Oculomotor-Related Measures Are Predictive of Reading Acquisition in First Grade Early Readers
Abstract
1. Introduction
2. Methods and Materials
2.1. Participants
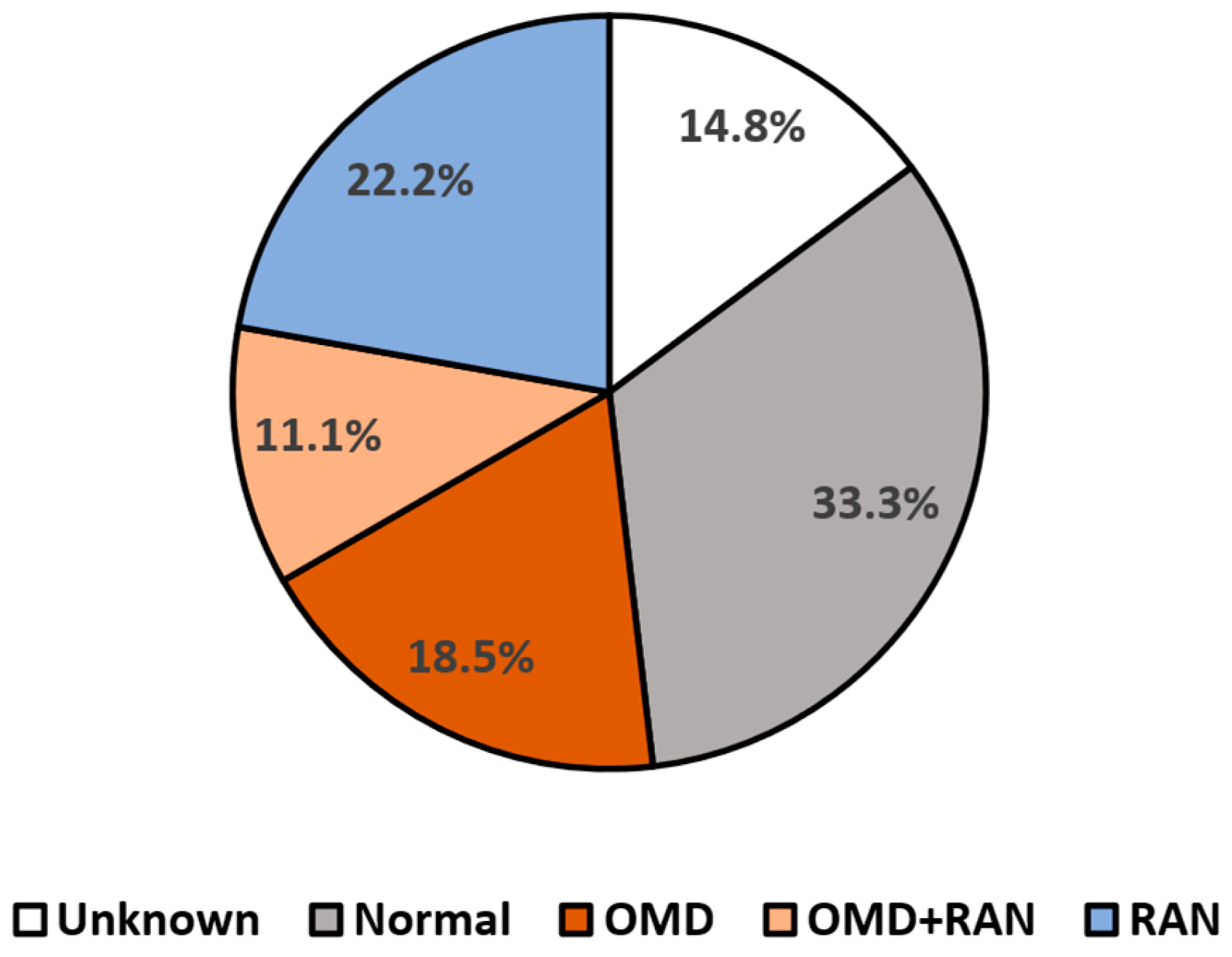
2.2. Visual Evaluation
- Normal language automaticity and oculomotor function.
- Normal language automaticity with oculomotor dysfunction.
- Abnormal language automaticity function but normal oculomotor function.
- A combination of both oculomotor and language automaticity dysfunctions.
2.3. Reading Assessment
2.4. Analyses
3. Results
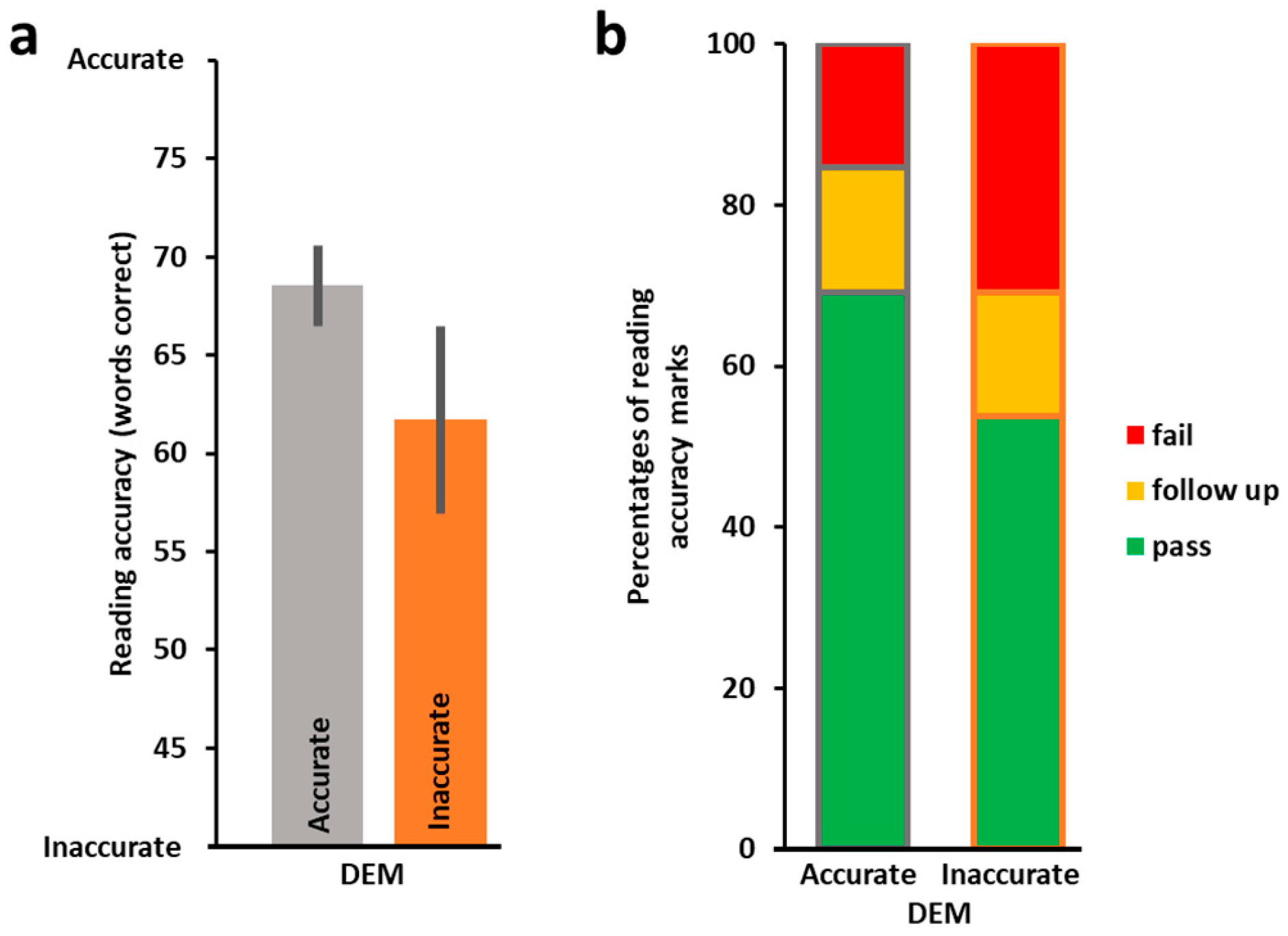
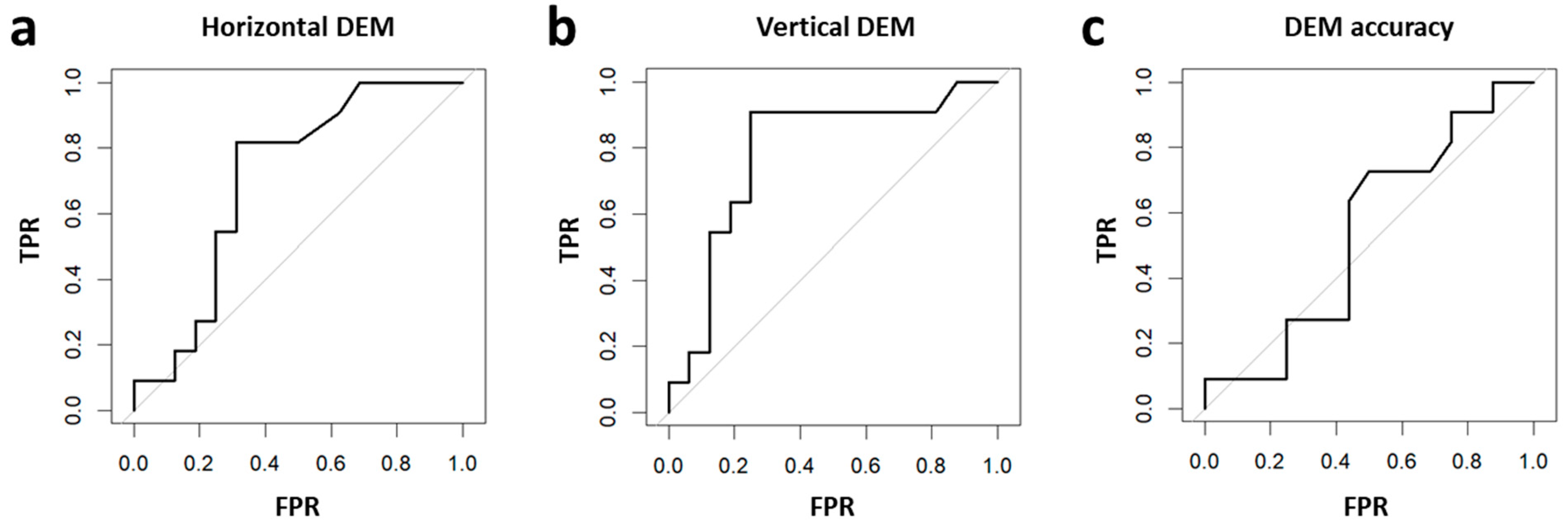
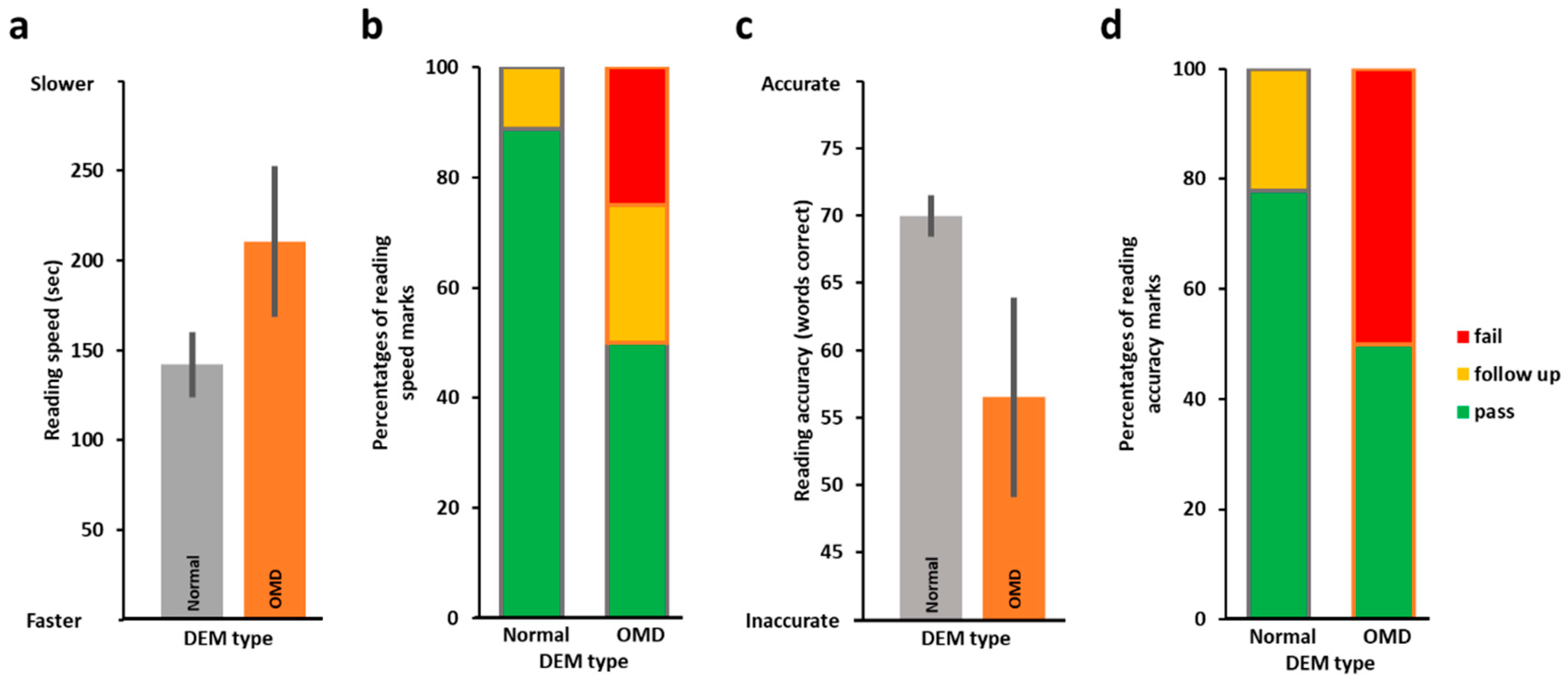
3.1. Oculomotor Evaluation by DEM and the Relation to Reading Performance
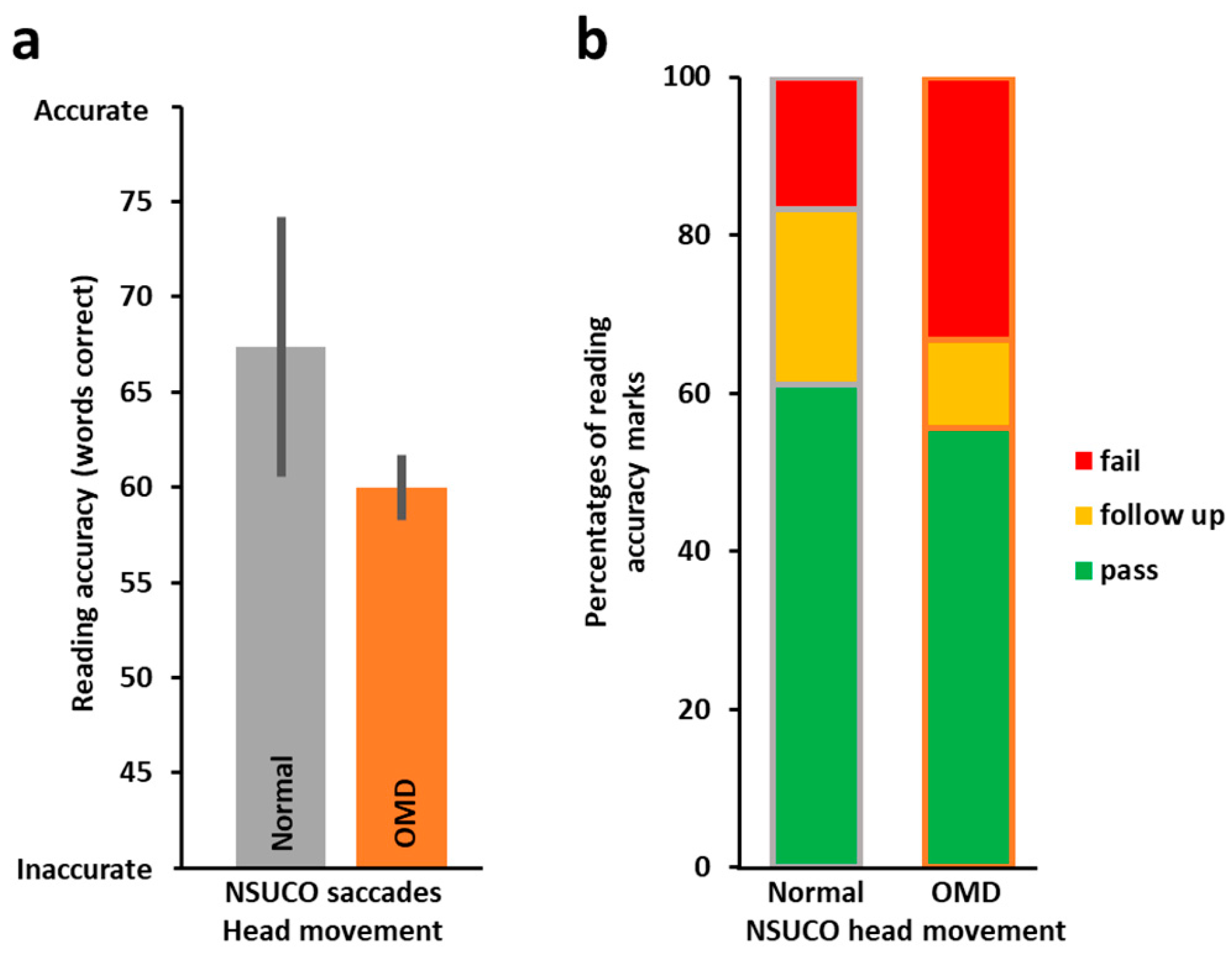
3.2. Oculomotor Evaluation by NSUCO and the Relation to Reading Performance
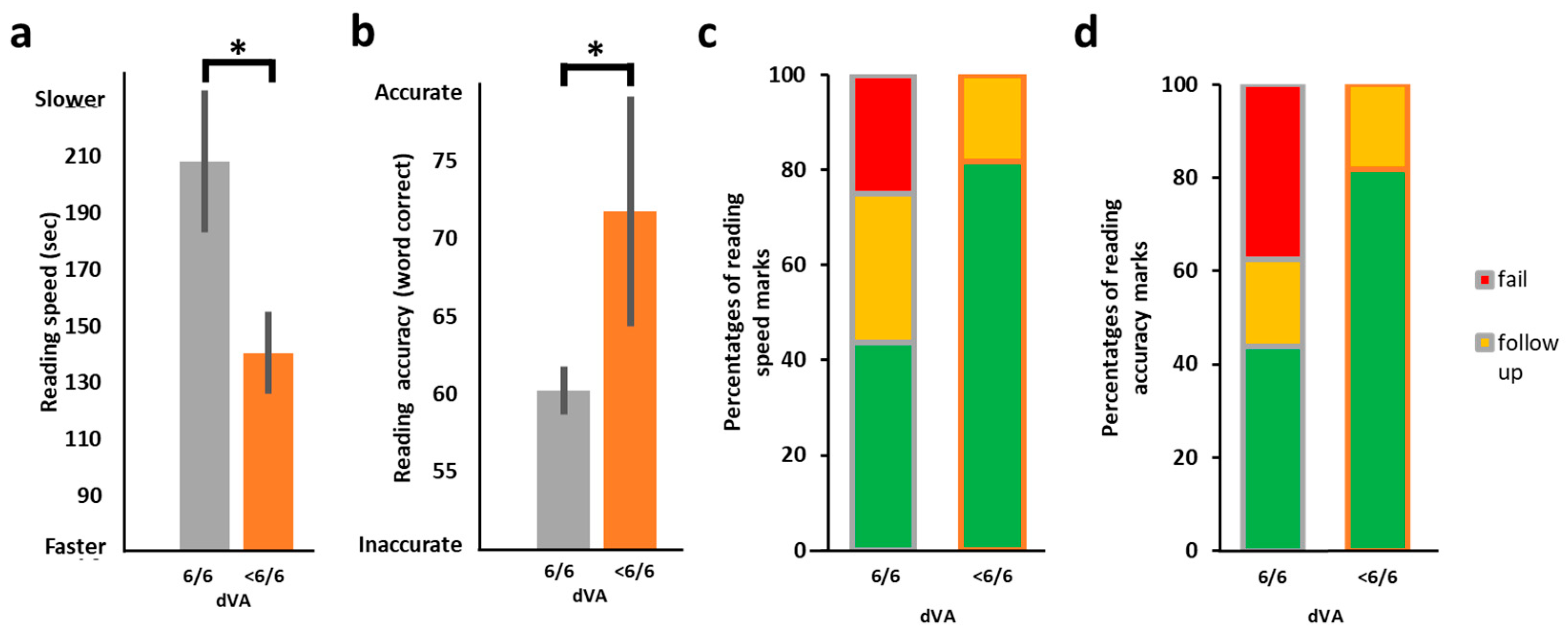
3.3. Distance Visual Acuity (VA) and the Relation to Reading Performance
3.4. Linear Regression Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Annie E. Casey Foundation. Kids Count Data Book: State Trends in Child Well-Being. Baltimore MD. 2024. Available online: https://assets.aecf.org/m/resourcedoc/aecf-2024kidscountdatabook-2024.pdf (accessed on 28 July 2024).
- Hernandez, D.J. Double Jeopardy: How Third-Grade Reading Skills and Poverty Influence High School Graduation; Annie E Casey Foundation: Baltimore, MD, USA, 2011. [Google Scholar]
- Tassinari, J.T. Untreated Oculomotor Dysfunction. Optom. Vis. Dev. 2007, 38, 121. [Google Scholar]
- Scheiman, M.; Wick, B. Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015. [Google Scholar]
- Hoffman, L.G. Incidence of vision difficulties in children with learning disabilities. J. Am. Optom. Assoc. 1980, 51, 447–451. [Google Scholar] [PubMed]
- Lieberman, S. The prevalence of visual disorders in a school for emotionally disturbed children. J. Am. Optom. Assoc. 1985, 56, 800–803. [Google Scholar] [PubMed]
- Sherman, A. Relating vision disorders to learning disability. J. Am. Optom. Assoc. 1973, 44, 140–141. [Google Scholar]
- Press, L.D. Applied Concepts in Vision Therapy; Optometric Extension Program Foundation: Timonium, MD, USA, 2008. [Google Scholar]
- Maples, W.C. NSUCO Oculomotor Test Manual; Willard, B., Corngold, S.M., Eds.; Optometric Extension Program; Optometric Extension Program Foundation: Santa Ana, CA, USA, 1995. [Google Scholar]
- Eden, G.; Stein, J.; Wood, H.; Wood, F. Differences in eye movements and reading problems in dyslexic and normal children. Vis. Res. 1994, 34, 1345–1358. [Google Scholar] [CrossRef]
- Biscaldi, M.; Gezeck, S.; Stuhr, V. Poor saccadic control correlates with dyslexia. Neuropsychologia 1998, 36, 1189–1202. [Google Scholar] [CrossRef]
- Dodick, D.; Starling, A.J.; Wethe, J.; Pang, Y.; Messner, L.V.; Smith, C.; Master, C.L.; Halker-Singh, R.B.; Vargas, B.B.; Bogle, J.M.; et al. The Effect of In-School Saccadic Training on Reading Fluency and Comprehension in First and Second Grade Students. J. Child Neurol. 2016, 32, 104–111. [Google Scholar] [CrossRef]
- Blythe, H.I.; Joseph, H.S.S.L. Children’s eye movements during reading. In The Oxford Handbook of Eye Movements; Liversedge, S.P., Gilchrist, L., Everling, S., Eds.; Online Edition; Oxford University Press: New York, NY, USA, 2011; pp. 643–662. [Google Scholar]
- Pavlidis, G.T. Do eye movements hold the key to dyslexia? Neuropsychologia 1981, 19, 57–64. [Google Scholar] [CrossRef]
- Raghuram, A.; Gowrisankaran, S.; Swanson, E.; Zurakowski, D.; Hunter, D.G.; Waber, D.P. Frequency of Visual Deficits in Children with Developmental Dyslexia. JAMA Ophthalmol. 2018, 136, 1089–1095. [Google Scholar] [CrossRef]
- De Luca, M.; Di Pace, E.; Judica, A.; Spinelli, D.; Zoccolotti, P. Eye movement patterns in linguistic and non-linguistic tasks in developmental surface dyslexia. Neuropsychologia 1999, 37, 1407–1420. [Google Scholar] [CrossRef]
- Sloot, F.; Hoeve, H.L.; De Kroon, M.L.; Goedegebure, A.; Carlton, J.; Griffiths, H.J.; Simonsz, H.J.; EUS€REEN Study Group. Inventory of current EU paediatric vision and hearing screening programmes. J. Med. Screen 2015, 22, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Carlson, N.; Kurtz, D. Clinical Procedures for Ocular Examination; McGraw Hill: New York, NY, USA, 2004. [Google Scholar]
- Gilaie-Dotan, S. Which visual functions depend on intermediate visual regions? Insights from a case of developmental visual form agnosia. Neuropsychologia 2016, 83, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Gilaie-Dotan, S.; Perry, A.; Bonneh, Y.; Malach, R.; Bentin, S. Seeing with Profoundly Deactivated Mid-level Visual Areas: Non-hierarchical Functioning in the Human Visual Cortex. Cereb. Cortex 2008, 19, 1687–1703. [Google Scholar] [CrossRef] [PubMed]
- Convergence Insufficiency Treatment Trial Study Group. Randomized Clinical Trial of Treatments for Sympto-matic Convergence Insufficiency in Children. Arch. Ophthalmol. 2008, 126, 1336–1349. [Google Scholar] [CrossRef]
- Scheiman, M.; Cotter, S.; Kulp, M.T.; Mitchell, G.L.; Cooper, J.; Gallaway, M.; Hopkins, K.B.; Bartuccio, M.; Chung, I.; Convergence Insufficiency Treatment Trial Study Group. Treatment of accommodative dysfunction in children: Results from a randomized clinical trial. Optom. Vis. Sci. 2011, 88, 1343–1352. [Google Scholar] [CrossRef]
- Convergence Insufficiency Treatment Trial (CITT) Study Group. The convergence insufficiency treatment trial: Design, methods, and baseline data. Ophthalmic Epidemiol. 2008, 15, 24–36. [Google Scholar] [CrossRef]
- Gilaie-Dotan, S.; Doron, R. Developmental visual perception deficits with no indications of prosopagnosia in a child with abnormal eye movements. Neuropsychologia 2017, 100, 64–78. [Google Scholar] [CrossRef]
- King, A.T. The Proposed King-Devick Test and Its Relation to the Pierce Saccade Test and Reading Levels; Carl Shepherd Memo-rial Library, Illinois College of Optometry: Chicago, IL, USA, 1976. [Google Scholar]
- Facchin, A. Spotlight on the Developmental Eye Movement (DEM) Test. Clin. Optom. 2021, 13, 73–81. [Google Scholar] [CrossRef]
- Garzia, R.P.; Richman, J.E.; Nicholson, S.B.; Gaines, C.S. A new visual-verbal saccade test: The Development Eye Movement Test (DEM). J. Am. Optom. Assoc. 1990, 61, 124–135. [Google Scholar]
- Powers, M.K. Paper Tools for Assessing Visual Function. Optom. Vis. Sci. 2009, 86, 613–618. [Google Scholar] [CrossRef]
- McLeod, H.M.; Chaglasian, E.L. Effect of Room Illumination on Manifest Refraction. Optom. Educ. 2018, 44, 2. [Google Scholar]
- Norton, E.S.; Wolf, M. Rapid Automatized Naming (RAN) and Reading Fluency: Implications for Understanding and Treatment of Reading Disabilities. Annu. Rev. Psychol. 2012, 63, 427–452. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.E. The Developmental Eye Movement Test Examiner’s Manual; The Bernell Corp.: Mishawaka, IN, USA, 2016; Volume 2.8. [Google Scholar]
- Mast, I.; Bar-On, A.; Yedidya, S. Reading and Writing Test for First Grade; RAMA—The National Authority for Measurement and Evaluation of Education (Ministry of Health): Jerusalem, Israel, 2014; pp. 36–37. [Google Scholar]
- Shany, M.; Bahat, A.; Lachman, D.; Shalem, Z.; Zeiger, T. ‘Aleph-Taph’: An Assessment System for Reading and Writing Disabilities; Yesod: Tel Aviv, Israel, 2006. [Google Scholar]
- Fernandez-Velazquez, F.J.; Fernandez-Fidalgo, M.J. Do DEM test scores change with respect to the language? Norms for Spanish-speaking population. Optom. Vis. Sci. 1995, 72, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Facchin, A.; Maffioletti, S. The Reliability of the DEM Test in the Clinical Environment. Front. Psychol. 2018, 9, 1279. [Google Scholar] [CrossRef]
- Palomo-Álvarez, C.; Puell, M.C. Relationship between oculomotor scanning determined by the DEM test and a contextual reading test in schoolchildren with reading difficulties. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 1243–1249. [Google Scholar] [CrossRef]
- Macdonald, D.; Luk, G.; Quintin, E.M. Early Word Reading of Preschoolers with ASD, Both with and Without Hyperlexia, Com-pared to Typically Developing Preschoolers. J. Autism Dev. Disord. 2021, 51, 1598–1612. [Google Scholar] [CrossRef]
- Ehm, J.-H.; Koerner, J.K.A.; Gawrilow, C.; Hasselhorn, M.; Schmiedek, F. The association of ADHD symptoms and reading acquisition during elementary school years. Dev. Psychol. 2016, 52, 1445–1456. [Google Scholar] [CrossRef]
- Moiroud, L.; Gerard, C.L.; Peyre, H.; Bucci, M.P. Developmental Eye Movement test and dyslexic children: A pilot study with eye movement recordings. PLoS ONE 2018, 13, e0200907. [Google Scholar] [CrossRef]
- Veuillet, E.; Bouilhol, C.; Thai-Van, H. Co-Morbidity of APD and Reading Disabilities. Curr. Pediatr. Rev. 2011, 7, 227–240. [Google Scholar] [CrossRef]
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Lancet 2012, 379, 1997–2007. [Google Scholar] [CrossRef]
- Polanczyk, G.; Silva de Lima, M.; Lessa Horta, B.; Biederman, J.; Augusto Rohde, L. The Worldwide Prevalence of ADHD: A Sys-tematic Review and Metaregression Analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Ayton, L.N.; Abel, L.A.; Fricke, T.R.; McBrien, N.A. Developmental Eye Movement Test: What is it Really Measuring? Optom. Vis. Sci. 2009, 88, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Orlansky, G.; Hopkins, K.B.; Mitchell, G.L.; Huang, K.; Frazier, M.; Heyman, C.; Scheiman, M. Reliability of the developmental eye movement test. Optom. Vis. Sci. 2011, 88, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Tanke, N.; Barsingerhorn, A.D.; Boonstra, F.N.; Goossens, J. Visual fixations rather than saccades dominate the developmental eye movement test. Sci. Rep. 2021, 11, 1162. [Google Scholar] [CrossRef]
- Wood, J.M.; Black, A.A.; Hopkins, S.; White, S.L.J. Vision and academic performance in primary school children. Ophthalmic Physiol. Opt. 2018, 38, 516–524. [Google Scholar] [CrossRef]
- Facchin, A.; Maffioletti, S. Validity Reassessment of Developmental Eye Movement (DEM) Test in the Italian Population. Op-Tometry Vision 2011, 42, 3. [Google Scholar]
- Eviatar, Z.; Ibrahim, R.; Ganayim, D. Orthography and the Hemispheres: Visual and Linguistic Aspects of Letter Processing. Neuropsychology 2004, 18, 174–184. [Google Scholar] [CrossRef]
- Eichler, R.; Scheiman, M.; Ben-Eli, H. Comparing the directional effect of number calling on the developmental eye movement test results in Hebrew-speaking children. Ophthalmic Physiol. Opt. 2023, 43, 623–628. [Google Scholar] [CrossRef]
- Vernet, M.; Jover, M.; Bellocchi, S.; Maziero, S.; Jucla, M.; Tallet, J.; Danna, J.; Chaix, Y.; Ducrot, S. Visual-processing deficits in children with neurofibromatosis type 1: A clinical marker of reading difficulties. Eur. J. Paediatr. Neurol. 2022, 38, 25–32. [Google Scholar] [CrossRef]
- Powers, M.; Grisham, D.; Riles, P. Saccadic tracking skills of poor readers in high school. Optom. J. Am. Optom. Assoc. 2008, 79, 228–234. [Google Scholar] [CrossRef]
- Cohen, Y.; Segal, O.; Barkana, Y.; Lederman, R.; Zadok, D.; Pras, E.; Morad, Y. Correlation between asthenopic symptoms and different measurements of convergence and reading comprehension and saccadic fixation eye movements. Optom. J. Am. Optom. Assoc. 2010, 81, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kulp, M.T.; Schmid, P.P. Effect of oculomotor and other visual skills on reading performance: A literature review. Optom. Vis. Sci. 1996, 73, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K. Eye Movements in Reading and Information Processing: 20 Years of Research. Psychol. Bull. 1998, 124, 372–422. [Google Scholar] [CrossRef] [PubMed]
- Al Dahhan, N.; Georgiou, G.K.; Hung, R.; Munoz, D.; Parrila, R.; Kirby, J.R. Eye movements of university students with and without reading difficulties during naming speed tasks. Ann. Dyslexia 2014, 64, 137–150. [Google Scholar] [CrossRef]
- McConkie, G.W.; Rayner, K. The span of the effective stimulus during a fixation in reading. Percept. Psychophys. 1975, 17, 578–586. [Google Scholar] [CrossRef]
- Strandberg, A.; Nilsson, M.; Östberg, P.; Seimyr, G.Ö. Eye movements are stable predictors of word reading ability in young readers. Front. Educ. 2023, 8, 1077882. [Google Scholar] [CrossRef]
- Bilbao, C.; Piñero, D.P. Clinical characterization of oculomotricity in children with and without specific learning disorders. Brain Sci. 2020, 10, 836. [Google Scholar] [CrossRef]
- Maples, W.C.; Ficklin, T.W. A preliminary study of the oculomotor skills of learning disabled, gifted and normal children. Op-Tometry Vis. Dev. 1989, 20, 9–13. [Google Scholar]
- Maples, W.C.; Atchley, J.; Ficklin, T. Northeastern State University College of Optometry’s oculomotor norms. J. Behav-Ioral Optometry 1992, 3, 143–150. [Google Scholar]
- Maples, W.C.; Ficklin, T. Comparison of Eye Movement Skills Between Above Average and Below Average Readers. J. Behav. Optom. 1990, 1, 87–91. [Google Scholar]
- Maples, W.C.; Ficklin, T. Test-retest reliability of the King-Devick Saccade and the NSUCO ocular motor tests. J. Behav. Optom. 1991, 3, 209–214. [Google Scholar]
- Zhao, J.; Pan, X.; Sui, R.; Munoz, S.R.; Sperduto, R.D.; Ellwein, L.B. Refractive error study in children: Results from Shunyi District, China. Arch. Ophthalmol. 2000, 129, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Murthy, G.V.S.; Gupta, S.K.; Ellwein, L.B.; Muñoz, S.R.; Pokharel, G.P.; Sanga, L.; Bachani, D. Refractive Error in Children in an Urban Population in New Delhi. Investig. Ophthalmol. Vis. Sci. 2002, 43, 623–631. [Google Scholar]
- Bruce, A.; Fairley, L.; Chambers, B.; Wright, J.; Sheldon, T.A. Impact of visual acuity on developing literacy at age 4–5 years: A cohort-nested cross-sectional study. BMJ Open 2016, 6, e010434. [Google Scholar] [CrossRef]
- Hopkins, S.; Narayanasamy, S.; Vincent, S.J.; Sampson, G.P.; Wood, J.M. Do reduced visual acuity and refractive error affect class-room performance? Clin. Exp. Optom. 2020, 103, 278–289. [Google Scholar] [CrossRef]
- Dirani, M.; Zhang, X.; Goh, L.K.; Young, T.L.; Lee, P.; Saw, S.M. The role of vision in academic school performance. Ophthalmic Epidemiol. 2010, 17, 18–24. [Google Scholar] [CrossRef]
- Vinuela-Navarro, V.; Erichsen, J.T.; Williams, C.; Woodhouse, J.M. Saccades and fixations in children with delayed reading skills. Ophthalmic Physiol. Opt. 2017, 37, 531–541. [Google Scholar] [CrossRef]
- West, S.K.; Rubin, G.S.; Broman, A.T.; Muñoz, B.; Bandeen-Roche, K.; Turano, K. How Does Visual Impairment Affect Performance on Tasks of Everyday Life? Arch. Ophthalmol. 2002, 120, 774–780. [Google Scholar] [CrossRef]
- Young, B.S.; Pollard, T.; Paynter, S.; Cox, R. Effect of eye exercises in improving control of eye movements during reading. J. Optom. Vis. Dev. 1982, 13, 4–7. [Google Scholar]
- Rounds, B.B.; Manley, C.W.; Norris, R.H. The effect of oculomotor training on reading efficiency. J. Am. Optom. Assoc. 1991, 62, 92–99. [Google Scholar]
- Thiagarajan, P.; Ciuffreda, K.J.; Capo-Aponte, J.E.; Ludlam, D.P.; Kapoor, N. Oculomotor neurorehabilitation for reading in mild traumatic brain injury (mTBI): An integrative approach. NeuroRehabilitation 2014, 34, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Suzuki, H.; Abe, M.; Sasaki, S.; Nagashima, J.; Kawate, N. Systematic review of visual rehabilitation interventions for oculomotor deficits in patients with brain injury. Brain Inj. 2019, 33, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Nabeel, T. Effects of Training of Eye Fixation Skills on the Reading Fluency of Children with Oculomotor Dysfunction. Pak. J. Educ. 2019, 36, 61–80. [Google Scholar]
- Solan, H.A. Eye movement problems in achieving readers: An update. Optom. Vis. Sci. 1985, 62, 812–819. [Google Scholar] [CrossRef]

| Sex | 10 girls, 17 boys | |||
| Education | 1st grade | |||
| Min. | Max. | Mean | SD | |
| dVA (Snellen) | 0.2 | 1 | 0.9 | 0.26 |
| DEM standardized vertical speed | 16 | 131 | 85.9 | 25.28 |
| DEM standardized horizontal speed | −285 | 126 | 57.4 | 87.02 |
| DEM standardized accuracy | 59 | 117 | 93.4 | 13.31 |
| DEM standardized ratio | −187 | 127 | 68.1 | 76.84 |
| Reading speed (time in sec) | 450 | 58 | 180.6 | 86.91 |
| Reading accuracy (accurate words) | 12 | 75 | 64.9 | 13.00 |
| READING ACCURACY | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| INTERCEPT | 75.43 ± 9.05 (1.52 × 10−8 ***) | 21.9304 ± 17.3171 (0.2175) | 58.74729 ± 3.02448 (3.47 × 10−16 ***) | 48.62904 ± 22.55831 (0.0423 *) |
| DVA | −12.60 ± 10.11 (0.224) | - | - | −7.13391 ± 9.32139 (0.4522) |
| DEM HORIZONTAL ACCURACY | - | 0.4609 ± 0.1852 (0.0202 *) | - | 0.19512 ± 0.24644 (0.4370) |
| DEM RATIO | - | - | 0.08743 ± 0.02936 (0.00654 **) | 0.06011 ± 0.04151 (0.1618) |
| OVERALL MODEL ESTIMATION | F(1,24) = 1.556, p = 0.2243, Adjusted R2 = 0.02174 | F(1,24) = 6.194 p = 0.02015 Adjusted R2 = 0.172 | F(1,24) = 8.867 p = 0.006543 Adjusted R2 = 0.2394 | F(3,22) = 3.245 p = 0.0414 Adjusted R2 = 0.2122 |
| READING SPEED | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| INTERCEPT | 91.0 ± 59.21 (0.137) | 212.2101 ± 17.9623 (1.73 × 10−11 ***) | 304.3051 ± 57.7368 (2.1 × 10−5 ***) | 232.7528 ± 58.8213 (0.00067 ***) |
| DVA | 107.05 ± 66.12 (0.119) | - | - | 117.2505 ± 53.8036 (0.04030 *) |
| DEM HORIZONTAL SPEED | - | −0.5290 ± 0.1724 (0.00526 **) | - | −1.5051 ± 0.5509 (0.01216 *) |
| DEM VERTICAL SPEED | - | - | −1.4333 ± 0.6532 (0.0381 *) | −0.4125 ± 0.1545 (0.01400 *) |
| OVERALL MODEL ESTIMATION | F(1,24) = 2.621, p = 0.1185, Adjusted R2 = 0.06089 | F(1,24) = 9.419, p = 0.005264, Adjusted R2 = 0.2519 | F(1,24) = 4.815, p = 0.03813, Adjusted R2 = 0.1324 | F(3,22) = 7.424, p = 0.001301, Adjusted R2 = 0.4353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portnoy, A.; Gilaie-Dotan, S. Oculomotor-Related Measures Are Predictive of Reading Acquisition in First Grade Early Readers. Vision 2025, 9, 48. https://doi.org/10.3390/vision9020048
Portnoy A, Gilaie-Dotan S. Oculomotor-Related Measures Are Predictive of Reading Acquisition in First Grade Early Readers. Vision. 2025; 9(2):48. https://doi.org/10.3390/vision9020048
Chicago/Turabian StylePortnoy, Avi, and Sharon Gilaie-Dotan. 2025. "Oculomotor-Related Measures Are Predictive of Reading Acquisition in First Grade Early Readers" Vision 9, no. 2: 48. https://doi.org/10.3390/vision9020048
APA StylePortnoy, A., & Gilaie-Dotan, S. (2025). Oculomotor-Related Measures Are Predictive of Reading Acquisition in First Grade Early Readers. Vision, 9(2), 48. https://doi.org/10.3390/vision9020048






