Anatomical and Functional Outcomes of Human-Amniotic Membrane Graft in Refractory Macular Hole Cases
Abstract
1. Introduction
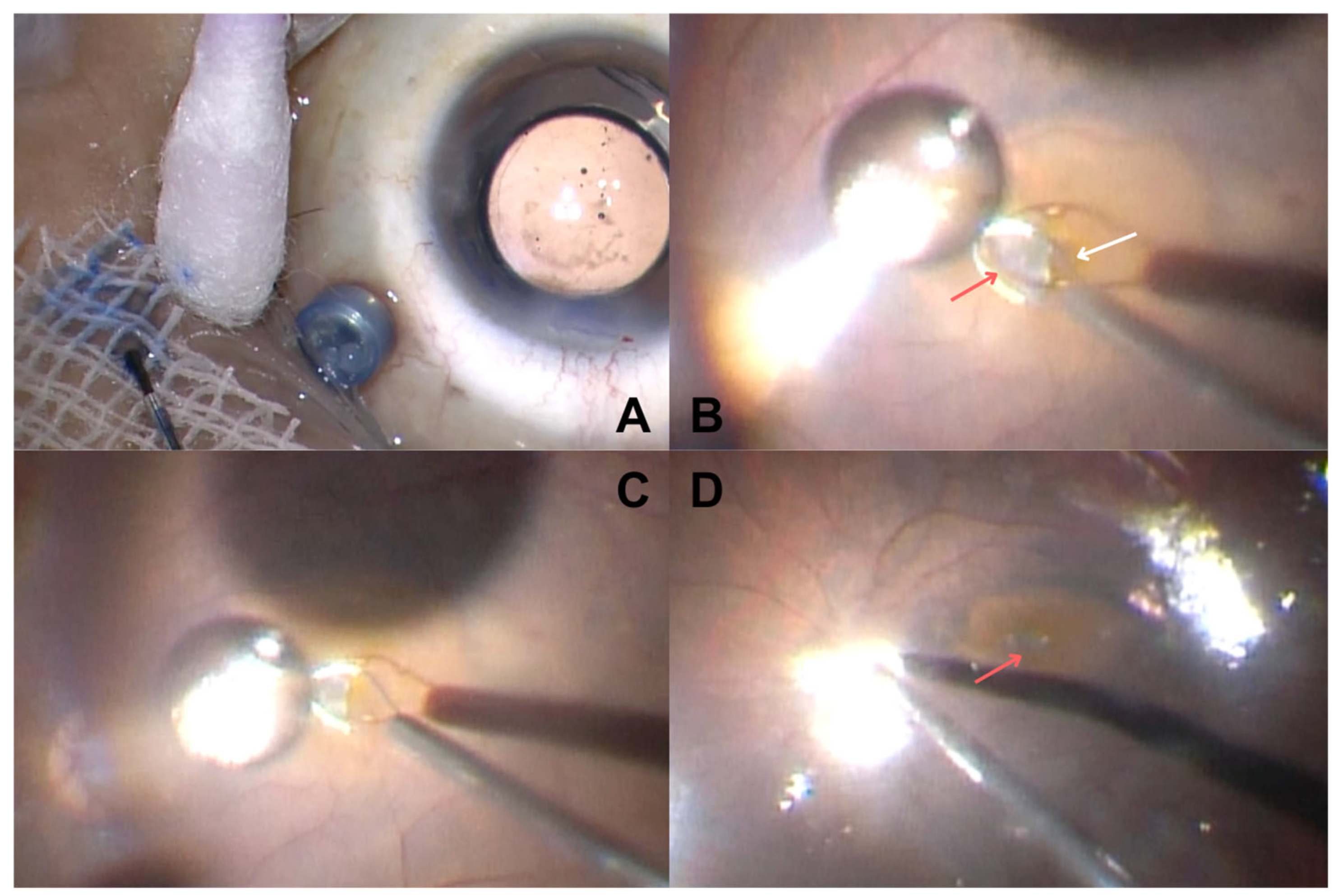
2. Materials and Methods
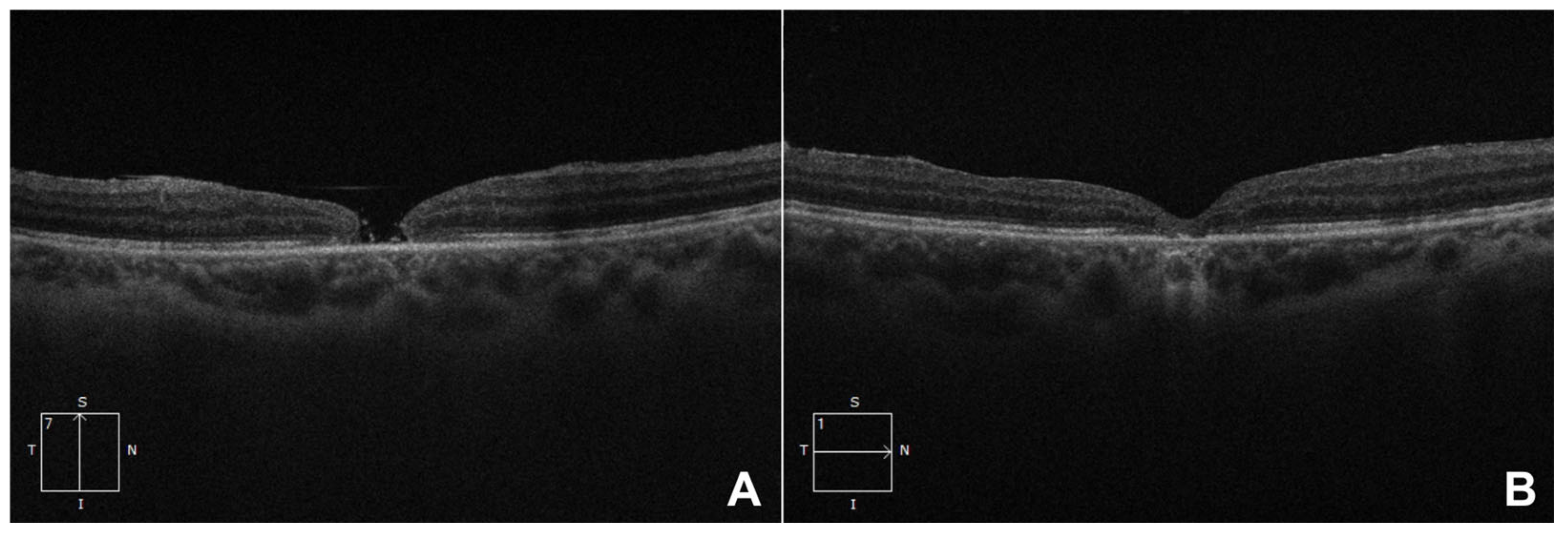
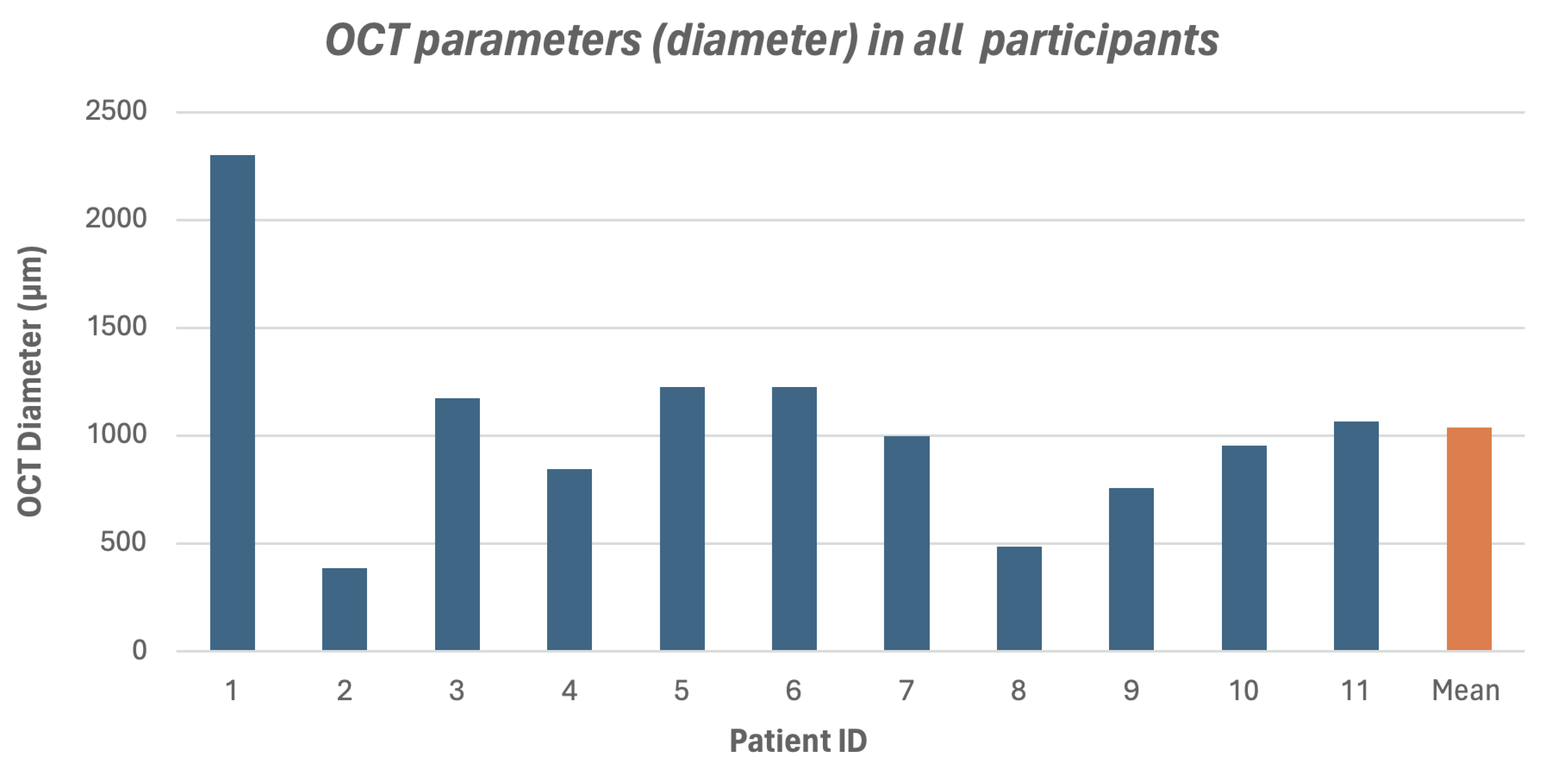
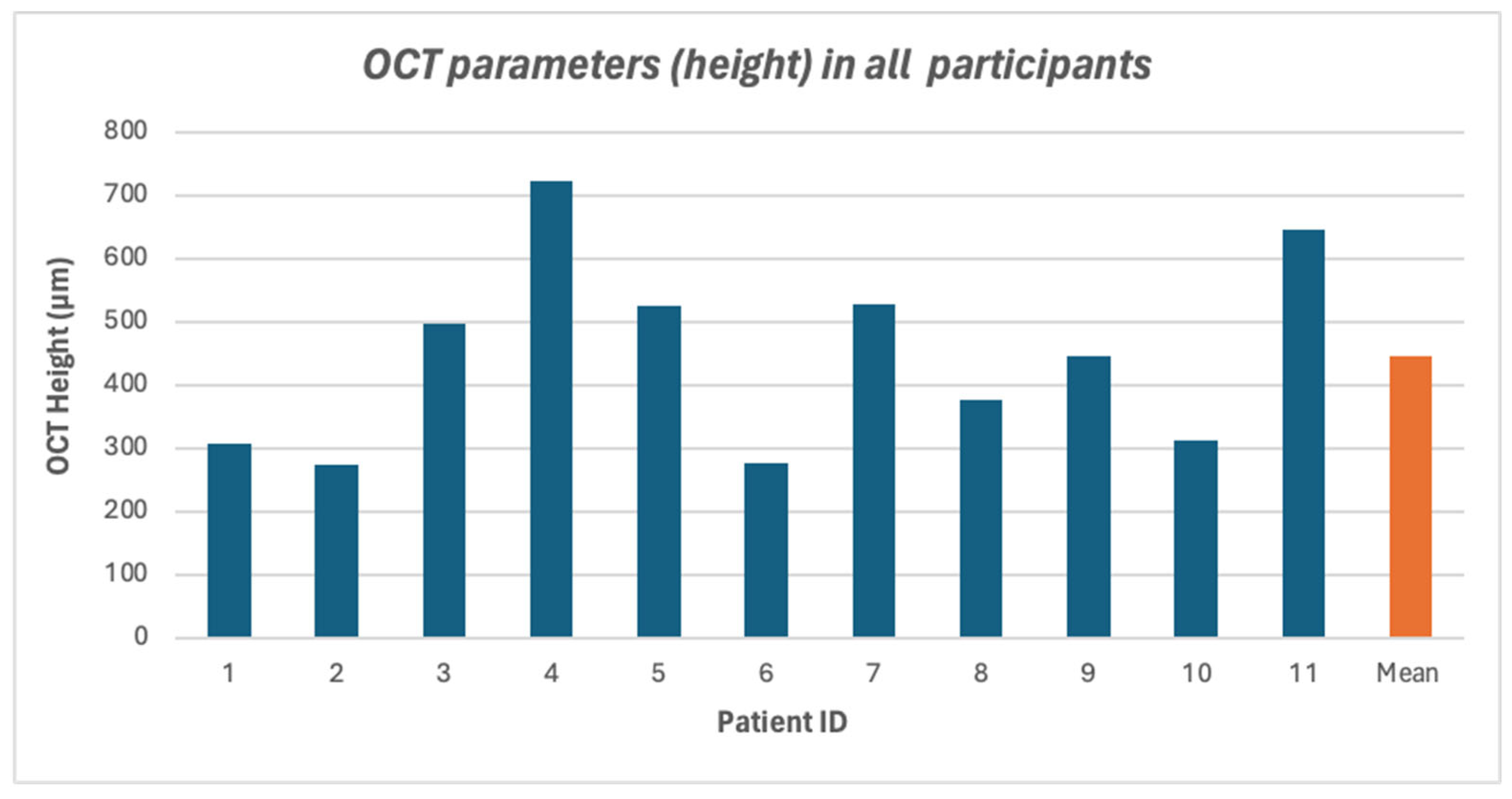
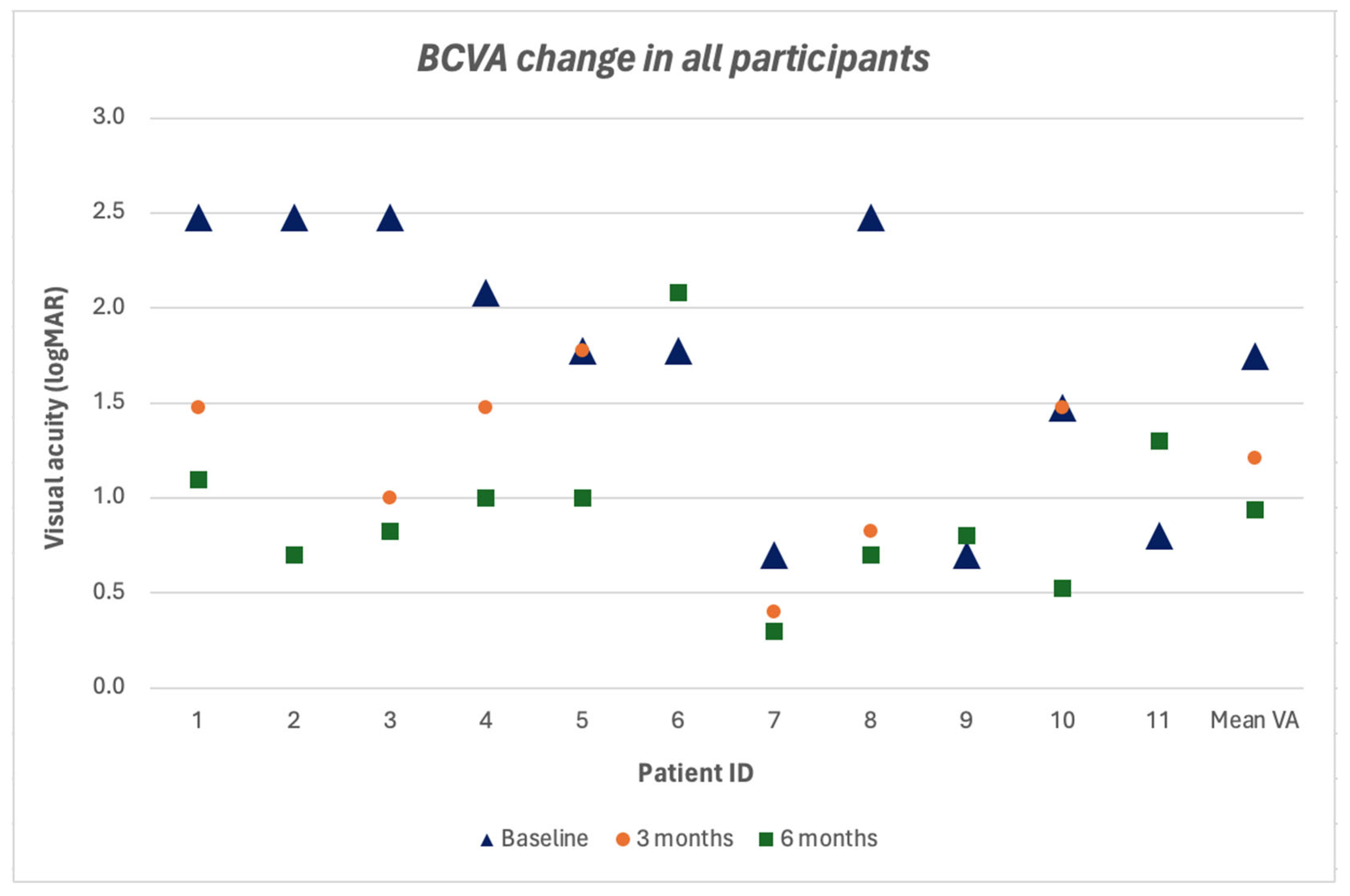
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MH | Macular hole |
| ILM | Internal limiting membrane |
| hAM | Human amniotic membrane |
| AMT | Amniotic membrane transplantation |
| BCVA | Best-corrected visual acuity |
| IOP | Intraocular pressure |
| OCT | Optical coherence tomography |
| logMAR | Logarithm of the minimum angle of resolution |
| PPV | Pars plana vitrectomy |
| FMH | Failed macular hole |
| SF6 | Sulfur hexafluoride |
| SO | Silicone oil |
| RPE | Retinal pigment epithelium |
| ART | Autologous retinal transplantation |
| PFC | Perfluorocarbon |
References
- Zhao, P.; Wang, S.; Liu, N.; Shu, Z.; Zhao, J. A review of surgical outcomes and advances for macular holes. J. Ophthalmol. 2018, 2018, 7389412. [Google Scholar] [CrossRef] [PubMed]
- Morizane, Y.; Shiraga, F.; Kimura, S.; Hosokawa, M.; Shiode, Y.; Kawata, T.; Hosogi, M.; Shirakata, Y.; Okanouchi, T. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am. J. Ophthalmol. 2014, 157, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Grewal, D.S.; Mahmoud, T.H. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016, 134, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.S.; Mahmoud, T.H. Subretinal transplantation of an autologous retinal free flap for chronic retinal detachment with proliferative vitreoretinopathy with and without macular hole. Retina 2018, 38, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Yang, C.M. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina 2016, 36, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, C.; Jin, H.; Zhang, H.; Zhao, P. Autologous lens capsular flap transplantation combined with autologous blood application in the management of refractory macular hole. Retina 2018, 38, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- van Herendael, B.J.; Oberti, C.; Brosens, I. Microanatomy of the human amniotic membranes. A light microscopic, transmission, and scanning electron microscopic study. Am. J. Obs. Obstet. Gynecol. 1978, 131, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Reyes, M.A.; Quiroz-Gonzalez, E.A.; Quiroz-Gonzalez, M.A.; Lima-Gomez, V. Safety and efficacy of human amniotic membrane plug transplantation in cases of macular hole: A scoping review. Int. J. Retin. Vitr. 2024, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Caporossi, T.; Tartaro, R.; Finocchio, L.; Franco, F.; Barca, F.; Giansanti, F. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina 2019, 39, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; Tartaro, R.; De Angelis, L.; Pacini, B.; Rizzo, S. A human amniotic membrane plug to repair retinal detachment associated with large macular tear. Acta Ophthalmol. 2019, 97, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; De Angelis, L.; Pacini, B.; Tartaro, R.; Finocchio, L.; Barca, F.; Rizzo, S. A human amniotic membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol. 2020, 98, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Grewal, D.S.; Charles, S.; Parolini, B.; Kadonosono, K.; Mahmoud, T.H. Autologous retinal transplant for refractory macular holes: Multicenter international collaborative study group. Ophthalmology 2019, 126, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; Pacini, B.; De Angelis, L.; Barca, F.; Peiretti, E.; Rizzo, S. Human amniotic membrane to close recurrent, high myopic macular holes in pathologic myopia with axial length of ≥30 mm. Retina 2020, 40, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; Pacini, B.; Bacherini, D.; Barca, F.; Faraldi, F.; Rizzo, S. Human amniotic membrane plug to promote failed macular hole closure. Sci. Rep. 2020, 10, 18264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Chen, G.; Han, F.; Jiang, W. Human amniotic membrane graft for refractory macular hole: A single-arm meta-analysis and systematic review. J. Fr. Ophtalmol. 2023, 46, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.A.; Maia, A.; Machado, A.J.; Ferreira, R.E.A.; Hagemann, L.F.; Júnior, P.H.E.R.; Rezende, F.A. Human amniotic membrane for the treatment of large and refractory macular holes: A retrospective, multicentric, interventional study. Int. J. Retin. Vitr. 2021, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Hanai, M.; Amaral, D.C.; Jacometti, R.; Aguiar, E.H.C.; Gomes, F.C.; Cyrino, L.G.; Alves, M.R.; Monteiro, M.L.R.; Fuganti, R.M.; Casella, A.M.B.; et al. Large macular hole and autologous retinal transplantation: A systematic review and meta-analysis. Int. J. Retin. Vitr. 2024, 10, 56. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Participants (n = 11) |
|---|---|
| Sex | |
| Male | 8 (72.7%) |
| Female | 3 (27.3%) |
| Age group (Years) | |
| 21–30 | 1 (9.1%) |
| 31–40 | 2 (18.2%) |
| 41–50 | 2 (18.2%) |
| 51–60 | 4 (36.4%) |
| >60 | 2 (18.2%) |
| Baseline VA | 1.75 ± 0.73 |
| (logMAR, mean ± SD) | |
| Number of surgery | |
| 1 | 9 (81.8%) |
| >1 | 2 (18.2%) |
| Lens Status | |
| Phakic | 2 (18.2%) |
| Pseudophakic | 8 (72.3%) |
| Aphakia | 1 (9.1%) |
| Tamponade used in first surgery | |
| Silicone Oil | 6 (54.6%) |
| Gas | 5 (45.4%) |
| Comorbidities | |
| Diabetes mellitus | 2 (18.2%) |
| Myopia | 1 (9.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soedarman, S.; Muslim, S.; Girsang, W.; Elvioza; Agustiawan, R.; Prasetya, A.D.B.; Triyoga, I.F. Anatomical and Functional Outcomes of Human-Amniotic Membrane Graft in Refractory Macular Hole Cases. Vision 2025, 9, 45. https://doi.org/10.3390/vision9020045
Soedarman S, Muslim S, Girsang W, Elvioza, Agustiawan R, Prasetya ADB, Triyoga IF. Anatomical and Functional Outcomes of Human-Amniotic Membrane Graft in Refractory Macular Hole Cases. Vision. 2025; 9(2):45. https://doi.org/10.3390/vision9020045
Chicago/Turabian StyleSoedarman, Soefiandi, Sandi Muslim, Waldensius Girsang, Elvioza, Referano Agustiawan, Alberthus Donni Budi Prasetya, and Ichsan Fauzi Triyoga. 2025. "Anatomical and Functional Outcomes of Human-Amniotic Membrane Graft in Refractory Macular Hole Cases" Vision 9, no. 2: 45. https://doi.org/10.3390/vision9020045
APA StyleSoedarman, S., Muslim, S., Girsang, W., Elvioza, Agustiawan, R., Prasetya, A. D. B., & Triyoga, I. F. (2025). Anatomical and Functional Outcomes of Human-Amniotic Membrane Graft in Refractory Macular Hole Cases. Vision, 9(2), 45. https://doi.org/10.3390/vision9020045





