The Influence of the Level of Monovision upon Early Outcomes Following the Bilateral Implantation of an Enhanced Monovision Intraocular Lens
Abstract
1. Introduction
2. Materials and Methods
2.1. Intraocular Lens
2.2. Surgical Technique
2.3. Statistical Analysis
3. Results
3.1. Refractive Outcomes
3.2. Visual Acuity
3.3. Reading Performance
3.4. Patient-Reported Outcomes
3.5. Stereo Acuity and Contrast Sensitivity
3.6. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDVA | Corrected distance visual acuity |
| EDoF | Extended depth of focus |
| IOLs | Intraocular lenses |
| QoV | Quality of vision |
| SE | Spherical equivalent |
| UDVA | Unaided distance visual acuity |
| UIVA | Unaided intermediate visual acuity |
| UNVA | Unaided near visual acuity |
References
- Finkelman, Y.M.; Ng, J.Q.; Barrett, G.D. Patient satisfaction and visual function after pseudophakic monovision. J. Cataract Refract. Surg. 2009, 35, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Venter, J.A.; Barclay, D.; Pelouskova, M.; Bull, C.E. Initial experience with a new refractive rotationally asymmetric multifocal intraocular lens. J. Refract. Surg. 2014, 31, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Webers, V.S.; Bauer, N.C.; Saelens, I.E.; Creten, O.J.; Berendschot, T.T.; van den Biggelaar, F.J.; Nuijts, R.M. Comparison of the intermediate distance of a trifocal IOL with an extended depth-of-focus IOL: Results of a prospective randomized trial. J. Cataract Refract. Surg. 2020, 46, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Megiddo-Barnir, E.; Alió, J.L. Latest Development in Extended Depth-of-Focus Intraocular Lenses: An Update. Asia-Pac. J. Ophthalmol. 2023, 12, 58–79. [Google Scholar] [CrossRef]
- Rampat, R.; Gatinel, D. Multifocal and Extended Depth-of-Focus Intraocular Lenses in 2020. Ophthalmology 2021, 128, e164–e185. [Google Scholar] [CrossRef]
- Auffarth, G.U.; Gerl, M.; Tsai, L.; Janakiraman, D.P.; Jackson, B.; Alarcon, A.; Dick, H.B. Clinical Evaluation of a New Monofocal IOL with Enhanced Intermediate Function in Patients with Cataract. J. Cataract Refract. Surg. 2021, 47, 184–191. [Google Scholar] [CrossRef]
- Beltraminelli, T.; Rizzato, A.; Toniolo, K.; Galli, A.; Menghini, M. Comparison of Visual Performances of Enhanced Monofocal versus Standard Monofocal IOLs in a Mini-Monovision Approach. BMC Ophthalmol. 2023, 23, 170. [Google Scholar] [CrossRef]
- McNeely, R.N.; Stewart, S.A.; Moore, J.E. Visual Performance and Subjective Experience 3 Months and 12 Months after Combined Implantation of 2 New Complementary Continuous Phase Multifocal Intraocular Lenses. J. Cataract Refract. Surg. 2023, 49, 921–928. [Google Scholar] [CrossRef]
- McAlinden, C.; Pesudovs, K.; Moore, J.E. The Development of an Instrument to Measure Quality of Vision: The Quality of Vision (QoV) Questionnaire. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5537–5545. [Google Scholar] [CrossRef]
- McNeely, R.N.; Moutari, S.; Palme, C.; Moore, J.E. Visual Outcomes and Subjective Experience after Combined Implantation of Extended Depth of Focus and Trifocal IOLs. J. Refract. Surg. 2020, 36, 326–333. [Google Scholar] [CrossRef]
- Mencucci, R.; Cennamo, M.; Venturi, D.; Vignapiano, R.; Favuzza, E. Visual Outcome, Optical Quality, and Patient Satisfaction with a New Monofocal IOL, Enhanced for Intermediate Vision: Preliminary Results. J. Cataract Refract. Surg. 2020, 46, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Hovanesian, J.A.; Jones, M.; Allen, Q. The Vivity Extended Range of Vision IOL vs. the PanOptix Trifocal, ReStor 2.5 Active Focus, and ReStor 3.0 Multifocal Lenses: A Comparison of Patient Satisfaction, Visual Disturbances, and Spectacle Independence. Clin. Ophthalmol. 2022, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Luedtke, H.; Borkenstein, A.F. Enhanced Depth-of-Focus Intraocular Lenses: Latest Wavefront-Shaped Optics versus Diffractive Optics. Optom. Vis. Sci. 2022, 99, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Tomagova, N.; Elahi, S.; Vandekerckhove, K. Clinical Outcomes of a New Non-Diffractive Extended Depth-of-Focus Intraocular Lens Targeted for Mini-Monovision. Clin. Ophthalmol. 2023, 17, 981–990. [Google Scholar] [CrossRef]
- Aristodemou, P.; Knox Cartwright, N.E.; Sparrow, J.M.; Johnston, R.L. Formula Choice: Hoffer Q, Holladay 1, or SRK/T and Refractive Outcomes in 8108 Eyes after Cataract Surgery with Biometry by Partial Coherence Interferometry. J. Cataract Refract. Surg. 2011, 37, 63–71. [Google Scholar] [CrossRef]
- Cochener, B.; Boutillier, G.; Lamard, M.; Auberger-Zagnoli, C. A Comparative Evaluation of a New Generation of Diffractive Trifocal and Extended Depth of Focus Intraocular Lenses. J. Refract. Surg. 2018, 34, 507–514. [Google Scholar] [CrossRef]
- Rodov, L.; Reitblat, O.; Levy, A.; Assia, E.I.; Kleinmann, G. Visual outcomes and patient satisfaction for trifocal, extended depth of focus and monofocal intraocular lenses. J. Refract. Surg. 2019, 35, 434–440. [Google Scholar] [CrossRef]
- García-Pérez, J.L.; Gros-Otero, J.; Sánchez-Ramos, C.; Blázquez, V.; Contreras, I. Short term visual outcomes of a new trifocal intraocular lens. BMC Ophthalmol. 2017, 17, 72. [Google Scholar] [CrossRef]
- McNeely, R.N.; Moutari, S.; Stewart, S.; Moore, J.E. Visual outcomes and patient satisfaction 1 and 12 months after combined implantation of extended depth of focus and trifocal intraocular lenses. Int. Ophthalmol. 2021, 41, 3985–3998. [Google Scholar] [CrossRef]
- Song, M.Y.; Kang, K.H.; Lee, H.; Kim, T.I.; Koh, K. A Comparative Study of Two Extended Depth of Focus Intraocular Lenses. Eye Contact Lens. 2022, 48, 433–438. [Google Scholar] [CrossRef]
- Ribeiro, F.; Ferreira, T.B. Comparison of clinical outcomes of 3 trifocal IOLs. J. Cataract Refract. Surg. 2020, 46, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Mendicute, J.; Kapp, A.; Pierre, L.; Krommes, G.; Arias-Puente, A.; Tomalla, M.; Barraquer, E.; Rozot, P.; Bouchut, P. Evaluation of visual outcomes and patient satisfaction after implantation of a diffractive trifocal intraocular lens. J. Cataract Refract. Surg. 2016, 42, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Schallhorn, S.C.; Schallhorn, J.M.; Pelouskova, M.; Venter, J.A.; Hettinger, K.A.; Hannan, S.J.; Teenan, D. Refractive lens exchange in younger and older presbyopes: Comparison of complication rates, 3 months clinical and patient-reported outcomes. Clin. Ophthalmol. 2017, 11, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lopez, V.; Barcala, X.; Zaytouny, A.; Dorronsoro, C.; Peli, E.; Marcos, S. Monovision Correction Preference and Eye Dominance Measurements. Transl. Vis. Sci. Technol. 2023, 12, 18. [Google Scholar] [CrossRef]
- Kim, J.; Shin, H.J.; Kim, H.C.; Shin, K.C. Comparison of conventional versus crossed monovision in pseudophakia. Br. J. Ophthalmol. 2015, 99, 391–395. [Google Scholar] [CrossRef]
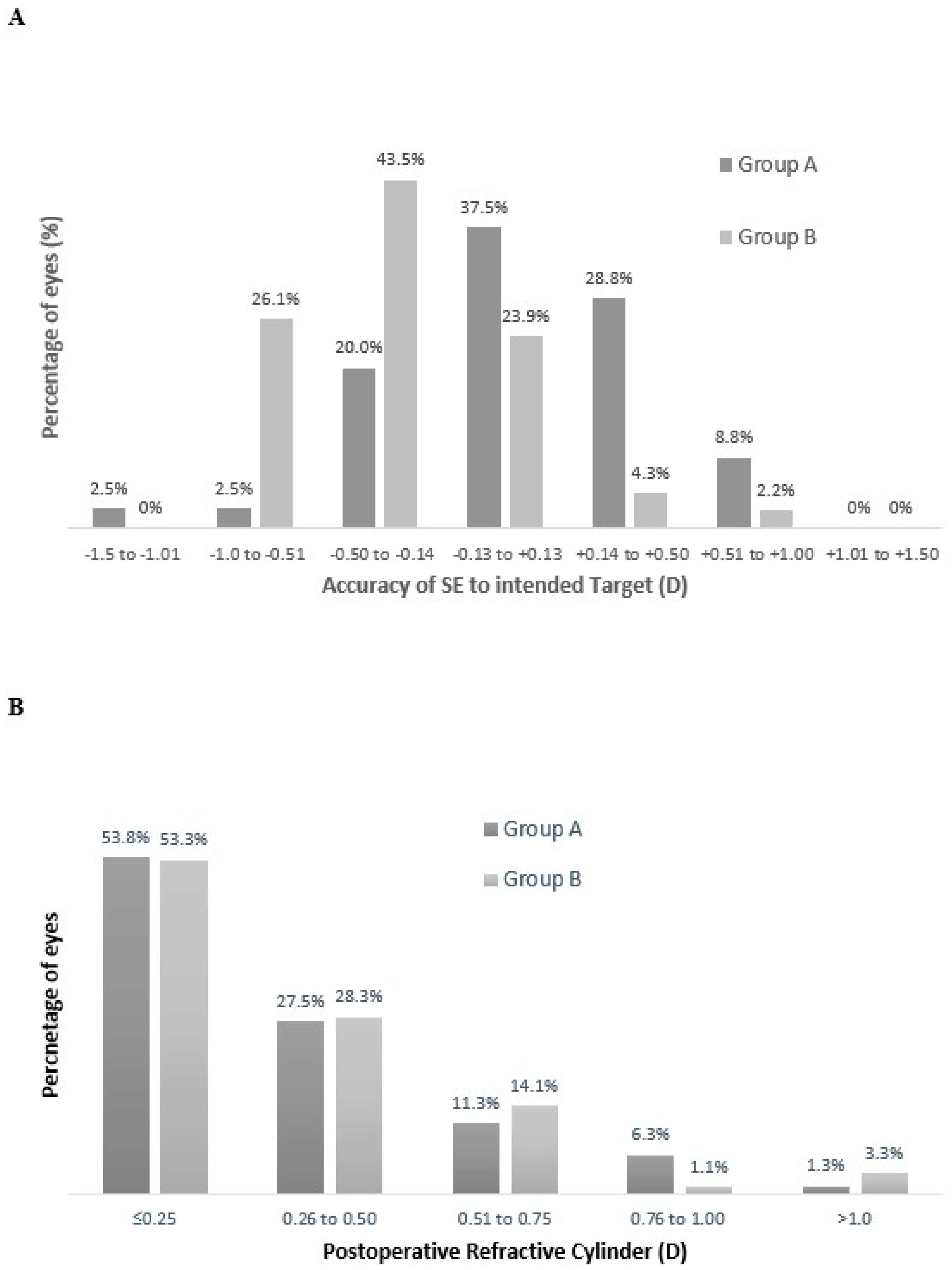
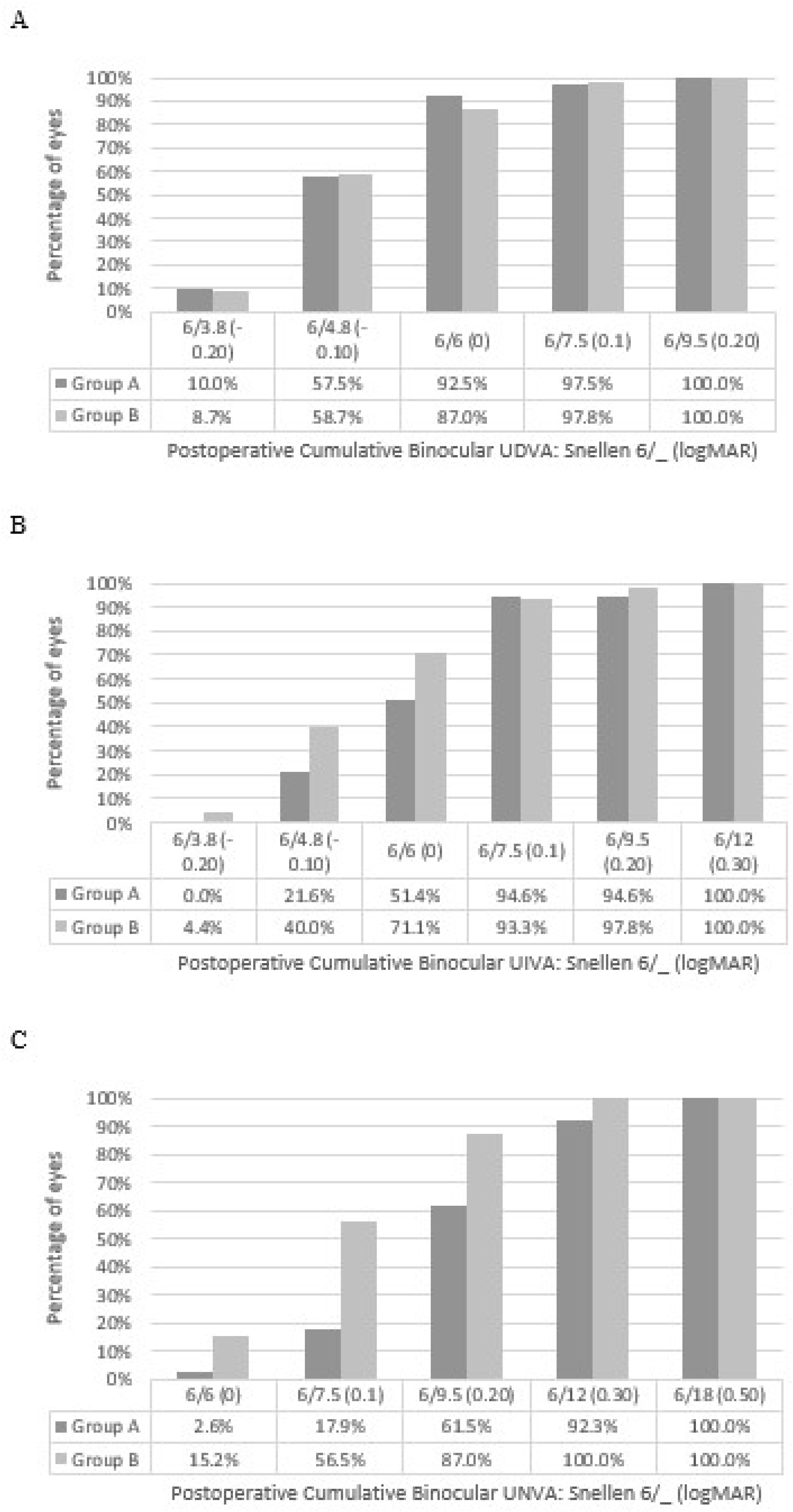
| Parameter | Group A | Group B |
|---|---|---|
| No. of patients (eyes) | 40 (80) | 46 (92) |
| Age (y), mean ± SD (range) | 62 ± 8.53 (51 to 79) | 58 ± 7.60 (47 to 77) |
| Gender, male/female (%) | 48.8/51.2 | 26.1/73.9 |
| Axial length (mm), mean ± SD (range) | 23.54 ± 1.29 (21.71, 27.23) | 23.56 ± 1.52 (20.49, 28.02) |
| Power of implanted IOL (D), mean ± SD (range) | 22.13 ± 3.59 (11.0, 28.0) | 21.54 ± 4.53 (10.5, 30) |
| No. of toric IOLs Power of IOL cylinder (D), mean ± SD (range) Power of IOL cylinder (D), mean ± SD (range) | 8 20.19 ± 4.58 (12.5, 25) 1.88 ± 1.13 (0.75, 3.75) | 14 20.07 ± 4.53 (11 to 25) 1.88 ± 1.01 (0.75, 3.75) |
| Clinical, mean ± SD (range) | ||
| Sphere (D) | 1.20 ± 2.97 (−8.75 to 6.50) | 0.76 ± 3.03 (−10.0 to 5.00) |
| Cylinder (D) | −0.63 ± 0.70 (−4.50 to 0) | −0.62 ± 0.76 (−4.75 to 0) |
| MSE (D) | 0.69 ± 3.13 (−9.13 to 6.13) | 0.44 ± 3.18 (−10.88 to 4.88) |
| CDVA | −0.01 ± 0.13 (−0.2 to 0.34) | −0.01 ± 0.15 (−0.2 to 0.80) |
| Parameter, Mean ± SD (Range) | Postoperative | |||||
|---|---|---|---|---|---|---|
| Group A | Group B | p Value | ||||
| Dominant | Nondominant Eye | Dominant | Nondominant Eye | Group A to Group B Comparison | ||
| Sphere (D) | 0.21 ± 0.39 (−0.50 to +1.50) | −0.61 ± 0.24 (−1.00 to 0) | 0.01 ± 0.31 (−0.75 to +1.25) | −1.12 ± 0.22 (−1.50 to −0.50) | 0.01 | <0.001 |
| Cylinder (D) | −0.38 ± 0.31 (−1.25 to 0) | −0.36 ± 0.31 (−1.00 to 0) | −0.35 ± 0.36 (−1.75 to 0) | −0.43 ± 0.32 (−1.75 to 0) | 0.70 | 0.32 |
| MSE (D) | 0.02 ± 0.37 (−1.13 to 1.13) | −0.78 ± 0.18 (−1.00 to −0.50) | −0.17 ± 0.31 (−0.75 to 0.88) | −1.34 ± 0.18 (−1.75 to −1.13) | 0.01 | <0.001 |
| UDVA (logMAR) | −0.03 ± 0.11 (−0.20 to 0.32) | 0.15 ± 0.11 (−0.12 to 0.50) | −0.04 ± 0.10 (−0.20 to 0.20) | 0.33 ± 0.14 (0.10 to 0.64) | 0.85 | <0.001 |
| Binocular UDVA (logMAR) | −0.05 ± 0.09 (−0.20 to 0.20) | −0.05 ± 0.08 (−0.20 to 0.16) | 0.87 | |||
| UIVA (logMAR) | 0.26 ± 0.14 (0 to 0.60) | 0.05 ± 0.11 (−0.10 to 0.40) | 0.22 ± 0.13 (0 to 0.50) | 0 ± 0.11 (−0.20 to 0.30) | 0.25 | 0.02 |
| Binocular UIVA (logMAR) | 0.05 ± 0.10 (−0.10 to 0.30) | −0.01 ± 0.11 (−0.20 to 0.30) | 0.03 | |||
| UNVA (logMAR) | 0.47 ± 0.15 (0.20 to 0.80) | 0.26 ± 0.10 (0.10 to 0.50) | 0.46 ± 0.17 (0.10 to 0.80) | 0.16 ± 0.10 (0 to 0.40) | 0.77 | <0.001 |
| Binocular UNVA (logMAR) | 0.23 ± 0.09 (0 to 0.40) | 0.14 ± 0.09 (0 to 0.30) | <0.001 | |||
| CDVA | −0.08 ± 0.06 (−0.20 to 0.10) | −0.07 ± 0.06 (−0.20 to 0.14) | −0.07 ± 0.07 (−0.20 to 0.20) | −0.07 ± 0.07 (−0.20 to 0.14) | 0.44 | 0.85 |
| Group A | Group B | |||
|---|---|---|---|---|
| Binocular Near Vision (40 cm) | Binocular Intermediate Vision (66 cm) | Binocular Near Vision (40 cm) | Binocular Intermediate Vision (66 cm) | |
| Reading acuity (logMAR) | 0.24 (0.17) | 0.03 (0.14) | 0.07 (0.12) | 0.01 (0.10) |
| Reading speed (wpm) | 93 (34.5) | 107 (18) | 101.5 (48) | 123 (77) |
| Reading duration (s) | 12.1 (4.45) | 10.2 (2.05) | 10.6 (3.6) | 9.6 (4.5) |
| Letter size | 2.00 (0.5) | 1.00 (0.23) | 1.13 (0.25) | 1.00 (0.2) |
| Group A | Group B | p Value | |
|---|---|---|---|
| Glare | 0.38 ± 0.77 (0, 3) | 0.35 ± 0.64 (0, 2) | 0.83 |
| Haloes | 0.18 ± 0.59 (0, 3) | 0.20 ± 0.54 (0, 2) | 0.67 |
| Starburst | 0.20 ± 0.52 (0, 2) | 0.24 ± 0.60 (0, 2) | 0.92 |
| Hazy vision | 0.18 ± 0.55 (0, 3) | 0 | 0.01 |
| Blurred vision | 0.15 ± 0.48 (0, 2) | 0.39 ± 0.68 (0, 2) | 0.04 |
| Distortion | 0.05 ± 0.32 (0, 2) | 0 | 0.29 |
| Double vision | 0 | 0.02 ± 0.15 (0, 1) | 0.36 |
| Vision fluctuate | 0.13 ± 0.40 (0, 2) | 0.13 ± 0.50 (0, 2) | 0.62 |
| Depth perception | 0 | 0 | 1 |
| QoV day | 8.77 ± 1.33 (3, 10) | 8.85 ± 0.99 (7, 10) | 0.76 |
| QoV night | 8.13 ± 1.34 (5, 10) | 7.85 ± 1.35 (4, 10) | 0.34 |
| Postoperative Assessment | Question | |||||
|---|---|---|---|---|---|---|
| How often do you require distance glasses? | ||||||
| Never | Occasionally | Quite often | Always | |||
| Group A | 95% | 5% | 0% | 0% | ||
| Group B | 100% | 0% | 0% | 0% | ||
| How often do you require reading glasses? | ||||||
| Never | Occasionally | Quite often | Always | |||
| Group A | 55% | 35% | 2.5% | 7.5% | ||
| Group B | 89.1% | 10.9% | 0% | 0% | ||
| How much difficulty do you have doing a regular task that requires you to see well in the distance? | ||||||
| Distance vision is clear | Slight problem | Moderate problem | Severe problem | Intolerable problem | ||
| Group A | Activity 1 | 95% | 5% | 0% | 0% | 0% |
| Activity 2 | 85% | 2.5% | 10% | 2.5% | 0% | |
| Group B | Activity 1 | 84.7% | 13.0% | 2.2% | 0% | 0% |
| Activity 2 | 73.9% | 21.7% | 4.3% | 0% | 0% | |
| How much difficulty do you have doing a regular task that requires you to see well at intermediate working distances? | ||||||
| Intermediate vision is clear | Slight problem | Moderate problem | Severe problem | Intolerable problem | ||
| Group A | Activity 1 | 92.5% | 7.5% | 0% | 0% | 0% |
| Activity 2 | 87.5% | 12.5% | 0% | 0% | 0% | |
| Group B | Activity 1 | 95.7% | 2.2% | 2.2% | 0% | 0% |
| Activity 2 | 100% | 0% | 0% | 0% | 0% | |
| How much difficulty do you have doing a regular task that requires you to see well at near working distances? | ||||||
| Near vision is clear | Slight problem | Moderate problem | Severe problem | Intolerable problem | ||
| Group A | Activity 1 | 67.5% | 17.5% | 15% | 0% | 0% |
| Activity 2 | 80.0% | 10% | 10% | 0% | 0% | |
| Group B | Activity 1 | 87% | 8.7% | 4.3% | 0% | 0% |
| Activity 2 | 91.3% | 8.7% | 0% | 0% | 0% | |
| How were your expectations fulfilled with the procedure? | ||||||
| More than fulfilled | Fulfilled | Sufficiently fulfilled | Not fulfilled at all | |||
| Group A | 37.5% | 40% | 22.5% | 0% | ||
| Group B | 28.3% | 50% | 21.7% | 0% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNeely, R.N.; Stewart, S.; Mandal, N.; Moutari, S.; Barsam, A.; Moore, J.E. The Influence of the Level of Monovision upon Early Outcomes Following the Bilateral Implantation of an Enhanced Monovision Intraocular Lens. Vision 2025, 9, 41. https://doi.org/10.3390/vision9020041
McNeely RN, Stewart S, Mandal N, Moutari S, Barsam A, Moore JE. The Influence of the Level of Monovision upon Early Outcomes Following the Bilateral Implantation of an Enhanced Monovision Intraocular Lens. Vision. 2025; 9(2):41. https://doi.org/10.3390/vision9020041
Chicago/Turabian StyleMcNeely, Richard N., Stephen Stewart, Niraj Mandal, Salissou Moutari, Allon Barsam, and Jonathan E. Moore. 2025. "The Influence of the Level of Monovision upon Early Outcomes Following the Bilateral Implantation of an Enhanced Monovision Intraocular Lens" Vision 9, no. 2: 41. https://doi.org/10.3390/vision9020041
APA StyleMcNeely, R. N., Stewart, S., Mandal, N., Moutari, S., Barsam, A., & Moore, J. E. (2025). The Influence of the Level of Monovision upon Early Outcomes Following the Bilateral Implantation of an Enhanced Monovision Intraocular Lens. Vision, 9(2), 41. https://doi.org/10.3390/vision9020041





