The Impact of Preoperative Corneal Epithelial Refraction Toricity on Transepithelial Photorefractive Keratectomy for the Treatment of Hyperopia or Mixed Astigmatism
Abstract
1. Introduction
2. Materials and Methods
3. Results
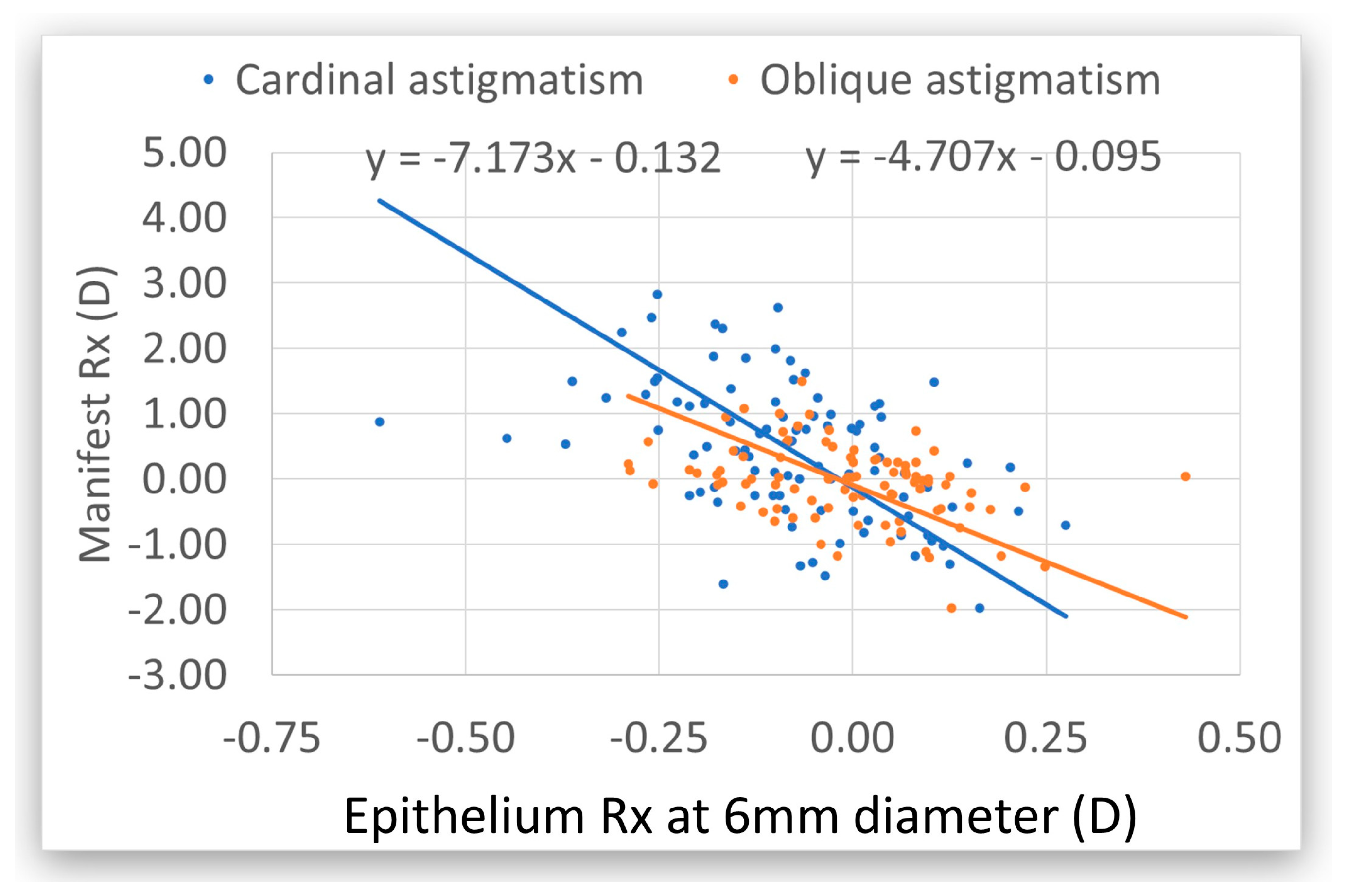
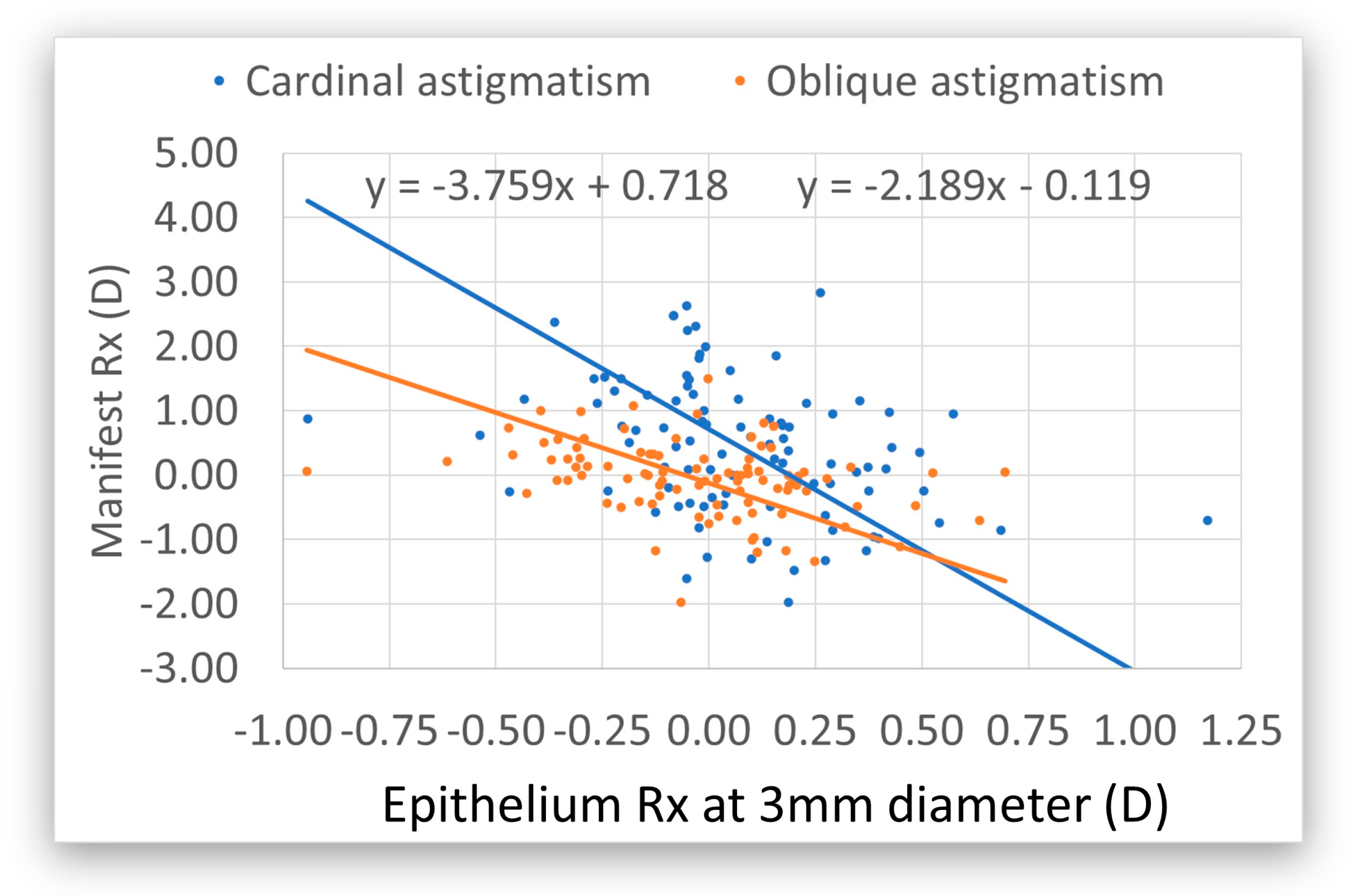
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spadea, L.; Sabetti, L.; D’Alessandri, L.; Balestrazzi, E. Photorefractive keratectomy and LASIK for the correction of hyperopia: 2-year follow-up. J. Refract. Surg. 2006, 22, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Settas, G.; Settas, C.; Minos, E.; Yeung, I.Y. Photorefractive keratectomy (PRK) versus laser assisted in situ keratomileusis (LASIK) for hyperopia correction. Cochrane Database Syst. Rev. 2012, 2012, CD007112. [Google Scholar] [CrossRef] [PubMed]
- Kezirian, G.M.; Moore, C.R.; Stonecipher, K.G.; SurgiVision® Consultants Inc WaveLight Investigator Group. Four-year Postoperative Results of the US ALLEGRETTO WAVE Clinical Trial for the Treatment of Hyperopia. J. Refract. Surg. 2008, 24, S431–S438. Available online: https://journals.healio.com/doi/10.3928/1081597X-20080401-21 (accessed on 9 March 2025). [CrossRef] [PubMed]
- Jaycock, P.D.; O’Brart, D.P.S.; Rajan, M.S.; Marshall, J. 5-year follow-up of LASIK for hyperopia. Ophthalmology 2005, 112, 191–199. [Google Scholar] [CrossRef]
- Humayun, S.; Ishaq, M.; Fawad, A.; Mashhadi, S.F.; Humayun, Q.; Arzoo, S. Assessment of Refractive Outcomes of Femtosecond-assisted Laser in Situ Keratomileusis (LASIK) for Hyperopia. J. Coll. Physicians Surg. Pak. 2021, 31, 434–439. [Google Scholar]
- Arba-Mosquera, S.; de Ortueta, D. LASIK for Hyperopia Using an Aberration-Neutral Profile With an Asymmetric Offset Centration. J. Refract. Surg. 2016, 32, 78–83. [Google Scholar] [CrossRef]
- Kaluzny, B.J.; Piotrowiak-Slupska, I.; Kaszuba-Modrzejewska, M.; Stachura, J.; Arba-Mosquera, S.; Verma, S. Three-year outcomes after high hyperopia correction using photorefractive keratectomy with a large ablation zone. Br. J. Ophthalmol. 2019, 103, 849–854. [Google Scholar] [CrossRef]
- Shahin, B.; Ojaghi, H.; Amani, F. One-year follow-up of patients with hyperopia undergoing photorefractive keratectomy with Allegretto WaveLight Eye Q 400. J. Med. Life 2022, 15, 489–498. [Google Scholar] [CrossRef]
- Sabhapandit, S.; Abdussamad, A.; Shaik, T.A.; Perumalla, S.R. Transepithelial Photorefractive Keratectomy for Hyperopia Correction: An Uncharted Territory. Clin. Ophthalmol. 2023, 17, 1497–1504. [Google Scholar] [CrossRef]
- Gaeckle, H.C. Early clinical outcomes and comparison between trans-PRK and PRK, regarding refractive outcome, wound healing, pain intensity and visual recovery time in a real-world setup. BMC Ophthalmol. 2021, 21, 181. [Google Scholar] [CrossRef]
- Aslanides, I.M.; Kymionis, G.D. Trans advanced surface laser ablation (TransPRK) outcomes using SmartPulseTechnology. Cont. Lens Anterior Eye 2017, 40, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Seiler, T.; Kriegerowski, M.; Schnoy, N.; Bende, T. Ablation rate of human corneal epithelium and Bowman’s layer with the excimer laser (193 nm). Refract. Corneal Surg. 1990, 6, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Goggin, M.; Stewart, P.; Andersons, V.; Criscenti, G. Fine tuning of the default depth and rate of ablation of the epithelium in customized trans-epithelial one-step superficial refractive excimer laser ablation. Eye Vis. 2019, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Arba-Mosquera, S.; de Ortueta, D. Geometrical analysis of the loss of ablation efficiency at non-normal incidence. Opt. Express 2008, 16, 3877–3895. [Google Scholar] [CrossRef]
- Simon, G.; Ren, Q.; Kervick, G.N.; Parel, J.M. Optics of the corneal epithelium. Refract. Corneal Surg. 1993, 9, 42–50. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M.; Silverman, R.H.; Coleman, D.J. Epithelial thickness in the normal cornea: Three-dimensional display with Artemis very high-frequency digital ultrasound. J. Refract. Surg. 2008, 24, 571–581. [Google Scholar] [CrossRef]
- Abtahi, M.-A.; Beheshtnejad, A.H.; Latifi, G.; Akbari-Kamrani, M.; Ghafarian, S.; Masoomi, A.; Abtahi, S.H. Corneal Epithelial Thickness Mapping: A Major Review. J. Ophthalmol. 2024, 2024, 6674747. [Google Scholar] [CrossRef]
- de Ortueta, D.; Arba Mosquera, S.; Baatz, H. Comparison of standard and aberration-neutral profiles for myopic LASIK with the SCHWIND ESIRIS platform. J. Refract. Surg. 2009, 25, 339–349. [Google Scholar] [CrossRef]
- Savini, G.; Schiano-Lomoriello, D.; Hoffer, K.J. Repeatability of automatic measurements by a new anterior segment optical coherence tomographer combined with Placido topography and agreement with 2 Scheimpflug cameras. J. Cataract. Refract. Surg. 2018, 44. Available online: https://journals.lww.com/jcrs/Fulltext/2018/04000/Repeatability_of_automatic_measurements_by_a_new.10.aspx (accessed on 9 March 2025). [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Randleman, J.B. JRS standard for reporting astigmatism outcomes of refractive surgery. J. Refract. Surg. 2014, 30, 654–659. [Google Scholar] [CrossRef]
- Khamar, P.; Rao, K.; Wadia, K.; Dalal, R.; Grover, T.; Versaci, F.; Gupta, K. Advanced epithelial mapping for refractive surgery. Indian J. Ophthalmol. 2020, 68, 2819. [Google Scholar] [CrossRef] [PubMed]
- de Ortueta, D.; von Rüden, D.; Verma, S.; Magnago, T.; Arba-Mosquera, S. Transepithelial Photorefractive Keratectomy in Moderate to High Astigmatism With a Non-wavefront–Guided Aberration-Neutral Ablation Profile. J. Refract. Surg. 2018, 34, 466–474. [Google Scholar] [CrossRef] [PubMed]
- de Ortueta, D. Transepithelial Photorefractive Keratektomy after a Clear Lens Exchange. Vision 2021, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- de Ortueta, D.; von Rüden, D.; Arba-Mosquera, S. Customized versus Standard Epithelium Profiles in Transepithelial Photorefractive Keratectomy. Optics 2021, 2, 266–275. [Google Scholar] [CrossRef]
- Thibos, L.N.; Wheeler, W.; Horner, D. Power vectors: An application of Fourier analysis to the description and statistical analysis of refractive error. Optom. Vis. Sci. 1997, 74, 367–375. [Google Scholar] [CrossRef]
- Amano, S.; Kashiwabuchi, K.; Sakisaka, T.; Inoue, K.; Toda, I.; Tsubota, K. Efficacy of Hyperopic Photorefractive Keratectomy Simultaneously Performed With Phototherapeutic Keratectomy for Decreasing Hyperopic Shift. Cornea 2016, 35, 1069–1072. [Google Scholar] [CrossRef]
- Gatinel, D.; Racine, L.; Hoang-Xuan, T. Contribution of the corneal epithelium to anterior corneal topography in patients having myopic photorefractive keratectomy. J. Cataract. Refract. Surg. 2007, 33, 1860–1865. [Google Scholar] [CrossRef]
- Benito, A.; Redondo, M.; Artal, P. Laser in situ keratomileusis disrupts the aberration compensation mechanism of the human eye. Am. J. Ophthalmol. 2009, 147, 424–431.e1. [Google Scholar] [CrossRef]
- Aslanides, I.M.; Padroni, S.; Arba Mosquera, S.; Ioannides, A.; Mukherjee, A. Comparison of single-step reverse transepithelial all-surface laser ablation (ASLA) to alcohol-assisted photorefractive keratectomy. Clin. Ophthalmol. 2012, 6, 973–980. [Google Scholar] [CrossRef]
- Arba Mosquera, S.; Awwad, S.T. Theoretical analyses of the refractive implications of transepithelial PRK ablations. Br. J. Ophthalmol. 2013, 97, 905–911. [Google Scholar] [CrossRef]
- Luger, M.H.A.; Ewering, T.; Arba-Mosquera, S. Consecutive myopia correction with transepithelial versus alcohol-assisted photorefractive keratectomy in contralateral eyes: One-year results. J. Cataract. Refract. Surg. 2012, 38, 1414–1423. [Google Scholar] [CrossRef]
- Salah-Mabed, I.; Saad, A.; Gatinel, D. Topography of the corneal epithelium and Bowman layer in low to moderately myopic eyes. J. Cataract. Refract. Surg. 2016, 42, 1190–1197. [Google Scholar] [CrossRef]

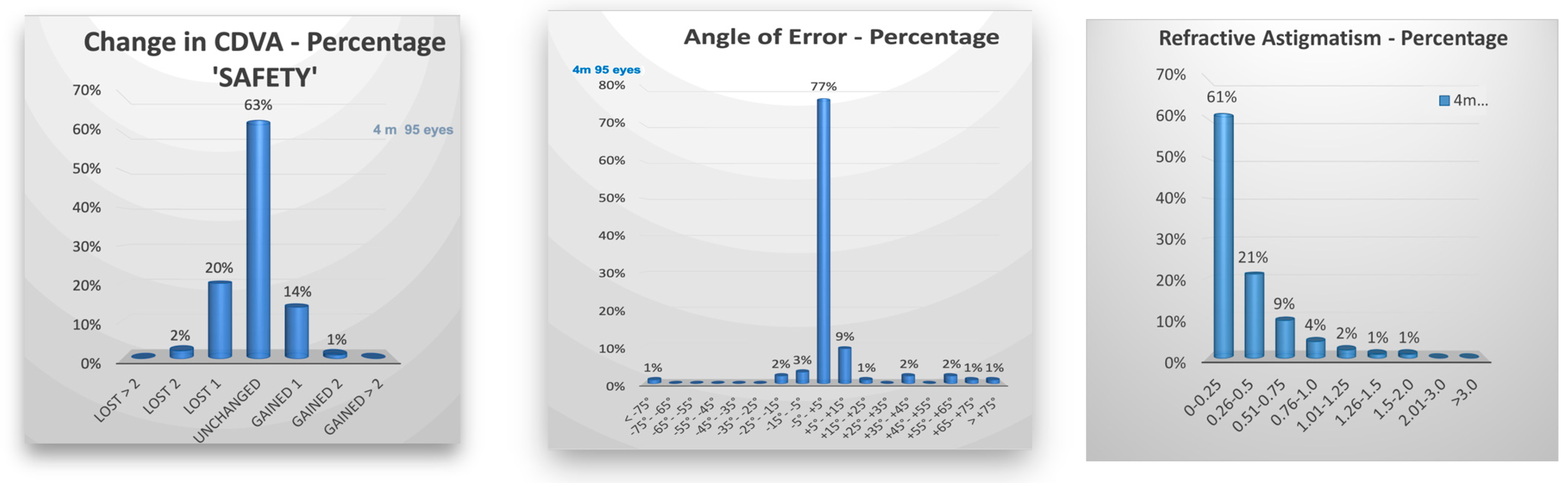
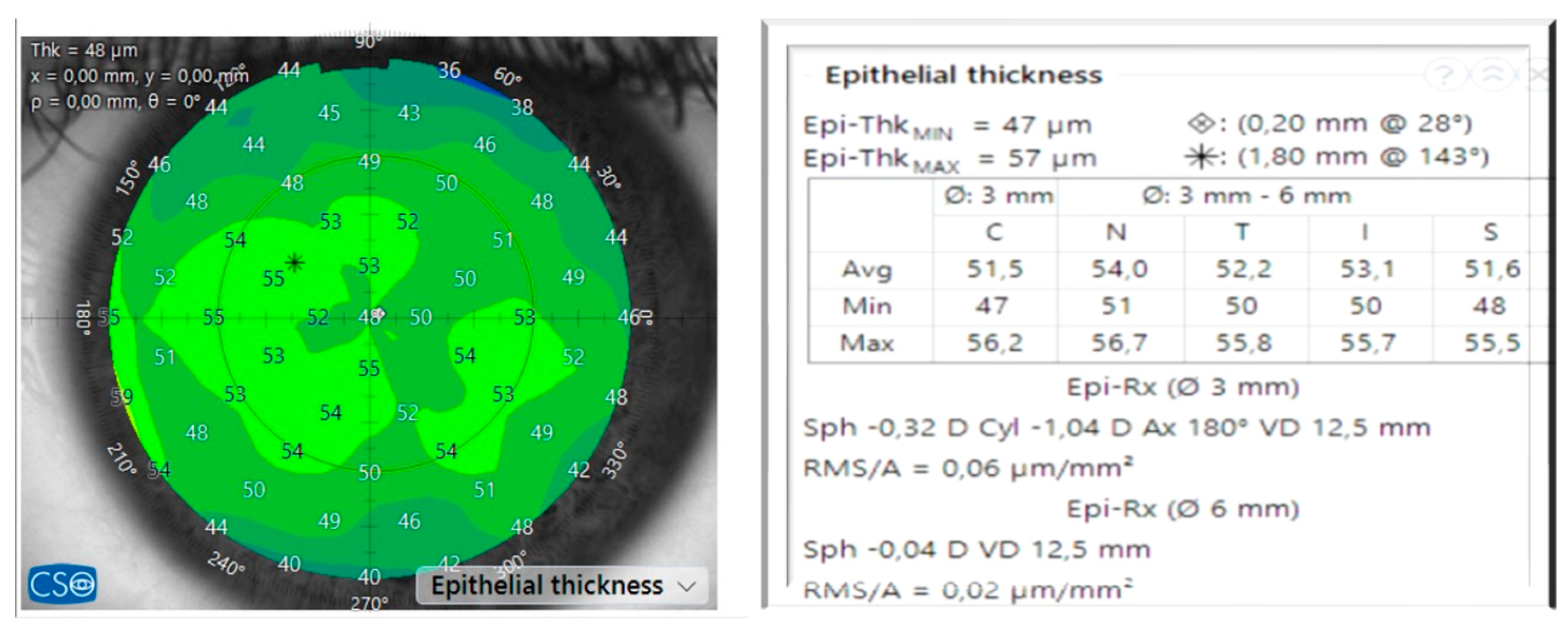
| n = 95 Eyes | ||||
|---|---|---|---|---|
| Gender | Female | 46.3% (44 eyes) | ||
| Male | 53.7% (51 eyes) | |||
| Age | 36 years | (18–64 years) | ||
| Eye | Left | 51.6% (49 eyes) | ||
| Right | 48.4% (46 eyes) | |||
| Preoperative | Range | Postoperative 4 m | Range | |
| SEQ | +0.53 ± 34 D | (−1.75 to +3.88 D) | −0.03 ± 0.3 D | (−1.13 D to +0.75 D) |
| Sphere | +1.6 ±1.16 D | (+0.25 to +6 D) | +0.13 ± 0.29 D | (−0.5 D to +1 D) |
| Cyl | −2.16 ± 1.32 D | (−5.75 to −0 D) | −0.33 ±0.38 D | (−1.75 D to 0 D) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Ortueta, D.; Arba-Mosquera, S. The Impact of Preoperative Corneal Epithelial Refraction Toricity on Transepithelial Photorefractive Keratectomy for the Treatment of Hyperopia or Mixed Astigmatism. Vision 2025, 9, 57. https://doi.org/10.3390/vision9030057
de Ortueta D, Arba-Mosquera S. The Impact of Preoperative Corneal Epithelial Refraction Toricity on Transepithelial Photorefractive Keratectomy for the Treatment of Hyperopia or Mixed Astigmatism. Vision. 2025; 9(3):57. https://doi.org/10.3390/vision9030057
Chicago/Turabian Stylede Ortueta, Diego, and Samuel Arba-Mosquera. 2025. "The Impact of Preoperative Corneal Epithelial Refraction Toricity on Transepithelial Photorefractive Keratectomy for the Treatment of Hyperopia or Mixed Astigmatism" Vision 9, no. 3: 57. https://doi.org/10.3390/vision9030057
APA Stylede Ortueta, D., & Arba-Mosquera, S. (2025). The Impact of Preoperative Corneal Epithelial Refraction Toricity on Transepithelial Photorefractive Keratectomy for the Treatment of Hyperopia or Mixed Astigmatism. Vision, 9(3), 57. https://doi.org/10.3390/vision9030057





