-Steven-Johnson syndrome symblepharon: A case report. Indian J. Ophthalmol. 2011, 59, 231–233. [Google Scholar] [CrossRef]
- Finkelstein, Y.; Soon, G.S.; Acuna, P.; George, M.; Pope, E.; Ito, S.; Shear, N.H.; Koren, G.; Shannon, M.W.; Garcia-Bournissen, F. Recurrence and outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatrics 2011, 128, 723–728. [Google Scholar] [CrossRef]
- Gregory, D.G. Treatment of acute stevensjohnson syndrome and toxic epidermal necrolysis using amniotic membrane: A review of 10 consecutive cases. Ophthalmology 2011, 118, 908–914. [Google Scholar] [CrossRef]
- Lau, B.; Mutyala, D.; Dhaliwal, D. A case report of doxycycline-induced Stevens-Johnson syndrome. Cornea 2011, 30, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheha, H.; Fu, Y.; Giegengack, M.; Tseng, S.C.G. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am. J. Ophthalmol. 2011, 152, 739–774.e1. [Google Scholar] [CrossRef] [PubMed]
- Onaran, Z.; Usta, G.; Koçak, M.; Örnek, K.; Büyükkoçak, U. Topical ophthalmic cyclosporine in the treatment of toxic epidermal necrolysis. Case Rep. Med. 2011, 2011, 416842. [Google Scholar] [CrossRef]
- Pujari, S.; Siddique, S.S.; Dohlman, C.H.; Chodosh, J. The boston keratoprosthesis type II: The massachusetts eye and ear infirmary experience. Cornea 2011, 30, 1298–1303. [Google Scholar] [CrossRef]
- Satake, Y.; Higa, K.; Tsubota, K.; Shimazaki, J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology 2011, 118, 1524–1530. [Google Scholar] [CrossRef]
- Reddy, S.C.; Tajunisah, I.; Tan, D.T. Osteo-odonto keratoprosthesis in Stevens-Johnson syndrome: A case report. Int. J. Ophthalmol. 2011, 4, 212–215. [Google Scholar]
- Uy, H.S.; Yu, E.N.; Sua, A.S. Histologic findings of bevacizumab-treated human conjunctiva in Stevens-Johnson syndrome. Cornea 2011, 30, 1273–1276. [Google Scholar] [CrossRef]
- Yagi, T.; Sotozono, C.; Tanaka, M.; Fuwa, M.; Sekiyama, E.; Ueta, M.; Tashiro, K.; Kinoshita, S. Cytokine storm arising on the ocular surface in a patient with Stevens—Johnson syndrome. Br. J. Ophthalmol. 2011, 95, 1030–1031. [Google Scholar] [CrossRef]
- Barua, A.; McKee, H.D.; Barbara, R.; Carley, F.; Biswas, S. Toxic epidermal necrolysis in a 15-month-old girl successfully treated with amniotic membrane transplantation. J. AAPOS 2012, 16, 478–480. [Google Scholar] [CrossRef]
- Hess, T.M.; Chew, H.F. Successful treatment of acute ocular involvement in Stevens-Johnson syndrome with amniotic membrane transplantation: A case report. Can. J. Ophthalmol. 2012, 47, e44–e46. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Jayaram, A.; Verner, R.; Lin, A.; Bouchard, C. Indications and outcomes of amniotic membrane transplantation in the management of acute stevens-johnson syndrome and toxic epidermal necrolysis: A case-control study. Cornea 2012, 31, 1394–1402. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Y.; Wang, L.; Du, G.; Yu, J.; Song, J.; Chiang, H.H. Long-term outcomes of MICOF keratoprosthesis in the end stage of autoimmune dry eyes: An experience in China. Br. J. Ophthalmol. 2012, 96, 28–33. [Google Scholar] [PubMed]
- Kesarwani, S.; Sahu, S.K.; Basu, S. Bilateral response after unilateral subconjunctival bevacizumab injection in a child with Stevens-Johnson syndrome. J AAPOS 2012, 16, 309–311. [Google Scholar] [CrossRef]
- Sant’ Anna, A.E.; Hazarbassanov, R.M.; de Freitas, D.; Gomes, J. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br. J. Ophthalmol. 2012, 96, 234–239. [Google Scholar] [CrossRef]
- Ciralsky, J.B.; Sippel, K.C. Prompt versus delayed amniotic membrane application in a patient with acute Stevens-Johnson syndrome. Clin. Ophthalmol. 2013, 7, 1031–1034. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, S.W.; Kim, M.K.; Wee, W.R. Effect of age and early intervention with a systemic steroid, intravenous immunoglobulin or amniotic membrane transplantation on the ocular outcomes of patients with Stevens-Johnson syndrome. Korean J. Ophthalmol. KJO 2013, 27, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Kolomeyer, A.M.; Do, B.K.; Tu, Y.; Chu, D.S. Placement of ProKera in the management of ocular manifestations of acute Stevens-Johnson syndrome in an outpatient. Eye Contact Lens 2013, 39, e7–e11. [Google Scholar] [CrossRef]
- Ling, J.D.; Gire, A.; Pflugfelder, S.C. PROSE therapy used to minimize corneal trauma in patients with corneal epithelial defects. Am. J. Ophthalmol 2013, 155, 615–619.e2. [Google Scholar] [CrossRef] [PubMed]
- Md Noh, U.K.; Then, K.Y. Spontaneous bilateral corneal perforation in stevens- johnsons syndrome—A challenge in management. Malays J. Med. Sci. 2013, 20, 84–87. [Google Scholar]
- Prabhasawat, P.; Tesavibul, N.; Karnchanachetanee, C.; Kasemson, S. Efficacy of cyclosporine 0.05% eye drops in Stevens Johnson syndrome with chronic dry eye. J. Ocul. Pharmacol. Ther. 2013, 29, 372–377. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Miyakoda, K.; Kaneda, H.; Fukushima, M.; et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology 2013, 120, 193–200. [Google Scholar] [CrossRef]
- Tomlins, P.J.; Parulekar, M.V.; Rauz, S. “Triple-TEN” in the treatment of acute ocular complications from toxic epidermal necrolysis. Cornea 2013, 32, 365–369. [Google Scholar] [CrossRef]
- Basu, S.; Sureka, S.; Shukla, R.; Sangwan, V. Boston type 1 based keratoprosthesis (Auro Kpro) and its modification (LVP Kpro) in chronic Stevens Johnson syndrome. BMJ Case Rep 2014, 2014, bcr2013202756. [Google Scholar] [CrossRef]
- de Oliveira, L.A.; Magalhães, F.P.; Hirai, F.E.; de Sousa, L.B. Experience with Boston keratoprosthesis type 1 in the developing world. Can. J. Ophthalmol. 2014, 49, 351–357. [Google Scholar] [CrossRef]
- Heur, M.; Bach, D.; Theophanous, C.; Chiu, G.B. Prosthetic replacement of the ocular surface ecosystem scleral lens therapy for patients with ocular symptoms of chronic Stevens-Johnson syndrome. Am. J. Ophthalmol. 2014, 158, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Srinivasan, B.; Agarwal, S.; Kamala Muralidharan, S.; Arumugam, S. Comprehensive approach to ocular consequences of Stevens Johnson Syndrome—The aftermath of a systemic condition. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 457–467. [Google Scholar] [CrossRef]
- López-García, J.S.; Rivas, L.; García-Lozano, I.; Conesa, E.; Elosua, I.; Murube, J. Amniotic membrane transplantation in acute toxic epidermal necrolysis: Histopathologic changes and ocular surface features after 1-year follow-up. Eur. J. Ophthalmol. 2014, 24, 667–675. [Google Scholar] [CrossRef]
- Pruet, C.M.; Queen, J.H.; Kim, G. Amnion doughnut: A novel method for sutureless fixation of amniotic membrane to the bulbar and palpebral conjunctiva in acute ocular-involving Stevens-Johnson syndrome. Cornea 2014, 33, 1240–1244. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Kaneda, H.; Fukushima, M.; Kinoshita, S. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol 2014, 92, e447–e453. [Google Scholar] [CrossRef] [PubMed]
- Sotozono, C.; Yamauchi, N.; Maeda, S.; Kinoshita, S. Tear Exchangeable Limbal Rigid Contact Lens for Ocular Sequelae Resulting From Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis. Am. J. Ophthalmol. 2014, 158, 983–993.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, S.; Wang, T.; Gao, H.; Shi, W. Modified tectonic keratoplasty with minimal corneal graft for corneal perforation in severe Stevens--Johnson syndrome: A case series study. BMC Ophthalmol. 2014, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Pratap, V.B. Amniotic membrane transplantation (AMT) without the use of sutures/fibrin glue. Nepal. J. Ophthalmol. 2015, 7, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.K.; Basak, S.K.; Padilla, M.D.; Yu, F.; Aldave, A.J. International Outcomes of the Boston Type I Keratoprosthesis in Stevens-Johnson Syndrome. Cornea 2015, 34, 1387–1394. [Google Scholar] [CrossRef]
- Papakostas, T.D.; Le, H.G.; Chodosh, J.; Jacobs, D.S. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of stevens-johnson syndrome/toxic epidermal necrolysis. Ophthalmology 2015, 122, 248–253. [Google Scholar] [CrossRef]
- Catt, C.J.; Hamilton, G.M.; Fish, J.; Mireskandari, K.; Ali, A. Ocular Manifestations of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Children. Am. J. Ophthalmol. 2016, 166, 68–75. [Google Scholar] [CrossRef]
- Cheung, C.S.Y.; Ali, A.; Chew, H.F. Successful treatment of acute ocular-involving toxic epidermal necrolysis using amniotic membrane suture fixated to custom designed symblepharon rings. Cornea 2016, 35, 578–581. [Google Scholar] [CrossRef]
- Gregory, D.G. New Grading System and Treatment Guidelines for the Acute Ocular Manifestations of Stevens-Johnson Syndrome. Ophthalmology 2016, 123, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Srinivasan, B.; Agarwal, S.; Pillai, V.S.; Ahuja, A. Treatment Modalities and Clinical Outcomes in Ocular Sequelae of Stevens-Johnson Syndrome Over 25 Years--A Paradigm Shift. Cornea 2016, 35, 46–50. [Google Scholar]
- La Porta Weber, S.; Becco de Souza, R.; Gomes JÁ, P.; Hofling-Lima, A.L. The Use of the Esclera Scleral Contact Lens in the Treatment of Moderate to Severe Dry Eye Disease. Am. J. Ophthalmol. 2016, 163, 167–173.e1. [Google Scholar]
- Ma, K.N.; Thanos, A.; Chodosh, J.; Shah, A.S.; Mantagos, I.S. A Novel Technique for Amniotic Membrane Transplantation in Patients with Acute Stevens-Johnson Syndrome. Ocul. Surf. 2016, 14, 31–36. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Frank, G.S.; Hink, E.M.; Palestine, A.G.; Gregory, D.G.; McCourt, E.A. Amniotic membrane transplants in the pediatric population. J. AAPOS 2017, 21, 215–218. [Google Scholar] [CrossRef]
- Ma, X.; Xiang, R.; Meng, X.; Qin, L.; Wu, Y.; Tain, L.; Jiang, Y.; Huang, Y.; Wang, L. Russian Keratoprosthesis in Stevens-Johnson Syndrome. Cornea 2017, 36, 304–309. [Google Scholar] [CrossRef]
- Barry, R.J.; Zanetto, U.; Kolli, S.; Morjaria, R. Toxic epidermal necrolysis: The red eye and red herrings in casualty. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef]
- Basu, S.; Shanbhag, S.S.; Gokani, A.; Kedar, R.; Bahuguna, C.; Sangwan, V.S. Chronic Ocular Sequelae of Stevens-Johnson Syndrome in Children: Long-term Impact of Appropriate Therapy on Natural History of Disease. Am. J. Ophthalmol. 2018, 189, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.; Benson, M.D.; Plemel, D.J.A.; Mahmood, M.N.; Chan, S.M. A diagnosis of Stevens-Johnson Syndrome (SJS) in a patient presenting with superficial keratitis. Am. J. Ophthalmol. Case Rep. 2018, 11, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; de Libero, C.; Zamma Gallarati, B.; Fortunato, P.; Piozzi, E. Propranolol eye drops in patients with corneal neovascularization. Medicine 2018, 97, e13002. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhai, J.; Liao, G.; Chen, J. Boston Type I Keratoprosthesis Implantation Following Autologous Submandibular Gland Transplantation for End Stage Ocular Surface Disorders. Ocul. Immunol. Inflamm. 2018, 26, 452–455. [Google Scholar]
- Kalhorn, A.J.; Tawse, K.L.; Shah, A.A.; Jung, J.L.; Gregory, D.G.; McCourt, E.A. Maternal Serum Eye Drops in the Management of Pediatric Persistent Corneal Epithelial Defects: A Case Series. Cornea 2018, 37, 912–915. [Google Scholar] [CrossRef]
- Nguyen, M.T.B.; Thakrar, V.; Chan, C.C. EyePrintPRO therapeutic scleral contact lens: Indications and outcomes. Can. J. Ophthalmol. 2018, 53, 66–70. [Google Scholar]
- OOsaki, T.H.; Sant’aNna, A.E.; Osaki, M.H.M.; Kikkawa, D.O.M.; Yabumoto, C.; Yang, P.; Korn, B.S.M. Management of Severe Cicatricial Entropion With Labial Mucous Membrane Graft in Cicatricial Ocular Surface Disorders. J. Craniofacial Surg. 2018, 29, 1531–1534. [Google Scholar] [CrossRef]
- Sato, S.; Kanbe, T.; Tamaki, Z.; Furuichi, M.; Uejima, Y.; Suganuma, E.; Takano, T.; Kawano, Y. Clinical features of Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr. Int. 2018, 60, 697–702. [Google Scholar]
- Sevik, M.O.; Turhan, S.A.; Toker, E. Topical Treatment of Persistent Epithelial Defects with a Matrix Regenerating Agent. J. Ocul. Pharmacol. Ther. 2018, 34, 621–627. [Google Scholar] [CrossRef]
- Baş, Z.; Uçakhan Gündüz, Ö. Sutureless Amniotic Membrane Transplantation in a Pediatric Patient with Acute Toxic Epidermal Necrolysis. Turk. J. Ophthalmol. 2019, 49, 356–360. [Google Scholar] [CrossRef]
- Choe, H.R.; Yoon, C.H.; Kim, M.K. Ocular Surface Reconstruction Using Circumferentially-trephined Autologous Oral Mucosal Graft Transplantation in Limbal Stem Cell Deficiency. Korean J. Ophthalmol. 2019, 33, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, V.; Agarwal, S.; Srinivasan, B.; Krishnakumar, S.; Krishnan, U.M.; Iyer, G. Clinical Outcome of Autologous Cultivated Oral Mucosal Epithelial Transplantation in Ocular Surface Reconstruction. Cornea 2019, 38, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Miyachi, H.; Kataoka, K.; Maru, Y.; Togawa, Y.; Matsue, H. Case of fertility treatment-induced Stevens-Johnson syndrome with a severe ocular complication. J. Dermatol. 2019, 46, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Srinivasan, B.; Agarwal, S.; Ravindran, R.; Rishi, E.; Rishi, P.; Krishnamoorthy, S. Boston Type 2 keratoprosthesis- mid term outcomes from a tertiary eye care centre in India. Ocul. Surf. 2019, 17, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Devrim, I.; Çağlar, I.; Bayram, N.; Kundak, S.; Apa, H.; Altan, E.V. Stevens-Johnson syndrome and toxic epidermal necrolysis: A report of six cases. Turk. J. Pediatr. 2019, 61, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, Y.J.; Choi, S.H.; Oh, J.Y.; Kim, M.K. Long-term effect of corneoscleral contact lenses on refractory ocular surface diseases. Contact Lens Anterior Eye 2019, 42, 399–405. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Chodosh, J.; Saeed, H.N. Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul. Surf. 2019, 17, 560–564. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, R.; Jacobs, D.S.; Saeed, H.N. Prosthetic Replacement of the Ocular Surface Ecosystem Treatment for Ocular Surface Disease in Pediatric Patients With Stevens-Johnson Syndrome. Am. J. Ophthalmol. 2019, 201, 1–8. [Google Scholar] [CrossRef]
- Xiang, Q.; Gao, X.; Fang, J.; Pi, L.; Chen, X.; Chen, L.; Liu, Q. Lacrimal passage irrigation in children with Stevens-Johnson syndrome or toxic epidermal necrolysis: A five-year retrospective study. BMC Ophthalmol. 2019, 19, 22. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, M.K.; Seo, K.Y.; Ueta, M.; Yoon, K.C. Effectiveness of photodynamic therapy with verteporfin combined with intrastromal bevacizumab for corneal neovascularization in Stevens-Johnson syndrome. Int. Ophthalmol. 2019, 39, 55–62. [Google Scholar] [CrossRef]
- Abrol, A.; Gulanikar, A.; Thakre, S.; Patel, A. Study of Ocular Manifestations of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Indian Dermatol. Online J. 2020, 11, 570–574. [Google Scholar]
- Alvarado-Villacorta, R.; García-Carmona, K.P.; Martínez-Pardo, M.E.; Vázquez-Maya, L. Allogeneic Limbal Epithelial Transplantation Modified With Solid Platelet-Rich Plasma for Bilateral Limbal Stem Cell Deficiency. Cornea 2020, 39, 1311–1314. [Google Scholar] [CrossRef]
- de la Sen-Corcuera, B.; Montero-Iruzubieta, J.; Sanchez-Avila, R.M.; Orive, G.; Anitua, E.; Caro-Magdaleno, M.; Merayo-Lloves, J. Plasma Rich in Growth Factors for the Treatment of Cicatrizing Conjunctivitis. Clin. Ophthalmol. 2020, 14, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Itoi, M.; Ueta, M.; Ogino, K.; Sumi, E.; Imai, K.; Teramukai, S.; Kinoshita, S.; Sotozono, C. Clinical trial to evaluate the therapeutic benefits of limbal-supported contact lens wear for ocular sequelae due to Stevens-Johnson syndrome/toxic epidermal necrolysis. Contact Lens Anterior Eye 2020, 43, 535–542. [Google Scholar] [PubMed]
- Nakatsuka, A.S.; Lin, A. Ocular management of acute Stevens-Johnson syndrome in a 14-month-old child. Can. J. Ophthalmol. 2020, 55, e213–e214. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Hall, L.; Chodosh, J.; Saeed, H.N. Long-term outcomes of amniotic membrane treatment in acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul. Surf. 2020, 18, 517–522. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Shah, S.; Singh, M.; Bahuguna, C.; Donthineni, P.R.; Basu, S. Lid-Related Keratopathy in Stevens-Johnson Syndrome: Natural Course and Impact of Therapeutic Interventions in Children and Adults. Am. J. Ophthalmol. 2020, 219, 357–365. [Google Scholar] [CrossRef]
- Shegaonkar, S.H. Bilateral panophthalmitis following toxic epidermal necrolysis: A case report. Indian J. Ophthalmol. 2020, 68, 538–540. [Google Scholar] [CrossRef]
- Shimazaki, J.; Satake, Y.; Higa, K.; Yamaguchi, T.; Noma, H.; Tsubota, K. Long-term outcomes of cultivated cell sheet transplantation for treating total limbal stem cell deficiency. Ocul. Surf. 2020, 18, 663–671. [Google Scholar] [CrossRef]
- Sudana, P.; Basu, S.; Shanbhag, S.S. Oral mucous membrane grafts for total symblepharon and lid margin keratinisation post Stevens-Johnson syndrome. BMJ Case Rep. 2020, 13, e239383. [Google Scholar] [CrossRef]
- Chan, S.; Gole, G.A.; Lee, G.A. Amniotic Membrane-Covered Conformer and Fibrin Glue for Toxic Epidermal Necrolysis. Cornea 2021, 40, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.M.; Gise, R.; Scelfo, C.; Mantagos, I.S. Amniotic membrane transplantation in a 2-month-old infant with toxic epidermal necrolysis. Am. J. Ophthalmol. Case Rep. 2021, 21, 101017. [Google Scholar] [CrossRef]
- Hall, L.N.; Shanbhag, S.S.; Rashad, R.; Chodosh, J.; Saeed, H.N. The effects of systemic cyclosporine in acute Stevens-Johnson syndrome/toxic epidermal necrolysis on ocular disease. Ocul. Surf. 2021, 19, 128–132. [Google Scholar] [CrossRef]
- Huhtanen, A.; Lindsay, R.G. Management of Stevens-Johnson syndrome using a mini-scleral contact lens. Clin. Exp. Optom. 2021, 104, 233–236. [Google Scholar] [CrossRef]
- Jabbour, S.; Din, N.; Logeswaran, A.; Taberno Sanchez, S.; Ahmad, S. Clinical Characteristics of Patients With Chronic Stevens-Johnson Syndrome Treated at a Major Tertiary Eye Hospital Within the United Kingdom. Front Med. 2021, 8, 644795. [Google Scholar] [CrossRef]
- Jovanovic, N.; Russell, W.W.; Heisel, C.J.; Hood, C.T.; Kahana, A. Direct Injection of 5-Fluorouracil Improves Outcomes in Cicatrizing Conjunctival Disorders Secondary to Systemic Disease. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.-K.; Tsai, T.-Y.; Pan, L.-Y.; Chen, S.-Y.; Hsiao, C.-H.; Yeh, L.-K.; Tan, H.-Y.; Lu, C.-W.; Chen, C.-B.; Chung, W.-H. Clinical Aspects of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis With Severe Ocular Complications in Taiwan. Front. Med. 2021, 8, 661891. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.-K.; Saeed, H.N.; Chodosh, J.; Wang, C.-W.; Chung, Y.-C.; Wei, L.-C.; Kuo, M.-T.; Liang, C.-M.; Chang, J.W.-C.; Chung, W.-H.; et al. Ocular manifestations of anti-neoplastic immune checkpoint inhibitor-associated Stevens-Johnson syndrome/toxic epidermal necrolysis in cancer patients. Ocul. Surf. 2021, 22, 47–50. [Google Scholar] [CrossRef]
- Mahmood, A.H.; Alharbi, A.S.; Almanea, B.A.; Alsaati, A.F. Sutureless Amniotic Membrane (ProKera®) and Intravenous Immunoglobulin in the Management of Ocular Complications of Stevens-Johnson Syndrome-Toxic Epidermal Necrolysis Overlap. Cureus 2021, 13, e16989. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Elsawah, K.; Dhillon, N.; Soliman, S.; Laginaf, M.; Lodhia, V.; Lake, D.; Hamada, S.; Elalfy, M. Management of Persistent Corneal Epithelial Defects with Human Amniotic Membrane-derived Dry Matrix. Clin. Ophthalmol. 2021, 15, 2231–2238. [Google Scholar] [CrossRef]
- Mieno, H.; Ueta, M.; Kinoshita, F.; Teramukai, S.; Kinoshita, S.; Sotozono, C. Corticosteroid Pulse Therapy for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Patients With Acute Ocular Involvement. Am. J. Ophthalmol. 2021, 231, 194–199. [Google Scholar] [CrossRef]
- Mimouni, M.; Trinh, T.; Sorkin, N.; Cohen, E.; Santaella, G.; Rootman, D.S.; Slomovic, A.R.; Chan, C.C. Sutureless dehydrated amniotic membrane for persistent epithelial defects. Eur. J. Ophthalmol. 2021, 32, 11206721211011354. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.M.; Hyon, J.Y.; Kim, M.K.; Oh, J.Y.; Choi, H.J. Large diameter scleral lens benefits for Asians with intractable ocular surface diseases: A prospective, single-arm clinical trial. Sci. Rep. 2021, 11, 2288. [Google Scholar] [CrossRef]
- Ngowyutagon, P.; Prabhasawat, P.; Chirapapaisan, C.; Jaru-Ampornpan, P.; Pornpanich, K.; Ekpo, P.; Sukon, N.; Matamnan, S.M. Successful Ocular Surface Reconstruction in Complete Ankyloblepharon With the Simple Oral Mucosal Epithelial Transplantation Technique: A Case Report. Cornea 2021, 40, 1482–1486. [Google Scholar] [CrossRef]
- Pushker, N.; Gorimanipalli, B.; Sharma, N.; Kashyap, S.; Bajaj, M.S. Mucous membrane grafting (fibrin glue vs. suture) for lid margin pathologies in Stevens-Johnson syndrome: Randomized comparative study. Eye 2021, 35, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, A.; Sant’Anna, E.B.; Osaki, T.H.; Pereira Gomes, J. A new option for treatment of severe cicatricial entropion in patients with Stevens-Johnson syndrome. Ocul. Surf. 2021, 22, 80–82. [Google Scholar] [CrossRef]
- Santamaria, J.A.; Cancio, L.C.; Reed, D.; Phillips, H.; Chen, S.; Carlton, D.K.; Johnson, A.J. Complete Fusion of Both Eyelids in Stevens-Johnson Syndrome: Case Report. J. Burn. Care Res. 2021, 42, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, T.; Hassanpour, K.; Arabi, A.; Ansari, I.; Sadoughi, M.M. Corona virus disease 2019-associated Stevens-Johnson syndrome: A case report. BMC Ophthalmol. 2021, 21, 274. [Google Scholar] [CrossRef]
- Utine, C.A.; Birlik, M.; Özizmirliler, D.; Karakaş, A.; Akbulut, B.; Durak, I. TNF-α Inhibitors for the Management of Intractable Corneal Melt: Report of Three Cases and Review of the Literature. Eye Contact Lens 2021, 47, 372–377. [Google Scholar] [CrossRef]
- Venugopal, R.; Nagpal, R.; Mohanty, S.; Sen, S.; Kashyap, S.; Agarwal, T.; Maharana, P.K.; Vajpayee, R.B.; Sharma, N. Outcomes of Cultivated Oral Mucosal Epithelial Transplantation in Eyes With Chronic Stevens-Johnson Syndrome Sequelae. Am. J. Ophthalmol. 2021, 222, 82–91. [Google Scholar] [CrossRef]
- Yang, Y.; Fung, S.S.M.; Chew, H.; Mireskandari, K.; Ali, A. Amniotic membrane transplantation for Stevens-Johnson syndrome/toxic epidermal necrolysis: The Toronto experience. Br. J. Ophthalmol. 2021, 105, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Aziza, Y.; Harada, K.; Ueta, M.; Fukuoka, H.; Kinoshita, S.; Sotozono, C. Challenges in the management of bilateral eyelid closure in Stevens-Johnson Syndrome. Am. J. Ophthalmol. Case Rep. 2022, 26, 101473. [Google Scholar] [CrossRef]
- Booranapong, W.; Kosrirukvongs, P.; Duangsa-Ard, S.; Kasetsinsombat, K.; Sa-Ngiamsuntorn, K.; Wongkajornsilp, A. Transplantation of autologous cultivated oral mucosal epithelial sheets for limbal stem cell deficiency at Siriraj Hospital: A case series. J. Med. Case Rep. 2022, 16, 298. [Google Scholar] [CrossRef]
- Iannetti, L.; Liberali, M.; Armentano, M.; Alisi, L.; Visioli, G.; Mastromarino, D.; Brauner, E.; Iannetti, G. Osteo-odonto-keratoprosthesis According to Strampelli Original Technique: A Retrospective Study With Up to 30 Years of Follow-up. Am. J. Ophthalmol. 2022, 242, 56–68. [Google Scholar] [CrossRef]
- Kanazawa, M.; Tominaga, K.; Kanamori, A.; Tanaka, T.; Masuyama, S.; Watanabe, S.; Abe, K.; Yamamiya, A.; Goda, K.; Irisawa, A. A Case of Stevens-Johnson Syndrome Complicated with Multimatrix System Mesalamine in Ulcerative Colitis. Medicina 2022, 58, 276. [Google Scholar] [CrossRef]
- Katz, E.A.; Sunshine, S.; Mun, C.; Sarwar, M.; Surenkhuu, B.; Pradeep, A.; Jain, S. Combinatorial therapy with immunosuppressive, immunomodulatory and tear substitute eyedrops (“Triple Play”) in Recalcitrant Immunological Ocular Surface Diseases. Ocul. Surf. 2022, 23, 1–11. [Google Scholar] [CrossRef]
- Komai, S.; Inatomi, T.; Nakamura, T.; Ueta, M.; Horiguchi, G.; Teramukai, S.; Kimura, Y.; Kagimura, T.; Fukushima, M.; Kinoshita, S.; et al. Long-term outcome of cultivated oral mucosal epithelial transplantation for fornix reconstruction in chronic cicatrising diseases. Br. J. Ophthalmol. 2022, 106, 1355–1362. [Google Scholar] [CrossRef]
- Liao, J.; Asghari, B.; Carrasquillo, K.G. Regression of corneal opacity and neovascularization in Stevens-Johnson syndrome and Toxic Epidermal Necrolysis with the use of prosthetic replacement of the ocular surface ecosystem (PROSE) treatment. Am. J. Ophthalmol Case Rep. 2022, 26, 101520. [Google Scholar] [CrossRef] [PubMed]
- Mitani, K.; Hida, S.; Fujino, H.; Sumimoto, S. Rare case of Stevens-Johnson syndrome with bronchiolitis obliterans as a chronic complication. BMJ Case Rep. 2022, 15, e249224. [Google Scholar] [CrossRef]
- Pradeep, T.G.; Shetti, S.A. Ocular manifestations in acute stage Stevens-Johnson syndrome/toxic epidermal necrolysis—A retrospective study in a tertiary hospital in South India. Taiwan J. Ophthalmol. 2022, 12, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Jun, I.; Kim, T.I.; Seo, K.Y.; Kim, E.K. Pembrolizumab-induced Stevens-Johnson Syndrome with Severe Ocular Complications. Ocul. Immunol. Inflamm. 2022, 30, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, S.; Basu, S.; Shanbhag, S.S. Chronic Ocular Sequelae and Subsequent Surgical Interventions in Stevens-Johnson Syndrome After Amniotic Membrane Transplantation. Cornea 2022, 41, 632–634. [Google Scholar] [CrossRef]
- Sims, J.R.; Kozlova, A.; Ostrovsky, A. Modified technique for sutureless amniotic membrane transplantation in acute Stevens-Johnson syndrome using fibrin sealant. Ocul. Surf. 2022, 25, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Susiyanti, M.; Kurnia, D.A.; Fasha, I.; Irawati, Y.; Rachmadi, L.; Liem, I.K.; Artini, W. Treatment of Severe Dry Eye in Stevens-Johnson Syndrome with Umbilical Cord Serum Eye Drops. Clin. Ophthalmol. 2022, 16, 4089–4095. [Google Scholar] [CrossRef]
- Arboleda, A.; Phansalkar, R.B.; Amescua, G.; Lee, W.-S.; Brandt, J.D.; Mannis, M.J.; Kossler, A.L.; Lin, C.C. Preparing the Ocular Surface for a Boston Keratoprosthesis Type 1 Through En Bloc Minor Salivary Gland Transplantation and Mucous Membrane Grafting in End-Stage Stevens-Johnson Syndrome. Cornea 2023, 42, 912–916. [Google Scholar] [CrossRef]
- Asghari, B.; Carrasquillo, K.G.; Kwok, A.; Sippel, K.C. Use of PROSE for long-term ocular surface support in patients with a permanent keratoprosthesis. Am. J. Ophthalmol. Case Rep. 2023, 32, 101919. [Google Scholar] [CrossRef]
- Bourke, C.M.; Cummings, B.K.; Hurley, D.J.; Murphy, C.C.; Chamney, S. Isolated Ocular Stevens-Johnson Syndrome Caused by Lymecycline in a Patient with Underlying Ulcerative Colitis. J. Clin. Med. 2023, 12, 5259. [Google Scholar] [CrossRef]
- Ceylan, A.; Mergen, B.; Aydin, F.O.; Avci, E.; Yildirim, Y. Sutureless Amniotic Membrane Transplantation Using Pediatric Nasogastric Tube for Patients With Acute Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Eye Contact Lens 2023, 49, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.I.; Tsai, C.C. Self-Retained, Sutureless Amniotic Membrane Transplantation for the Management of Ocular Surface Diseases. J. Clin. Med. 2023, 12, 6222. [Google Scholar] [CrossRef]
- Doctor, M.B.; Rajagopal, R.N.; Basu, S. Simple oral mucosal epithelial transplantation (SOMET) for ocular surface reconstruction in Stevens-Johnson Syndrome: A case report. Int. J. Surg. Case Rep. 2023, 110, 108643. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Singh, G.; Bashir, H.; Sangwan, V.; Mathur, U. Managing chronic inflammation in ocular sequelae of Stevens Johnson Syndrome to restore vision. Ocul. Surf. 2023, 28, 40–41. [Google Scholar] [CrossRef]
- Hooshmandi, S.; Hassanpour, K.; Veisi, A.; Movafaghi, V.; Langari, F.; Sadoughi, M.-M.; Javadi, M.A. Management of Large Conjunctival Cysts in a Patient with Stevens-Johnson Syndrome: A Case Report and Review of the Literature. Case Rep. Ophthalmol. 2023, 14, 528–534. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ueta, M.; Inatomi, T.; Fukuoka, H.; Mieno, H.; Tamagawa-Mineoka, R.; Katoh, N.; Kinoshita, S.; Sotozono, C. Topical Betamethasone Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis with Ocular Involvement in the Acute Phase. Am. J. Ophthalmol. 2023, 253, 142–151. [Google Scholar]
- Mimouni, M.; Cole, E.; Kim, S.J.; Schiff, J.; Cardella, C.; Tinckam, K.J.; Slomovic, A.R.; Chan, C.C. Outcomes of keratolimbal allograft from ABO compatible donors for severe bilateral limbal stem cell deficiency. Ocul. Surf. 2023, 27, 48–53. [Google Scholar] [CrossRef]
- Mortensen, X.M.; Shenkute, N.T.; Zhang, A.Y.; Banna, H. Clinical Outcome of Amniotic Membrane Transplant in Ocular Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis at a Major Burn Unit. Am. J. Ophthalmol. 2023, 256, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, A.P.; Sinha, R.; Bari, A.; Velpandian, T.; Sen, S.; Agarwal, T.; Sharma, N.; Titiyal, J.S. Retinol palmitate in management of chronic Steven-Johnson Syndrome with ocular surface keratinization. Ocul. Surf. 2023, 30, 160–167. [Google Scholar] [CrossRef]
- Shamloul, G.; Desai, M.; Laslett, N. An Unusual Case of Stevens-Johnson/Toxic Epidermal Necrolysis Overlap Syndrome in HER2 (Human Epidermal Growth Factor Receptor 2)-Positive Breast Cancer Patient Treated With Docetaxel. Cureus 2023, 15, e37590. [Google Scholar] [CrossRef] [PubMed]
- Shlager, G.; Nakhla, M.N.; Pritchett, D.; Brocks, D. Case report: Concomitant use of nightly vitamin A ointment with daily PROSE wear for ocular surface disease associated with chronic Stevens-Johnson syndrome. Am. J. Ophthalmol. Case Rep. 2023, 32, 101943. [Google Scholar] [CrossRef] [PubMed]
- Shree, N.; Das, S.; Arya, D.; Srivastava, A.; Singh, A.; Sangwan, V. Single-Staged Surgical Correction of Eyelid Sequelae Along With Lid Margin Mucous Membrane Grafting in Stevens-Johnson Syndrome and Other Cicatricial Ocular Surface Diseases. Cornea 2023, 42, 404–411. [Google Scholar] [CrossRef]
- Singh, S.; Basu, S. 5-Fluorouracil as a targeted lacrimal gland therapy for chronic Stevens-Johnson syndrome: A pilot study. Indian J. Ophthalmol. 2023, 71, 1626–1629. [Google Scholar] [CrossRef]
- Singh, S.; Basu, S.; Jakati, S. Cicatricial Entropion in Chronic Cicatrizing Conjunctivitis: Potential Pathophysiologic Mechanisms and Long-Term Outcomes of a Modified Technique. Ophthalmic Plast. Reconstr. Surg. 2023, 39, 563–569. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, Y.; Imai, Y.; Yamaguchi, Y. Eyelid and Vaginal Adhesions as Severe Sequelae of Toxic Epidermal Necrolysis. Cureus 2023, 15, e41496. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Chang, H.-T.; Weng, S.-W.; Chu, C.-C.; Wang, Y.-C.; Zhao, Z.; Mai, E.L.-C. Ocular surface reconstruction of Steven Johnson syndrome/toxic epidermal necrolysis affected eye—A case report. Heliyon 2023, 9, e12590. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Ueta, M.; Kinoshita, S.; Kida, T.; Sotozono, C. Long-Term Benefits of Tear Exchangeable Limbal-Rigid Contact Lens Wear Therapy in Stevens-Johnson Syndrome Cases. Eye Contact Lens 2023, 49, 247–253. [Google Scholar] [CrossRef]
- Zhang, N.; Geng, X.; Liu, R.; Liu, X.; Cui, H.; Dou, R.; Hou, S.; Li, J.; Zhu, L.; Li, Z. Novel technique for amniotic membrane transplantation for acute Stevens-Johnson syndrome/toxic epidermal necrolysis patients. Heliyon 2023, 9, e18853. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Qiu, W.Y.; Xu, Y.S.; Yao, Y.F. Clinical efficacy of a new surgical technique of oral mucosal epithelial transplantation for severe ocular surface disorders. BMC Ophthalmol. 2023, 23, 145. [Google Scholar] [CrossRef]
- Abulfateh, F.K.; AlHaqbani, Y.J.; Albuainain, A.S. Early Recognition and Management of Ocular Manifestations of Toxic Epidermal Necrolysis in a Pediatric Patient: A Case Report. Cureus 2024, 16, e70323. [Google Scholar] [CrossRef]
- Aziza, Y.; Imai, K.; Itoi, M.; Yoshioka, H.; Komai, S.; Kitazawa, K.; Sitompul, R.; Ueta, M.; Fukuoka, H.; Inatomi, T.; et al. Strategic combination of cultivated oral mucosal epithelial transplantation and postoperative limbal-rigid contact lens-wear for end-stage ocular surface disease: A retrospective cohort study. Br. J. Ophthalmol. 2024, 108, 1177–1183. [Google Scholar] [CrossRef]
- Bai, H.; Wang, X.; Wang, Y.; Li, Y.; Guo, W.; Lv, J.; Li, Y.; Hao, Z.; Pan, X. Ornidazole induced Stevens-Johnson syndrome without body surface involved: A case report. Medicine 2024, 103, e37164. [Google Scholar]
- Chen, Y.K.; Chi, C.L.; Lai, C.H.; Wu, P.L. Conjunctival squamous metaplasia on amniotic membrane in Stevens-Johnson syndrome: A case report. BMC Ophthalmol. 2024, 24, 484. [Google Scholar] [CrossRef] [PubMed]
- Foo, V.H.X.; Yueh, L.H.; Mehta, J.S.; Ong, H.S. Acute and chronic ocular outcomes in SJS/TEN patients treated with oral ciclosporin vs intravenous immunoglobulin. Front. Med. 2024, 11, 1398506. [Google Scholar] [CrossRef] [PubMed]
- Gueudry, J.; Terkmane, M.N.; Tétart, F.; Muraine, M.; Ingen-Housz-Oro, S. Promising response to Wharton’s jelly eye drops in severe ocular involvement during acute phase of epidermal necrolysis and erythema multiforme major. J. Eur. Acad. Dermatol. Venereol. 2024, 39, e75–e77. [Google Scholar] [CrossRef] [PubMed]
- Kwong, E.Y.L.; Kuok, M.C.I.; Lam, K.F.; Chan, W.K.Y. Case Report: Multi-targeted therapy in the treatment of severe toxic epidermal necrolysis. Front. Pediatr. 2024, 12, 1460579. [Google Scholar] [CrossRef]
- Pan, L.-Y.; Wang, C.-W.; Tsai, T.-Y.; Chen, S.-Y.; Ma, K.S.-K.; Chung, W.-H.; Chen, C.-B.; Sun, C.-C.; Yeh, L.-K.; Chen, H.-C.; et al. Post hoc Analysis of Role of Etanercept in Ocular Sequelae of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Ophthalmology 2024, 131, 864–866. [Google Scholar] [CrossRef]
- Peng, R.; Chi, M.; Xiao, G.; Qu, H.; Shen, Z.; Zhao, Y.; Hong, J. The outcomes of corneal sight rehabilitating surgery in Stevens-Johnson syndrome: Case series. BMC Ophthalmol. 2024, 24, 205. [Google Scholar] [CrossRef] [PubMed]
- Rashad, R.; Kwan, J.T.; Shanbhag, S.S.; Ngowyutagon, P.; Saeed, M.; A Tahboub, M.; Haseeb, A.; Chodosh, J.; Saeed, H.N. Long-term outcomes of glued (sutureless) amniotic membrane transplantation in acute Stevens-Johnson syndrome/toxic epidermal necrolysis: A comparative study. Br. J. Ophthalmol. 2024, 108, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumar, V.; Bari, A.; Venugopal, R.; Sharma, S.; Agarwal, T.; Dada, T.; Pushker, N. The clinical outcomes of minor salivary gland transplantation for severe dry eye disease secondary to chronic Stevens-Johnson syndrome. Ocul. Surf. 2024, 34, 277–282. [Google Scholar] [CrossRef]
- Sharma, N.; Venugopal, R.; Nagpal, R.; K, P.; Verma, K.K.; Biswas, N.; Velpandian, T.; Sen, S.; Dwivedi, S.; Tandon, R.; et al. Evaluation of adjuvant role of topical cyclosporine 1% in acute Stevens-Johnson syndrome: A randomised control trial. Br. J. Ophthalmol. 2024, 109, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Oie, Y.; Takayanagi, H.; Matsubara, S.; Yamada, T.; Nomura, M.; Yoshinaga, Y.; Maruyama, K.; Watanabe, A.; Takashima, K.; et al. Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: A single-arm, open-label, first-in-human interventional study in Japan. Lancet 2024, 404, 1929–1939. [Google Scholar]
- Subramanian, M.; Balaji, J. Piggyback Scleral Contact Lens to Enhance Cosmesis and Comfort in Uniocular Stevens-Johnson Syndrome. Eye Contact Lens 2024, 51, e157–e159. [Google Scholar]
- Wróblewska-Czajka, E.; Dobrowolski, D.; Wylęgała, A.; Jurkunas, U.V.; Wylęgała, E. Outcomes of Boston Keratoprosthesis Type I Implantation in Poland: A Retrospective Study on 118 Patients. J. Clin. Med. 2024, 13, 975. [Google Scholar] [CrossRef]
- Yao, A.; Singh, S.; Bagga, B.; Patel, B.; Malhotra, R. Oral mucous membrane tarsal patch grafting: Broadening indications and long-term outcomes. Orbit 2024, 44, 454–459. [Google Scholar] [CrossRef]
- Aromataris, E.L.C.; Porritt, K.; Pilla, B.; Jordan, Z. JBI Manual for Evidence Synthesis: JBI. 2024. Available online: https://jbi-global-wiki.refined.site/space/MANUAL/355599504/Downloadable+PDF+-+current+version (accessed on 23 August 2025).
- Chang, V.S.; Chodosh, J.; Papaliodis, G.N. Chronic Ocular Complications of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: The Role of Systemic Immunomodulatory Therapy. In Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2016; Volume 31, pp. 178–187. [Google Scholar]
- Shanbhag, S.S.; Singh, S.; Koshy, P.G.; Donthineni, P.R.; Basu, S. A beginner’s guide to mucous membrane grafting for lid margin keratinization: Review of indications, surgical technique and clinical outcomes. Indian J. Ophthalmol. 2021, 69, 794–805. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, N.; Basu, S.; Iyer, G.; Ueta, M.; Sotozono, C.; Kannabiran, C.; Rathi, V.M.; Gupta, N.; Kinoshita, S.; et al. Stevens-Johnson syndrome: The role of an ophthalmologist. Surv. Ophthalmol. 2016, 61, 369–399. [Google Scholar] [CrossRef]
- Dua, H.S.; Saini, J.S.; Azuara-Blanco, A.; Gupta, P. Limbal stem cell deficiency: Concept, aetiology, clinical presentation, diagnosis and management. Indian J. Ophthalmol. 2000, 48, 83–92. [Google Scholar]
- Singh, S.; Basu, S.; Geerling, G. Salivary gland transplantation for dry eye disease: Indications, techniques, and outcomes. Ocul. Surf. 2022, 26, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.D.; Nichols, K.K. Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape. Ophthalmol. Ther. 2023, 12, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, T.; Moon, H.S.; Choi, W.; Yoon, H.J.; Ji, Y.S.; Ueta, M.; Yoon, K.C. Characteristics of meibomian gland dysfunction in patients with Stevens-Johnson syndrome. Medicine 2019, 98, e16155. [Google Scholar] [PubMed]
| Treatment Modality | Total N (Eyes) | BCVA Improvement (n = Eyes) | Epithelial Regeneration | Improved Ocular Symptoms | Reported Complications (n = Eyes) |
|---|---|---|---|---|---|
| Topical Treatment (corticosteroids, antibiotics, lubricants, beta blockers, maternal serum drops, plasma drops, retinol, vitamin A, Wharton’s jelly drops, cyclosporine) | 1424 | 497 (34.9%) | 263 (18.5%) | 264 (18.5%) | 382 (26.8%) |
| Mucosal Graft | 1220 | 464 (38%) | 139 (11.4%) | 531 (43.5%) | 24 (2%) |
| Contact Lens | 1134 | 1024 (90.3%) | 71 (6.3%) | 492 (43.4%) | 55 (4.9%) |
| Amniotic Membrane Transplantation (AMT) | 889 (110 ProKera) | 624 (70.2%; 97 ProKera) | 258 (29%; 22 ProKera) | 181 (20.4%; 18 ProKera) | 180 (20.2%; 5 ProKera) |
| Medical Management (oral corticosteroids, oral immunosuppressants, oral antihistamines, oral antibiotics) | 524 | 207 (39.5%) | 43 (8.2%) | 36 (6.9%) | 63 (12%) |
| Punctal Occlusion | 456 | 219 (48%) | 125 (27.4%) | - | 2 (0.4%) |
| Keratoprosthesis | 225 | 173 (76.9%) | - | - | 43 (19.1%) |
| Cultivated Oral Mucosal Epithelial Transplantation (COMET) | 179 | 92 (51.4%) | 69 (38.5%) | 97 (54.2%) | 11 (6.1%) |
| Limbal Stem Cell Transplant | 154 | 79 (51.3%) | 82 (53.2%) | 40 (26%) | 35 (22.7%) |
| Intravenous Immunoglobulin (IVIG) | 142 | 26 (18.3%) | 12 (8.5%) | 24 (16.9%) | 26 (18.3%) |
| IV Steroids | 127 | 51 (40.2%) | 35 (27.6%) | 2 (1.6%) | 12 (9.4%) |
| Salivary Gland Transplantation | 70 | 30 (42.9%) | 21 (30%) | 59 (84.3%) | - |
| TNF-Alpha Inhibitor | 66 | 62 (93.9%) | 64 (97%) | - | 2 (3%) |
| Lacrimal System Irrigation | 42 | - | - | - | 4 (9.5%) |
| 5-Fluorouracil | 17 | - | - | 17 (100%) | - |
| Anti-VEGF | 14 | 6 (42.9%) | - | 14 (100%) | 2 (14.3%) |
| Photodynamic Therapy (PDT) | 8 | - | - | 8 (100%) | 2 (25%) |
| Debridement | 7 | 4 (57.1%) | 2 (28.6%) | - | 2 (28.6%) |
| Treatment Modality | SJS/TEN Stage | Indications | Efficacy | Potential Complications |
|---|---|---|---|---|
| Amniotic Membrane Transplantation (AMT) | Acute | To promote epithelial regeneration and healing of corneal defects | BCVA improvement = 70.2% Epithelial regeneration = 29.0% Symptom improvement = 20.4% | Membrane displacement, infection, persistent epithelial defect, corneal melt, scarring, conjunctivalization |
| Debridement | Acute | Prevention of adhesions via removal of fibrin, pseudomembranes, and necrotic epithelium from the ocular surface | BCVA improved in 4 of 7 eyes and promoted epithelial regeneration in 2 of 7 eyes | Mechanical trauma, inflammation, subconjunctival hemorrhage |
| Intravenous Immunoglobulin (IVIG) | Acute | Manage systemic disease burden and limit progression of mucocutaneous damage | BCVA improvement = 18.3% Epithelial regeneration = 8.5% Symptom improvement = 16.9% | Thromboembolic events, renal dysfunction, hemolytic anemia, infusion reactions (fever, headache, nausea, hypotension) |
| IV Steroids | Acute | Reduce systemic inflammation and limit progression of mucocutaneous damage | BCVA improvement = 40.1% Epithelial regeneration = 27.6% Symptom improvement = 1.6% | ↑ Risk of infection, impaired wound healing, GI bleeding, insomnia, mood alteration, osteoporosis, ↑ IOP/glaucoma, cataract |
| Lacrimal System Irrigation | Acute | Epiphora secondary to nasolacrimal duct occlusion | Significantly decrease lacrimal passage obstruction and rates of epiphora | Not reported |
| Medical Management | Acute | Manage systemic disease burden and limit progression of mucocutaneous damage | BCVA improvement = 39.5% Epithelial regeneration = 8.2% Symptom improvement = 6.9% | Adverse drug reactions |
| Contact Lens | Acute and Chronic | Severe dry eye, enhancing corneal healing, and protecting the ocular surface from damage caused by sequelae of SJS/TEN (e.g., posterior eyelid margin changes, trichiasis, distichiasis, and other adnexal changes) | BCVA improvement = 90.3% Epithelial regeneration = 6.3% Symptom improvement = 43.4% | Infection, corneal hypoxia, corneal neovascularization, epithelial defects (abrasion) |
| TNF-Alpha Inhibitor | Acute and Chronic | 1. Acute SJS/TEN as an alternative to corticosteroids 2. Corneal melt | Etanercept-treated eyes demonstrated superior outcomes compared to those treated with prednisolone in nearly all aspects of the OSGS, BCVA, and Schirmer test | Infection, ↑ risk of malignancy, infusion site reaction, cytopenia |
| Topical Treatment | Acute and Chronic | To provide adequate lubrication, reduce epithelial injury, prevent infection, and decrease inflammation | BCVA improvement = 34.9% Epithelial regeneration = 18.5% Symptom improvement = 18.5% | Overall minimal Steroid: ↑ IOP, cataract with prolonged use ABX: microbial resistance with prolonged use |
| Anti-VEGF + Photodynamic Therapy (PDT) | Chronic | Refractory corneal neovascularization | At 3 and 6 months after treatment, all eyes showed regression of corneal neovascularization; complete regression was achieved in five eyes (62.5%) and partial regression in three eyes (37.5%) | Corneal edema, hemorrhage around neovascularization |
| Cultivated Oral Mucosal Epithelial Transplantation (COMET) | Chronic | Limbal stem cell deficiency | BCVA improvement = 51.4% Epithelial regeneration = 38.5% Symptom improvement = 54.2% | Graft failure, infection, recurrent LSCD, persistent epithelial defect, corneal neovascularization |
| Keratoprosthesis | Chronic | Visual rehabilitation in end-stage cases with ocular surface changes such as severe corneal opacification, conjunctivalization, or keratinization refractory to other treatments | BCVA improvement = 76.9% Epithelial regeneration = not reported Symptom improvement = not reported | Retroprosthetic membrane (RPM), elevated intraocular pressure/glaucoma, endophthalmitis, retinal detachment (RD), device extrusion |
| Limbal Stem Cell Transplant | Chronic | Limbal stem cell deficiency | BCVA improvement = 51.3% Epithelial regeneration = 53.2% Symptom improvement = 26.0% | Graft rejection/failure, infection, recurrent LSCD, persistent epithelial defect, corneal neovascularization |
| Mucosal Graft | Chronic | Lid margin keratinization | BCVA improvement = 38.0% Epithelial regeneration = 11.4% Symptom improvement = 43.5% | Graft failure, graft displacement, infection, scarring, persistent keratinization |
| Punctal Occlusion | Chronic | Dry eye secondary to meibomian/lacrimal gland dysfunction | BCVA improvement = 48% Epithelial regeneration = 27.4% Symptom improvement = Not reported | Epiphora, infection (especially with punctal plugs), extrusion (punctal plugs), recanalization |
| Salivary Gland Transplantation | Chronic | Dry eye secondary to meibomian/lacrimal gland dysfunction | BCVA improvement = 42.9% Epithelial regeneration = 30% Symptom improvement = 84.3% | Graft failure, infection, epiphora |
| 5-Fluorouracil | Chronic | Dry eye secondary to conjunctival scarring of lacrimal glands located in the supertemporal fornix | Improved visual acuity, ocular surface disease index (OSDI) scores, and decreased corneal scarring | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawires, K.; Tao, B.K.; Nithianandan, H.; Menant-Tay, L.; O’Connor, M.; Yan, P.; Arjmand, P. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review of Ophthalmic Management and Treatment. Vision 2025, 9, 78. https://doi.org/10.3390/vision9030078
Sawires K, Tao BK, Nithianandan H, Menant-Tay L, O’Connor M, Yan P, Arjmand P. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review of Ophthalmic Management and Treatment. Vision. 2025; 9(3):78. https://doi.org/10.3390/vision9030078
Chicago/Turabian StyleSawires, Korolos, Brendan K. Tao, Harrish Nithianandan, Larena Menant-Tay, Michael O’Connor, Peng Yan, and Parnian Arjmand. 2025. "Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review of Ophthalmic Management and Treatment" Vision 9, no. 3: 78. https://doi.org/10.3390/vision9030078
APA StyleSawires, K., Tao, B. K., Nithianandan, H., Menant-Tay, L., O’Connor, M., Yan, P., & Arjmand, P. (2025). Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review of Ophthalmic Management and Treatment. Vision, 9(3), 78. https://doi.org/10.3390/vision9030078





