Visual Deficits and Diagnostic and Therapeutic Strategies for Neurofibromatosis Type 1: Bridging Science and Patient-Centered Care
Abstract
1. Introduction
2. Phenotypic Manifestations of NF1 Affecting Vision
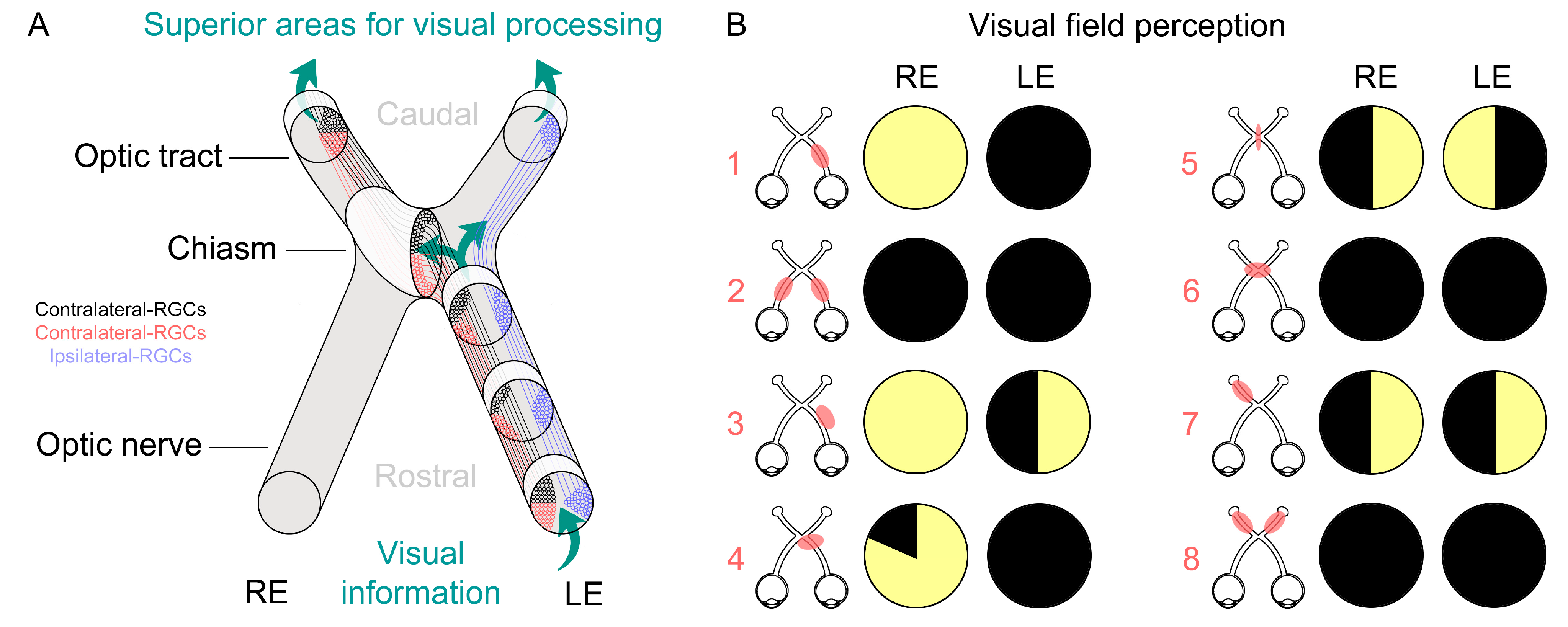
3. Diagnosis and Monitoring Methodologies
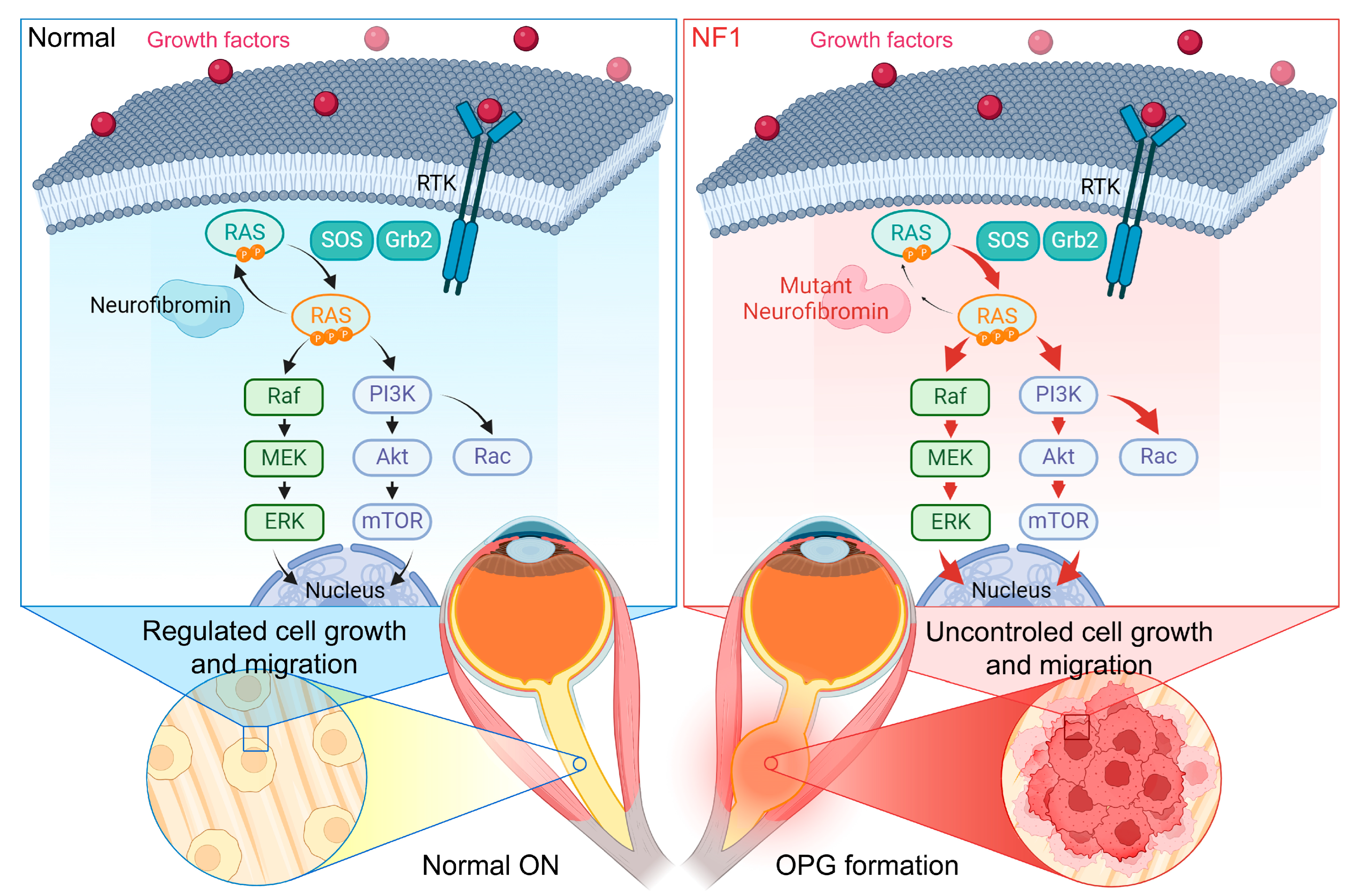
4. Therapeutic Strategies
5. The Role of Animal Models to Uncover Underlying Mechanisms of NF1 and to Develop Novel Therapies
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ricker, C.A.; Pan, Y.; Gutmann, D.H.; Keller, C. Challenges in Drug Discovery for Neurofibromatosis Type 1-Associated Low-Grade Glioma. Front. Oncol. 2016, 6, 259. [Google Scholar] [CrossRef]
- Riccardi, V.M. Neurofibromatosis: Past, Present, and Future. N. Engl. J. Med. 1991, 324, 1283–1285. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y. Research Update and Recent Developments in the Management of Scoliosis in Neurofibromatosis Type 1. Orthopedics 2010, 33, 335–341. [Google Scholar] [CrossRef]
- Ars, E.; Kruyer, H.; Morell, M.; Pros, E.; Serra, E.; Ravella, A.; Estivill, X.; Lázaro, C. Recurrent Mutations in the NF1 Gene Are Common among Neurofibromatosis Type 1 Patients. J. Med. Genet. 2003, 40, e82. [Google Scholar] [CrossRef]
- Cichowski, K.; Jacks, T. NF1 Tumor Suppressor Gene Function: Narrowing the GAP. Cell 2001, 104, 593–604. [Google Scholar] [CrossRef]
- Yap, Y.-S.; McPherson, J.R.; Ong, C.-K.; Rozen, S.G.; Teh, B.-T.; Lee, A.S.G.; Callen, D.F. The NF1 Gene Revisited—From Bench to Bedside. Oncotarget 2014, 5, 5873–5892. [Google Scholar] [CrossRef]
- Ratner, N.; Miller, S.J. A RASopathy Gene Commonly Mutated in Cancer: The Neurofibromatosis Type 1 Tumour Suppressor. Nat. Rev. Cancer 2015, 15, 290–301. [Google Scholar] [CrossRef]
- Listernick, R.; Ferner, R.E.; Liu, G.T.; Gutmann, D.H. Optic Pathway Gliomas in Neurofibromatosis-1: Controversies and Recommendations. Ann. Neurol. 2007, 61, 189–198. [Google Scholar] [CrossRef]
- Nix, J.S.; Blakeley, J.; Rodriguez, F.J. An Update on the Central Nervous System Manifestations of Neurofibromatosis Type 1. Acta Neuropathol. 2020, 139, 625–641. [Google Scholar] [CrossRef]
- Vitale, M.G.; Guha, A.; Skaggs, D.L. Orthopaedic Manifestations of Neurofibromatosis in Children: An Update. Clin. Orthop. Relat. Res. 2002, 401, 107–118. [Google Scholar] [CrossRef]
- Toro, G.; Santoro, C.; Ambrosio, D.; Landi, G.; Scilipoti, M.; Moretti, A.; Paoletta, M.; Liguori, S.; Schiavone Panni, A.; Picariello, S.; et al. Natural History of Scoliosis in Children with NF1: An Observation Study. Healthcare 2021, 9, 881. [Google Scholar] [CrossRef]
- Schindera, C.; Wingeier, K.; Goeggel Simonetti, B.; Diepold, M.; Nauer, C.B.; Fleischhauer, J.; Steinlin, M. Macrocephaly in Neurofibromatosis Type 1: A Sign Post for Optic Pathway Gliomas? Childs Nerv. Syst. 2011, 27, 2107–2111. [Google Scholar] [CrossRef][Green Version]
- Jett, K.; Friedman, J.M. Clinical and Genetic Aspects of Neurofibromatosis 1. Genet. Med. 2010, 12, 1–11. [Google Scholar] [CrossRef]
- North, K.N.; Riccardi, V.; Samango-Sprouse, C.; Ferner, R.; Moore, B.; Legius, E.; Ratner, N.; Denckla, M.B. Cognitive Function and Academic Performance in Neurofibromatosis. 1: Consensus Statement from the NF1 Cognitive Disorders Task Force. Neurology 1997, 48, 1121–1127. [Google Scholar] [CrossRef]
- Hyman, S.L.; Shores, A.; North, K.N. The Nature and Frequency of Cognitive Deficits in Children with Neurofibromatosis Type 1. Neurology 2005, 65, 1037–1044. [Google Scholar] [CrossRef]
- Torres Nupan, M.M.; Velez Van Meerbeke, A.; López Cabra, C.A.; Herrera Gomez, P.M. Cognitive and Behavioral Disorders in Children with Neurofibromatosis Type 1. Front. Pediatr. 2017, 5, 227. [Google Scholar] [CrossRef]
- Fisher, M.J.; Avery, R.A.; Allen, J.C.; Ardern-Holmes, S.L.; Bilaniuk, L.T.; Ferner, R.E.; Gutmann, D.H.; Listernick, R.; Martin, S.; Ullrich, N.J.; et al. Functional Outcome Measures for NF1-Associated Optic Pathway Glioma Clinical Trials. Neurology 2013, 81, S15–S24. [Google Scholar] [CrossRef]
- Stasheff, S.F.; Nadal-Nicolas, F.; Jecrois, E.; Li, W.; Bornhorst, M.; Zhu, Y. Physiologic Dysfunction, Demyelination, and Retinal Ganglion Cell Loss in Mice with Neurofibromatosis and Optic Pathway Gliomas. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3101. [Google Scholar]
- Ramón y Cajal, S. La Rétine Des Vertébrés; Typ. de Joseph van In & Cie.: Gradignan, France, 1892; pp. 122–255. Available online: https://books.google.com/books?id=kmBHnQEACAAJ (accessed on 11 February 2024).
- Hubel, D.H.; Wiesel, T.N. Brain Mechanisms of Vision. Sci. Am. 1979, 241, 150–162. [Google Scholar] [CrossRef]
- Güler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.-W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin Cells Are the Principal Conduits for Rod-Cone Input to Non-Image-Forming Vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef]
- Lucas, R.J.; Hattar, S.; Takao, M.; Berson, D.M.; Foster, R.G.; Yau, K.-W. Diminished Pupillary Light Reflex at High Irradiances in Melanopsin-Knockout Mice. Science 2003, 299, 245–247. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The Retina as a Window to the Brain-from Eye Research to CNS Disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- MacLaren, R.E. Re-Establishment of Visual Circuitry after Optic Nerve Regeneration. Eye 1999, 13, 277–284. [Google Scholar] [CrossRef]
- Rojnueangnit, K.; Xie, J.; Gomes, A.; Sharp, A.; Callens, T.; Chen, Y.; Liu, Y.; Cochran, M.; Abbott, M.-A.; Atkin, J.; et al. High Incidence of Noonan Syndrome Features Including Short Stature and Pulmonic Stenosis in Patients Carrying NF1 Missense Mutations Affecting p.Arg1809: Genotype-Phenotype Correlation. Hum. Mutat. 2015, 36, 1052–1063. [Google Scholar] [CrossRef]
- Pasmant, E.; Sabbagh, A.; Spurlock, G.; Laurendeau, I.; Grillo, E.; Hamel, M.-J.; Martin, L.; Barbarot, S.; Leheup, B.; Rodriguez, D.; et al. NF1 Microdeletions in Neurofibromatosis Type 1: From Genotype to Phenotype. Hum. Mutat. 2010, 31, E1506–E1518. [Google Scholar] [CrossRef]
- Wang, Q.; Montmain, G.; Ruano, E.; Upadhyaya, M.; Dudley, S.; Liskay, R.M.; Thibodeau, S.N.; Puisieux, A. Neurofibromatosis Type 1 Gene as a Mutational Target in a Mismatch Repair-Deficient Cell Type. Hum. Genet. 2003, 112, 117–123. [Google Scholar] [CrossRef]
- Kehrer-Sawatzki, H.; Mautner, V.-F.; Cooper, D.N. Emerging Genotype-Phenotype Relationships in Patients with Large NF1 Deletions. Hum. Genet. 2017, 136, 349–376. [Google Scholar] [CrossRef]
- Easton, D.F.; Ponder, M.A.; Huson, S.M.; Ponder, B.A. An Analysis of Variation in Expression of Neurofibromatosis (NF) Type 1 (NF1): Evidence for Modifying Genes. Am. J. Hum. Genet. 1993, 53, 305–313. [Google Scholar]
- Makino, S.; Tampo, H. Optical Coherence Tomography Imaging of Choroidal Abnormalities in Neurofibromatosis Type 1. Case Rep. Ophthalmol. Med. 2013, 2013, 292981. [Google Scholar] [CrossRef]
- Vagge, A.; Camicione, P.; Pellegrini, M.; Gatti, G.; Capris, P.; Severino, M.; Di Maita, M.; Panarello, S.; Traverso, C.E. Role of Visual Evoked Potentials and Optical Coherence Tomography in the Screening for Optic Pathway Gliomas in Patients with Neurofibromatosis Type I. Eur. J. Ophthalmol. 2021, 31, 698–703. [Google Scholar] [CrossRef]
- Wang, M.X.; Dillman, J.R.; Guccione, J.; Habiba, A.; Maher, M.; Kamel, S.; Panse, P.M.; Jensen, C.T.; Elsayes, K.M. Neurofibromatosis from Head to Toe: What the Radiologist Needs to Know. Radiographics 2022, 42, 1123–1144. [Google Scholar] [CrossRef]
- Huang, M.; Patel, J.; Patel, B.C. Optic Nerve Glioma; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Eid, H.; Crevier-Sorbo, G.; Aldraihem, A.; Menegotto, F.; Wilson, N. Neurofibromatosis Type 1: Description of a Novel Diagnostic Scoring System in Pediatric Optic Nerve Glioma. AJR Am. J. Roentgenol. 2019, 212, 892–898. [Google Scholar] [CrossRef]
- Beres, S.J.; Avery, R.A. Optic Pathway Gliomas Secondary to Neurofibromatosis Type 1. Semin Pediatr. Neurol. 2017, 24, 92–99. [Google Scholar] [CrossRef]
- Zeid, J.L.; Charrow, J.; Sandu, M.; Goldman, S.; Listernick, R. Orbital Optic Nerve Gliomas in Children with Neurofibromatosis Type 1. J. AAPOS 2006, 10, 534–539. [Google Scholar] [CrossRef]
- Pisapia, J.M.; Akbari, H.; Rozycki, M.; Thawani, J.P.; Storm, P.B.; Avery, R.A.; Vossough, A.; Fisher, M.J.; Heuer, G.G.; Davatzikos, C. Predicting Pediatric Optic Pathway Glioma Progression Using Advanced Magnetic Resonance Image Analysis and Machine Learning. Neuro-Oncol. Adv. 2020, 2, vdaa090. [Google Scholar] [CrossRef]
- Cummings, T.J.; Provenzale, J.M.; Hunter, S.B.; Friedman, A.H.; Klintworth, G.K.; Bigner, S.H.; McLendon, R.E. Gliomas of the Optic Nerve: Histological, Immunohistochemical (MIB-1 and P53), and MRI Analysis. Acta Neuropathol. 2000, 99, 563–570. [Google Scholar] [CrossRef]
- Perry, A.; Wesseling, P. Histologic Classification of Gliomas. Handb. Clin. Neurol. 2016, 134, 71–95. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a Marker of Retinal Ganglion Cells: Qualitative and Quantitative Time Course Studies in Naive and Optic Nerve-Injured Retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Galindo-Romero, C.; Lucas-Ruiz, F.; Marsh-Amstrong, N.; Li, W.; Vidal-Sanz, M.; Agudo-Barriuso, M. Pan-Retinal Ganglion Cell Markers in Mice, Rats, and Rhesus Macaques. Zool. Res. 2023, 44, 226–248. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis Type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef]
- Huson, S.; Jones, D.; Beck, L. Ophthalmic Manifestations of Neurofibromatosis. Br. J. Ophthalmol. 1987, 71, 235–238. [Google Scholar] [CrossRef]
- Maharaj, A.; Singh, V.R.; Lalchan, S.A. Lisch and the Importance of His Nodules. West Indian Med. J. 2014, 63, 799–802. [Google Scholar] [CrossRef][Green Version]
- Hernáiz Driever, P.; von Hornstein, S.; Pietsch, T.; Kortmann, R.; Warmuth-Metz, M.; Emser, A.; Gnekow, A.K. Natural History and Management of Low-Grade Glioma in NF-1 Children. J. Neuro-Oncol. 2010, 100, 199–207. [Google Scholar] [CrossRef]
- Fisher, M.J.; Jones, D.T.W.; Li, Y.; Guo, X.; Sonawane, P.S.; Waanders, A.J.; Phillips, J.J.; Weiss, W.A.; Resnick, A.C.; Gosline, S.; et al. Integrated Molecular and Clinical Analysis of Low-Grade Gliomas in Children with Neurofibromatosis Type 1 (NF1). Acta Neuropathol. 2021, 141, 605–617. [Google Scholar] [CrossRef]
- Lohkamp, L.-N.; Parkin, P.; Puran, A.; Bartels, U.K.; Bouffet, E.; Tabori, U.; Rutka, J.T. Optic Pathway Glioma in Children with Neurofibromatosis Type 1: A Multidisciplinary Entity, Posing Dilemmas in Diagnosis and Management Multidisciplinary Management of Optic Pathway Glioma in Children with Neurofibromatosis Type 1. Front. Surg. 2022, 9, 886697. [Google Scholar] [CrossRef]
- Shofty, B.; Ben Sira, L.; Constantini, S. Neurofibromatosis 1-Associated Optic Pathway Gliomas. Childs Nerv. Syst. 2020, 36, 2351–2361. [Google Scholar] [CrossRef]
- Taylor, T.; Jaspan, T.; Milano, G.; Gregson, R.; Parker, T.; Ritzmann, T.; Benson, C.; Walker, D. PLAN Study Group Radiological Classification of Optic Pathway Gliomas: Experience of a Modified Functional Classification System. Br. J. Radiol. 2008, 81, 761–766. [Google Scholar] [CrossRef]
- Listernick, R.; Charrow, J.; Greenwald, M.; Mets, M. Natural History of Optic Pathway Tumors in Children with Neurofibromatosis Type 1: A Longitudinal Study. J. Pediatr. 1994, 125, 63–66. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Perry, A.; Gutmann, D.H.; O’Neill, B.P.; Leonard, J.; Bryant, S.; Giannini, C. Gliomas in Neurofibromatosis Type 1: A Clinicopathologic Study of 100 Patients. J. Neuropathol. Exp. Neurol. 2008, 67, 240–249. [Google Scholar] [CrossRef]
- Azizi, A.A.; Walker, D.A.; Liu, J.-F.; Sehested, A.; Jaspan, T.; Pemp, B.; Simmons, I.; Ferner, R.; Grill, J.; Hargrave, D.; et al. NF1 Optic Pathway Glioma: Analyzing Risk Factors for Visual Outcome and Indications to Treat. Neuro-Oncology 2021, 23, 100–111. [Google Scholar] [CrossRef]
- Angelova-Toshkina, D.; Decker, J.A.; Traunwieser, T.; Holzapfel, J.; Bette, S.; Huber, S.; Schimmel, M.; Vollert, K.; Bison, B.; Kröncke, T.; et al. Comprehensive Neurological Evaluation of a Cohort of Patients with Neurofibromatosis Type 1 from a Single Institution. Eur. J. Paediatr. Neurol. 2023, 43, 52–61. [Google Scholar] [CrossRef]
- Parsa, C.F.; Hoyt, C.S.; Lesser, R.L.; Weinstein, J.M.; Strother, C.M.; Muci-Mendoza, R.; Ramella, M.; Manor, R.S.; Fletcher, W.A.; Repka, M.X.; et al. Spontaneous Regression of Optic Gliomas: Thirteen Cases Documented by Serial Neuroimaging. Arch. Ophthalmol. 2001, 119, 516–529. [Google Scholar] [CrossRef]
- Senthilkumar, V.A.; Tripathy, K. Lisch Nodules; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Papageorgiou, E.; Tsironi-Malizou, E. Types of Homonymous Visual Field Defects. In Homonymous Visual Field Defects; Skorkovská, K., Ed.; Springer: Cham, Switzerland, 2017; pp. 65–94. ISBN 978-3-319-52282-1. [Google Scholar]
- Walker, D.A.; Aquilina, K.; Spoudeas, H.; Pilotto, C.; Gan, H.-W.; Meijer, L. A New Era for Optic Pathway Glioma: A Developmental Brain Tumor with Life-Long Health Consequences. Front. Pediatr. 2023, 11, 1038937. [Google Scholar] [CrossRef]
- Silva, M.M.; Goldman, S.; Keating, G.; Marymont, M.A.; Kalapurakal, J.; Tomita, T. Optic Pathway Hypothalamic Gliomas in Children under Three Years of Age: The Role of Chemotherapy. Pediatr. Neurosurg. 2000, 33, 151–158. [Google Scholar] [CrossRef]
- Fuss, M.; Hug, E.B.; Schaefer, R.A.; Nevinny-Stickel, M.; Miller, D.W.; Slater, J.M.; Slater, J.D. Proton Radiation Therapy (PRT) for Pediatric Optic Pathway Gliomas: Comparison with 3D Planned Conventional Photons and a Standard Photon Technique. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 1117–1126. [Google Scholar] [CrossRef]
- Balcer, L.J.; Liu, G.T.; Heller, G.; Bilaniuk, L.; Volpe, N.J.; Galetta, S.L.; Molloy, P.T.; Phillips, P.C.; Janss, A.J.; Vaughn, S.; et al. Visual Loss in Children with Neurofibromatosis Type 1 and Optic Pathway Gliomas: Relation to Tumor Location by Magnetic Resonance Imaging. Am. J. Ophthalmol. 2001, 131, 442–445. [Google Scholar] [CrossRef]
- Falzon, K.; Drimtzias, E.; Picton, S.; Simmons, I. Visual Outcomes after Chemotherapy for Optic Pathway Glioma in Children with and without Neurofibromatosis Type 1: Results of the International Society of Paediatric Oncology (SIOP) Low-Grade Glioma 2004 Trial UK Cohort. Br. J. Ophthalmol. 2018, 102, 1367–1371. [Google Scholar] [CrossRef]
- Lobbous, M.; Bernstock, J.D.; Coffee, E.; Friedman, G.K.; Metrock, L.K.; Chagoya, G.; Elsayed, G.; Nakano, I.; Hackney, J.R.; Korf, B.R.; et al. An Update on Neurofibromatosis Type 1-Associated Gliomas. Cancers 2020, 12, 114. [Google Scholar] [CrossRef]
- Friedman, J.M. Neurofibromatosis 1. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Parrozzani, R.; Pilotto, E.; Clementi, M.; Frizziero, L.; Leonardi, F.; Convento, E.; Miglionico, G.; Pulze, S.; Perrini, P.; Trevisson, E.; et al. Retinal Vascular Abnormalities in a Large Cohort of Patients Affected by Neurofibromatosis Type 1: A Study Using Optical Coherence Tomography Angiography. Retina 2018, 38, 585–593. [Google Scholar] [CrossRef]
- Moramarco, A.; Miraglia, E.; Mallone, F.; Roberti, V.; Iacovino, C.; Bruscolini, A.; Giustolisi, R.; Giustini, S. Retinal Microvascular Abnormalities in Neurofibromatosis Type 1. Br. J. Ophthalmol. 2019, 103, 1590–1594. [Google Scholar] [CrossRef]
- Mallone, F.; Lucchino, L.; Giustini, S.; Lambiase, A.; Moramarco, A. An Update on Choroidal Abnormalities and Retinal Microvascular Changes in Neurofibromatosis Type 1. Orphanet J. Rare Dis. 2022, 17, 223. [Google Scholar] [CrossRef]
- Hoyt, W.F.; Luis, O. The Primate Chiasm. Details of Visual Fiber Organization Studied by Silver Impregnation Techniques. Arch. Ophthalmol. 1963, 70, 69–85. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Ligon, A.H.; Horkayne-Szakaly, I.; Rushing, E.J.; Ligon, K.L.; Vena, N.; Garcia, D.I.; Cameron, J.D.; Eberhart, C.G. BRAF Duplications and MAPK Pathway Activation Are Frequent in Gliomas of the Optic Nerve Proper. J. Neuropathol. Exp. Neurol. 2012, 71, 789–794. [Google Scholar] [CrossRef]
- Robert-Boire, V.; Rosca, L.; Samson, Y.; Ospina, L.H.; Perreault, S. Clinical Presentation and Outcome of Patients with Optic Pathway Glioma. Pediatr. Neurol. 2017, 75, 55–60. [Google Scholar] [CrossRef]
- Modrzejewska, M.; Olejnik-Wojciechowska, J.; Roszyk, A.; Szychot, E.; Konczak, T.D.; Szemitko, M.; Peregud-Pogorzelski, J.W. Optic Pathway Gliomas in Pediatric Population-Current Approach in Diagnosis and Management: Literature Review. J. Clin. Med. 2023, 12, 6709. [Google Scholar] [CrossRef]
- Ronsley, R.; Hounjet, C.D.; Cheng, S.; Rassekh, S.R.; Duncan, W.J.; Dunham, C.; Gardiner, J.; Ghag, A.; Ludemann, J.P.; Wensley, D.; et al. Trametinib Therapy for Children with Neurofibromatosis Type 1 and Life-Threatening Plexiform Neurofibroma or Treatment-Refractory Low-Grade Glioma. Cancer Med. 2021, 10, 3556–3564. [Google Scholar] [CrossRef]
- Manoharan, N.; Choi, J.; Chordas, C.; Zimmerman, M.A.; Scully, J.; Clymer, J.; Filbin, M.; Ullrich, N.J.; Bandopadhayay, P.; Chi, S.N.; et al. Trametinib for the Treatment of Recurrent/Progressive Pediatric Low-Grade Glioma. J. Neuro-Oncol. 2020, 149, 253–262. [Google Scholar] [CrossRef]
- Jones, D.T.W.; Hutter, B.; Jäger, N.; Korshunov, A.; Kool, M.; Warnatz, H.-J.; Zichner, T.; Lambert, S.R.; Ryzhova, M.; Quang, D.A.K.; et al. Recurrent Somatic Alterations of FGFR1 and NTRK2 in Pilocytic Astrocytoma. Nat. Genet. 2013, 45, 927–932. [Google Scholar] [CrossRef]
- Sharma, M.K.; Zehnbauer, B.A.; Watson, M.A.; Gutmann, D.H. RAS Pathway Activation and an Oncogenic RAS Mutation in Sporadic Pilocytic Astrocytoma. Neurology 2005, 65, 1335–1336. [Google Scholar] [CrossRef]
- Czyzyk, E.; Jóźwiak, S.; Roszkowski, M.; Schwartz, R.A. Optic Pathway Gliomas in Children with and without Neurofibromatosis 1. J. Child Neurol. 2003, 18, 471–478. [Google Scholar] [CrossRef]
- Listernick, R.; Darling, C.; Greenwald, M.; Strauss, L.; Charrow, J. Optic Pathway Tumors in Children: The Effect of Neurofibromatosis Type 1 on Clinical Manifestations and Natural History. J. Pediatr. 1995, 127, 718–722. [Google Scholar] [CrossRef]
- Rasool, N.; Odel, J.G.; Kazim, M. Optic Pathway Glioma of Childhood. Curr. Opin. Ophthalmol. 2017, 28, 289–295. [Google Scholar] [CrossRef]
- Singhal, S.; Birch, J.M.; Kerr, B.; Lashford, L.; Evans, D.G.R. Neurofibromatosis Type 1 and Sporadic Optic Gliomas. Arch. Dis. Child 2002, 87, 65–70. [Google Scholar] [CrossRef]
- Astrup, J. Natural History and Clinical Management of Optic Pathway Glioma. Br. J. Neurosurg. 2003, 17, 327–335. [Google Scholar] [CrossRef]
- Chateil, J.F.; Soussotte, C.; Pédespan, J.M.; Brun, M.; Le Manh, C.; Diard, F. MRI and Clinical Differences between Optic Pathway Tumours in Children with and without Neurofibromatosis. Br. J. Radiol. 2001, 74, 24–31. [Google Scholar] [CrossRef]
- Shamji, M.F.; Benoit, B.G. Syndromic and Sporadic Pediatric Optic Pathway Gliomas: Review of Clinical and Histopathological Differences and Treatment Implications. Neurosurg. Focus 2007, 23, E3. [Google Scholar] [CrossRef]
- Kornreich, L.; Blaser, S.; Schwarz, M.; Shuper, A.; Vishne, T.H.; Cohen, I.J.; Faingold, R.; Michovitz, S.; Koplewitz, B.; Horev, G. Optic Pathway Glioma: Correlation of Imaging Findings with the Presence of Neurofibromatosis. AJNR Am. J. Neuroradiol. 2001, 22, 1963–1969. [Google Scholar]
- Grill, J.; Laithier, V.; Rodriguez, D.; Raquin, M.A.; Pierre-Kahn, A.; Kalifa, C. When Do Children with Optic Pathway Tumours Need Treatment? An Oncological Perspective in 106 Patients Treated in a Single Centre. Eur. J. Pediatr. 2000, 159, 692–696. [Google Scholar] [CrossRef]
- Wan, M.J.; Ullrich, N.J.; Manley, P.E.; Kieran, M.W.; Goumnerova, L.C.; Heidary, G. Long-Term Visual Outcomes of Optic Pathway Gliomas in Pediatric Patients without Neurofibromatosis Type 1. J. Neuro-Oncol. 2016, 129, 173–178. [Google Scholar] [CrossRef]
- NIH. National Institutes of Health Consensus Development Conference Statement: Neurofibromatosis, Bethesda, Md., USA, July 13–15, 1987. Neurofibromatosis 1988, 1, 172–178. [Google Scholar]
- Kang, E.; Yoon, H.M.; Lee, B.H. Neurofibromatosis Type I: Points to Be Considered by General Pediatricians. Clin. Exp. Pediatr. 2021, 64, 149–156. [Google Scholar] [CrossRef]
- de Blank, P.M.K.; Fisher, M.J.; Liu, G.T.; Gutmann, D.H.; Listernick, R.; Ferner, R.E.; Avery, R.A. Optic Pathway Gliomas in Neurofibromatosis Type 1: An Update: Surveillance, Treatment Indications, and Biomarkers of Vision. J. Neuroophthalmol. 2017, 37 (Suppl. S1), S23–S32. [Google Scholar] [CrossRef]
- Tang, Y.; Gutmann, D.H. Neurofibromatosis Type 1-Associated Optic Pathway Gliomas: Current Challenges and Future Prospects. Cancer Manag. Res. 2023, 15, 667–681. [Google Scholar] [CrossRef]
- Lubs, M.L.; Bauer, M.S.; Formas, M.E.; Djokic, B. Lisch Nodules in Neurofibromatosis Type 1. N. Engl. J. Med. 1991, 324, 1264–1266. [Google Scholar] [CrossRef]
- Daoudi, C.; Daoudi, R. Lisch nodules in Von Recklinghausen disease. Pan Afr. Med. J. 2014, 19, 173. [Google Scholar] [CrossRef]
- Binning, M.J.; Liu, J.K.; Kestle, J.R.W.; Brockmeyer, D.L.; Walker, M.L. Optic Pathway Gliomas: A Review. Neurosurg. Focus 2007, 23, E2. [Google Scholar] [CrossRef]
- Fletcher, W.A.; Imes, R.K.; Hoyt, W.F. Chiasmal Gliomas: Appearance and Long-Term Changes Demonstrated by Computerized Tomography. J. Neurosurg. 1986, 65, 154–159. [Google Scholar] [CrossRef]
- Moreno, L.; Bautista, F.; Ashley, S.; Duncan, C.; Zacharoulis, S. Does Chemotherapy Affect the Visual Outcome in Children with Optic Pathway Glioma? A Systematic Review of the Evidence. Eur. J. Cancer 2010, 46, 2253–2259. [Google Scholar] [CrossRef]
- Maloney, E.; Perez, F.A.; Iyer, R.S.; Otto, R.K.; Wright, J.N.; Menashe, S.J.; Hippe, D.S.; Shaw, D.W.W.; Stanescu, A.L. Non-Inferiority of a Non-Gadolinium-Enhanced Magnetic Resonance Imaging Follow-up Protocol for Isolated Optic Pathway Gliomas. Pediatr. Radiol. 2022, 52, 539–548. [Google Scholar] [CrossRef]
- Walrath, J.D.; Engelbert, M.; Kazim, M. Magnetic Resonance Imaging Evidence of Optic Nerve Glioma Progression into and beyond the Optic Chiasm. Ophthalmic Plast. Reconstr. Surg. 2008, 24, 473–475. [Google Scholar] [CrossRef]
- Fisher, M.J.; Loguidice, M.; Gutmann, D.H.; Listernick, R.; Ferner, R.E.; Ullrich, N.J.; Packer, R.J.; Tabori, U.; Hoffman, R.O.; Ardern-Holmes, S.L.; et al. Visual Outcomes in Children with Neurofibromatosis Type 1-Associated Optic Pathway Glioma Following Chemotherapy: A Multicenter Retrospective Analysis. Neuro-Oncol. 2012, 14, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-J.; Yan, C.; Tang, Y.; Wang, W.; Gu, Y.-H.; Ren, J.-Y.; Cui, X.-W.; Lian, X.; Liu, J.; Wang, H.-J.; et al. Computed Tomography-Based Differentiation of Benign and Malignant Craniofacial Lesions in Neurofibromatosis Type I Patients: A Machine Learning Approach. Front. Oncol. 2020, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.A.; Mansoor, A.; Idrees, R.; Trimboli-Heidler, C.; Ishikawa, H.; Packer, R.J.; Linguraru, M.G. Optic Pathway Glioma Volume Predicts Retinal Axon Degeneration in Neurofibromatosis Type 1. Neurology 2016, 87, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, G.; Lafforgue, M.-P.; Lion-François, L.; Kemlin, I.; Rodriguez, D.; Castelnau, P.; Carneiro, M.; Meyer, P.; Rivier, F.; Barbarot, S.; et al. Systematic MRI in NF1 Children under Six Years of Age for the Diagnosis of Optic Pathway Gliomas. Study and Outcome of a French Cohort. Eur. J. Paediatr. Neurol. 2016, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Cassina, M.; Frizziero, L.; Opocher, E.; Parrozzani, R.; Sorrentino, U.; Viscardi, E.; Miglionico, G.; Midena, E.; Clementi, M.; Trevisson, E. Optic Pathway Glioma in Type 1 Neurofibromatosis: Review of Its Pathogenesis, Diagnostic Assessment, and Treatment Recommendations. Cancers 2019, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Parrozzani, R.; Clementi, M.; Kotsafti, O.; Miglionico, G.; Trevisson, E.; Orlando, G.; Pilotto, E.; Midena, E. Optical Coherence Tomography in the Diagnosis of Optic Pathway Gliomas. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8112–8118. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.A.; Cnaan, A.; Schuman, J.S.; Trimboli-Heidler, C.; Chen, C.-L.; Packer, R.J.; Ishikawa, H. Longitudinal Change of Circumpapillary Retinal Nerve Fiber Layer Thickness in Children with Optic Pathway Gliomas. Am. J. Ophthalmol. 2015, 160, 944–952.e1. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.A.; Liu, G.T.; Fisher, M.J.; Quinn, G.E.; Belasco, J.B.; Phillips, P.C.; Maguire, M.G.; Balcer, L.J. Retinal Nerve Fiber Layer Thickness in Children with Optic Pathway Gliomas. Am. J. Ophthalmol. 2011, 151, 542–549.e2. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhao, T.; Miyagishima, K.J.; Chen, S.; Li, W.; Nadal-Nicolás, F.M. Establishing the Ground Squirrel as a Superb Model for Retinal Ganglion Cell Disorders and Optic Neuropathies. Lab. Investig. 2021, 101, 1289–1303. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Miyagishima, K.J.; Li, W. In Search for the “Idyllic” Animal Model to Evaluate Ocular Pathologies and Translate New Therapies to Improve Human Health. Neural Regen. Res. 2022, 17, 2697–2699. [Google Scholar] [CrossRef]
- Barkana, Y.; Burgansky-Eliash, Z.; Gerber, Y.; Melamed, S.; Neudorfer, M.; Avni, I.; Bartov, E.; Morad, Y. Inter-Device Variability of the Stratus Optical Coherence Tomography. Am. J. Ophthalmol. 2009, 147, 260–266. [Google Scholar] [CrossRef]
- Yang, H.; Lee, H.S.; Bae, H.W.; Seong, G.J.; Kim, C.Y.; Lee, S.Y. Effect of Image Quality Fluctuations on the Repeatability of Thickness Measurements in Swept-Source Optical Coherence Tomography. Sci. Rep. 2020, 10, 13897. [Google Scholar] [CrossRef]
- Rovere, G.; Nadal-Nicolás, F.M.; Agudo-Barriuso, M.; Sobrado-Calvo, P.; Nieto-López, L.; Nucci, C.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Comparison of Retinal Nerve Fiber Layer Thinning and Retinal Ganglion Cell Loss after Optic Nerve Transection in Adult Albino Rats. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4487–4498. [Google Scholar] [CrossRef]
- Moramarco, A.; Giustini, S.; Nofroni, I.; Mallone, F.; Miraglia, E.; Iacovino, C.; Calvieri, S.; Lambiase, A. Near-Infrared Imaging: An in Vivo, Non-Invasive Diagnostic Tool in Neurofibromatosis Type 1. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 307–311. [Google Scholar] [CrossRef]
- Ratnam, K.; Carroll, J.; Porco, T.C.; Duncan, J.L.; Roorda, A. Relationship between Foveal Cone Structure and Clinical Measures of Visual Function in Patients with Inherited Retinal Degenerations. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5836–5847. [Google Scholar] [CrossRef]
- Foote, K.G.; Loumou, P.; Griffin, S.; Qin, J.; Ratnam, K.; Porco, T.C.; Roorda, A.; Duncan, J.L. Relationship Between Foveal Cone Structure and Visual Acuity Measured with Adaptive Optics Scanning Laser Ophthalmoscopy in Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3385–3393. [Google Scholar] [CrossRef]
- Bensinger, E.; Rinella, N.; Saud, A.; Loumou, P.; Ratnam, K.; Griffin, S.; Qin, J.; Porco, T.C.; Roorda, A.; Duncan, J.L. Loss of Foveal Cone Structure Precedes Loss of Visual Acuity in Patients with Rod-Cone Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3187–3196. [Google Scholar] [CrossRef]
- North, K.; Cochineas, C.; Tang, E.; Fagan, E. Optic Gliomas in Neurofibromatosis Type 1: Role of Visual Evoked Potentials. Pediatr. Neurol. 1994, 10, 117–123. [Google Scholar] [CrossRef]
- Falsini, B.; Ziccardi, L.; Lazzareschi, I.; Ruggiero, A.; Placentino, L.; Dickmann, A.; Liotti, L.; Piccardi, M.; Balestrazzi, E.; Colosimo, C.; et al. Longitudinal Assessment of Childhood Optic Gliomas: Relationship between Flicker Visual Evoked Potentials and Magnetic Resonance Imaging Findings. J. Neurooncol. 2008, 88, 87–96. [Google Scholar] [CrossRef]
- Kelly, J.P.; Weiss, A.H. Detection of Tumor Progression in Optic Pathway Glioma with and without Neurofibromatosis Type 1. Neuro-Oncology 2013, 15, 1560–1567. [Google Scholar] [CrossRef]
- Bowman, R.; Walters, B.; Smith, V.; Prise, K.L.; Handley, S.E.; Green, K.; Mankad, K.; O’Hare, P.; Dahl, C.; Jorgensen, M.; et al. Visual Outcomes and Predictors in Optic Pathway Glioma: A Single Centre Study. Eye 2023, 37, 1178–1183. [Google Scholar] [CrossRef]
- Barrea, C.; Vaessen, S.; Bulk, S.; Harvengt, J.; Misson, J.-P. Phenotype-Genotype Correlation in Children with Neurofibromatosis Type 1. Neuropediatrics 2018, 49, 180–184. [Google Scholar] [CrossRef]
- Riccardi, V.M. Pathophysiology of Neurofibromatosis. IV. Dermatologic Insights into Heterogeneity and Pathogenesis. J. Am. Acad. Dermatol. 1980, 3, 157–166. [Google Scholar] [CrossRef]
- Barker, D.; Wright, E.; Nguyen, K.; Cannon, L.; Fain, P.; Goldgar, D.; Bishop, D.T.; Carey, J.; Baty, B.; Kivlin, J. Gene for von Recklinghausen Neurofibromatosis Is in the Pericentromeric Region of Chromosome 17. Science 1987, 236, 1100–1102. [Google Scholar] [CrossRef]
- Koczkowska, M.; Callens, T.; Gomes, A.; Sharp, A.; Chen, Y.; Hicks, A.D.; Aylsworth, A.S.; Azizi, A.A.; Basel, D.G.; Bellus, G.; et al. Expanding the Clinical Phenotype of Individuals with a 3-Bp in-Frame Deletion of the NF1 Gene (c.2970_2972del): An Update of Genotype-Phenotype Correlation. Genet. Med. 2019, 21, 867–876. [Google Scholar] [CrossRef]
- Koczkowska, M.; Chen, Y.; Callens, T.; Gomes, A.; Sharp, A.; Johnson, S.; Hsiao, M.-C.; Chen, Z.; Balasubramanian, M.; Barnett, C.P.; et al. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844-848. Am. J. Hum. Genet. 2018, 102, 69–87. [Google Scholar] [CrossRef]
- Zhu, B.; Zheng, T.; Wang, W.; Gu, Y.; Wei, C.; Li, Q.; Wang, Z. Genotype-Phenotype Correlations of Neurofibromatosis Type 1: A Cross-Sectional Study from a Large Chinese Cohort. J. Neurol. 2023, 271, 1893–1900. [Google Scholar] [CrossRef]
- Bildirici, Y.; Kocaaga, A.; Karademir-Arslan, C.N.; Yimenicioglu, S. Evaluation of Molecular and Clinical Findings in Children with Neurofibromatosis Type 1: Identification of 15 Novel Variants. Pediatr. Neurol. 2023, 149, 69–74. [Google Scholar] [CrossRef]
- Pacot, L.; Sabbagh, A.; Sohier, P.; Hadjadj, D.; Ye, M.; Boland-Auge, A.; Bacq-Daian, D.; Laurendeau, I.; Briand-Suleau, A.; Deleuze, J.-F.; et al. Identification of Potential Common Genetic Modifiers of Neurofibromas: A Genome-Wide Association Study in 1333 Patients with Neurofibromatosis Type 1. Br. J. Dermatol. 2024, 190, 226–243. [Google Scholar] [CrossRef]
- Bettegowda, C.; Upadhayaya, M.; Evans, D.G.; Kim, A.; Mathios, D.; Hanemann, C.O. REiNS International Collaboration Genotype-Phenotype Correlations in Neurofibromatosis and Their Potential Clinical Use. Neurology 2021, 97, S91–S98. [Google Scholar] [CrossRef]
- Trevisson, E.; Morbidoni, V.; Forzan, M.; Daolio, C.; Fumini, V.; Parrozzani, R.; Cassina, M.; Midena, E.; Salviati, L.; Clementi, M. The Arg1038Gly Missense Variant in the NF1 Gene Causes a Mild Phenotype without Neurofibromas. Mol. Genet. Genom. Med. 2019, 7, e616. [Google Scholar] [CrossRef]
- Kehrer-Sawatzki, H.; Cooper, D.N. Classification of NF1 Microdeletions and Its Importance for Establishing Genotype/Phenotype Correlations in Patients with NF1 Microdeletions. Hum. Genet. 2021, 140, 1635–1649. [Google Scholar] [CrossRef]
- Well, L.; Döbel, K.; Kluwe, L.; Bannas, P.; Farschtschi, S.; Adam, G.; Mautner, V.-F.; Salamon, J. Genotype-Phenotype Correlation in Neurofibromatosis Type-1: NF1 Whole Gene Deletions Lead to High Tumor-Burden and Increased Tumor-Growth. PLoS Genet. 2021, 17, e1009517. [Google Scholar] [CrossRef]
- Svensson, C.K.; Drobitch, R.K.; Kloss, K.A. Effect of Glutathione Depletion on the in Vivo Inhibition of Drug Metabolism by Agents Forming an Inactive Cytochrome P-450 Fe(II):Metabolite Complex. Studies with Amiodarone and Troleandomycin. J. Pharm. Sci. 1991, 80, 225–228. [Google Scholar] [CrossRef]
- Chong, A.L.; Pole, J.D.; Scheinemann, K.; Hukin, J.; Tabori, U.; Huang, A.; Bouffet, E.; Bartels, U. Optic Pathway Gliomas in Adolescence--Time to Challenge Treatment Choices? Neuro-Oncology 2013, 15, 391–400. [Google Scholar] [CrossRef]
- Peng, F.; Juhasz, C.; Bhambhani, K.; Wu, D.; Chugani, D.C.; Chugani, H.T. Assessment of Progression and Treatment Response of Optic Pathway Glioma with Positron Emission Tomography Using Alpha-[(11)C]Methyl-L-Tryptophan. Mol. Imaging Biol. 2007, 9, 106–109. [Google Scholar] [CrossRef]
- Revere, K.E.; Katowitz, W.R.; Katowitz, J.A.; Rorke-Adams, L.; Fisher, M.J.; Liu, G.T. Childhood Optic Nerve Glioma: Vision Loss Due to Biopsy. Ophthalmic Plast. Reconstr. Surg. 2017, 33, S107–S109. [Google Scholar] [CrossRef]
- Oderich, G.S.; Sullivan, T.M.; Bower, T.C.; Gloviczki, P.; Miller, D.V.; Babovic-Vuksanovic, D.; Macedo, T.A.; Stanson, A. Vascular Abnormalities in Patients with Neurofibromatosis Syndrome Type I: Clinical Spectrum, Management, and Results. J. Vasc. Surg. 2007, 46, 475–484. [Google Scholar] [CrossRef]
- Hivelin, M.; Plaud, B.; Hemery, F.; Boulat, C.; Ortonne, N.; Valleyrie-Allanore, L.; Wolkenstein, P.; Lantieri, L. Low Rates of Blood Transfusion in Elective Resections of Neurofibromas in a Cohort Study: Neurofibroma Length as a Predictor of Transfusion Requirement. Plast. Reconstr. Surg. 2016, 137, 700e–711e. [Google Scholar] [CrossRef]
- Wang, D.; Ge, L.; Guo, Z.; Li, Y.; Zhu, B.; Wang, W.; Wei, C.; Li, Q.; Wang, Z. Efficacy and Safety of Trametinib in Neurofibromatosis Type 1-Associated Plexiform Neurofibroma and Low-Grade Glioma: A Systematic Review and Meta-Analysis. Pharmaceuticals 2022, 15, 956. [Google Scholar] [CrossRef]
- Farazdaghi, M.K.; Katowitz, W.R.; Avery, R.A. Current Treatment of Optic Nerve Gliomas. Curr. Opin. Ophthalmol. 2019, 30, 356–363. [Google Scholar] [CrossRef]
- Bataini, J.P.; Delanian, S.; Ponvert, D. Chiasmal Gliomas: Results of Irradiation Management in 57 Patients and Review of Literature. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 615–623. [Google Scholar] [CrossRef]
- Cappelli, C.; Grill, J.; Raquin, M.; Pierre-Kahn, A.; Lellouch-Tubiana, A.; Terrier-Lacombe, M.J.; Habrand, J.L.; Couanet, D.; Brauner, R.; Rodriguez, D.; et al. Long-Term Follow up of 69 Patients Treated for Optic Pathway Tumours before the Chemotherapy Era. Arch. Dis. Child 1998, 79, 334–338. [Google Scholar] [CrossRef]
- Lacaze, E.; Kieffer, V.; Streri, A.; Lorenzi, C.; Gentaz, E.; Habrand, J.-L.; Dellatolas, G.; Kalifa, C.; Grill, J. Neuropsychological Outcome in Children with Optic Pathway Tumours When First-Line Treatment Is Chemotherapy. Br. J. Cancer 2003, 89, 2038–2044. [Google Scholar] [CrossRef]
- Sutton, L.N.; Molloy, P.T.; Sernyak, H.; Goldwein, J.; Phillips, P.L.; Rorke, L.B.; Moshang, T.; Lange, B.; Packer, R.J. Long-Term Outcome of Hypothalamic/Chiasmatic Astrocytomas in Children Treated with Conservative Surgery. J. Neurosurg. 1995, 83, 583–589. [Google Scholar] [CrossRef]
- Rosser, T.L.; Vezina, G.; Packer, R.J. Cerebrovascular Abnormalities in a Population of Children with Neurofibromatosis Type 1. Neurology 2005, 64, 553–555. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Robertson, R.; Kinnamon, D.D.; Scott, R.M.; Kieran, M.W.; Turner, C.D.; Chi, S.N.; Goumnerova, L.; Proctor, M.; Tarbell, N.J.; et al. Moyamoya Following Cranial Irradiation for Primary Brain Tumors in Children. Neurology 2007, 68, 932–938. [Google Scholar] [CrossRef]
- Grabenbauer, G.G.; Schuchardt, U.; Buchfelder, M.; Rödel, C.M.; Gusek, G.; Marx, M.; Doerr, H.G.; Fahlbusch, R.; Huk, W.J.; Wenzel, D.; et al. Radiation Therapy of Optico-Hypothalamic Gliomas (OHG)--Radiographic Response, Vision and Late Toxicity. Radiother. Oncol. 2000, 54, 239–245. [Google Scholar] [CrossRef]
- Horwich, A.; Bloom, H.J. Optic Gliomas: Radiation Therapy and Prognosis. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 1067–1079. [Google Scholar] [CrossRef]
- Awdeh, R.M.; Kiehna, E.N.; Drewry, R.D.; Kerr, N.C.; Haik, B.G.; Wu, S.; Xiong, X.; Merchant, T.E. Visual Outcomes in Pediatric Optic Pathway Glioma after Conformal Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 46–51. [Google Scholar] [CrossRef]
- El-Shehaby, A.M.N.; Reda, W.A.; Abdel Karim, K.M.; Emad Eldin, R.M.; Nabeel, A.M. Single-Session Gamma Knife Radiosurgery for Optic Pathway/Hypothalamic Gliomas. J. Neurosurg. 2016, 125, 50–57. [Google Scholar] [CrossRef]
- Dong, M.-J.; Yang, Z.-K.; Yang, J.; Guo, R.-Q.; Xiao, Y.-Y.; Liu, H. Gamma Knife Radiotherapy in a Neurofibromatosis Type 1 Chinese Pedigrees with NF1 Gene Frameshift Mutation: A Case Report. Medicine 2022, 101, e29280. [Google Scholar] [CrossRef]
- Saran, F.H.; Baumert, B.G.; Khoo, V.S.; Adams, E.J.; Garré, M.L.; Warrington, A.P.; Brada, M. Stereotactically Guided Conformal Radiotherapy for Progressive Low-Grade Gliomas of Childhood. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 43–51. [Google Scholar] [CrossRef]
- Combs, S.E.; Schulz-Ertner, D.; Moschos, D.; Thilmann, C.; Huber, P.E.; Debus, J. Fractionated Stereotactic Radiotherapy of Optic Pathway Gliomas: Tolerance and Long-Term Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 814–819. [Google Scholar] [CrossRef]
- Hug, E.B.; Muenter, M.W.; Archambeau, J.O.; DeVries, A.; Liwnicz, B.; Loredo, L.N.; Grove, R.I.; Slater, J.D. Conformal Proton Radiation Therapy for Pediatric Low-Grade Astrocytomas. Strahlenther. Onkol. 2002, 178, 10–17. [Google Scholar] [CrossRef]
- Thirunavu, V.M.; Mohammad, L.M.; Kandula, V.; Beestrum, M.; Lam, S.K. Vision Outcomes for Pediatric Patients with Optic Pathway Gliomas Associated with Neurofibromatosis Type I: A Systematic Review of the Clinical Evidence. J. Pediatr. Hematol. Oncol. 2021, 43, 135–143. [Google Scholar] [CrossRef]
- Packer, R.J.; Ater, J.; Allen, J.; Phillips, P.; Geyer, R.; Nicholson, H.S.; Jakacki, R.; Kurczynski, E.; Needle, M.; Finlay, J.; et al. Carboplatin and Vincristine Chemotherapy for Children with Newly Diagnosed Progressive Low-Grade Gliomas. J. Neurosurg. 1997, 86, 747–754. [Google Scholar] [CrossRef]
- Lafay-Cousin, L.; Holm, S.; Qaddoumi, I.; Nicolin, G.; Bartels, U.; Tabori, U.; Huang, A.; Bouffet, E. Weekly Vinblastine in Pediatric Low-Grade Glioma Patients with Carboplatin Allergic Reaction. Cancer 2005, 103, 2636–2642. [Google Scholar] [CrossRef]
- Massimino, M.; Spreafico, F.; Cefalo, G.; Riccardi, R.; Tesoro-Tess, J.D.; Gandola, L.; Riva, D.; Ruggiero, A.; Valentini, L.; Mazza, E.; et al. High Response Rate to Cisplatin/Etoposide Regimen in Childhood Low-Grade Glioma. J. Clin. Oncol. 2002, 20, 4209–4216. [Google Scholar] [CrossRef]
- Ater, J.L.; Zhou, T.; Holmes, E.; Mazewski, C.M.; Booth, T.N.; Freyer, D.R.; Lazarus, K.H.; Packer, R.J.; Prados, M.; Sposto, R.; et al. Randomized Study of Two Chemotherapy Regimens for Treatment of Low-Grade Glioma in Young Children: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 2641–2647. [Google Scholar] [CrossRef]
- Felix, C.A. Secondary Leukemias Induced by Topoisomerase-Targeted Drugs. Biochim. Biophys. Acta 1998, 1400, 233–255. [Google Scholar] [CrossRef]
- Perry, J.R.; Brown, M.T.; Gockerman, J.P. Acute Leukemia Following Treatment of Malignant Glioma. J. Neuro-Oncol. 1998, 40, 39–46. [Google Scholar] [CrossRef]
- Bouffet, E.; Jakacki, R.; Goldman, S.; Hargrave, D.; Hawkins, C.; Shroff, M.; Hukin, J.; Bartels, U.; Foreman, N.; Kellie, S.; et al. Phase II Study of Weekly Vinblastine in Recurrent or Refractory Pediatric Low-Grade Glioma. J. Clin. Oncol. 2012, 30, 1358–1363. [Google Scholar] [CrossRef]
- Lassaletta, A.; Scheinemann, K.; Zelcer, S.M.; Hukin, J.; Wilson, B.A.; Jabado, N.; Carret, A.S.; Lafay-Cousin, L.; Larouche, V.; Hawkins, C.E.; et al. Phase II Weekly Vinblastine for Chemotherapy-Naïve Children with Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J. Clin. Oncol. 2016, 34, 3537–3543. [Google Scholar] [CrossRef]
- Cappellano, A.M.; Petrilli, A.S.; da Silva, N.S.; Silva, F.A.; Paiva, P.M.; Cavalheiro, S.; Bouffet, E. Single Agent Vinorelbine in Pediatric Patients with Progressive Optic Pathway Glioma. J. Neurooncol. 2015, 121, 405–412. [Google Scholar] [CrossRef]
- Wisinski, K.B.; Flamand, Y.; Wilson, M.A.; Luke, J.J.; Tawbi, H.A.; Hong, F.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.; Gray, R.J.; et al. Trametinib in Patients with NF1-, GNAQ-, or GNA11-Mutant Tumors: Results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocols S1 and S2. JCO Precis. Oncol. 2023, 7, e2200421. [Google Scholar] [CrossRef]
- Gururangan, S.; Fisher, M.J.; Allen, J.C.; Herndon, J.E.; Quinn, J.A.; Reardon, D.A.; Vredenburgh, J.J.; Desjardins, A.; Phillips, P.C.; Watral, M.A.; et al. Temozolomide in Children with Progressive Low-Grade Glioma. Neuro-Oncology 2007, 9, 161–168. [Google Scholar] [CrossRef]
- De Vita, S.; De Matteis, S.; Laurenti, L.; Chiusolo, P.; Reddiconto, G.; Fiorini, A.; Leone, G.; Sica, S. Secondary Ph+ Acute Lymphoblastic Leukemia after Temozolomide. Ann. Hematol. 2005, 84, 760–762. [Google Scholar] [CrossRef]
- Shofty, B.; Ben-Sira, L.; Kesler, A.; Jallo, G.; Groves, M.L.; Iyer, R.R.; Lassaletta, A.; Tabori, U.; Bouffet, E.; Thomale, U.-W.; et al. Isolated Optic Nerve Gliomas: A Multicenter Historical Cohort Study. J. Neurosurg. Pediatr. 2017, 20, 549–555. [Google Scholar] [CrossRef]
- Listernick, R.; Ferner, R.E.; Piersall, L.; Sharif, S.; Gutmann, D.H.; Charrow, J. Late-Onset Optic Pathway Tumors in Children with Neurofibromatosis 1. Neurology 2004, 63, 1944–1946. [Google Scholar] [CrossRef]
- Presta, L.G.; Chen, H.; O’Connor, S.J.; Chisholm, V.; Meng, Y.G.; Krummen, L.; Winkler, M.; Ferrara, N. Humanization of an Anti-Vascular Endothelial Growth Factor Monoclonal Antibody for the Therapy of Solid Tumors and Other Disorders. Cancer Res. 1997, 57, 4593–4599. [Google Scholar]
- Hwang, E.I.; Jakacki, R.I.; Fisher, M.J.; Kilburn, L.B.; Horn, M.; Vezina, G.; Rood, B.R.; Packer, R.J. Long-Term Efficacy and Toxicity of Bevacizumab-Based Therapy in Children with Recurrent Low-Grade Gliomas. Pediatr. Blood Cancer 2013, 60, 776–782. [Google Scholar] [CrossRef]
- Kalra, M.; Heath, J.A.; Kellie, S.J.; Dalla Pozza, L.; Stevens, M.M.; Swamy, S.; McCowage, G.B. Confirmation of Bevacizumab Activity, and Maintenance of Efficacy in Retreatment after Subsequent Relapse, in Pediatric Low-Grade Glioma. J. Pediatr. Hematol. Oncol. 2015, 37, e341–e346. [Google Scholar] [CrossRef]
- Packer, R.J.; Jakacki, R.; Horn, M.; Rood, B.; Vezina, G.; MacDonald, T.; Fisher, M.J.; Cohen, B. Objective Response of Multiply Recurrent Low-Grade Gliomas to Bevacizumab and Irinotecan. Pediatr. Blood Cancer 2009, 52, 791–795. [Google Scholar] [CrossRef]
- Couec, M.-L.; André, N.; Thebaud, E.; Minckes, O.; Rialland, X.; Corradini, N.; Aerts, I.; Marec Bérard, P.; Bourdeaut, F.; Leblond, P.; et al. Bevacizumab and Irinotecan in Children with Recurrent or Refractory Brain Tumors: Toxicity and Efficacy Trends. Pediatr. Blood Cancer 2012, 59, 34–38. [Google Scholar] [CrossRef]
- Zhukova, N.; Rajagopal, R.; Lam, A.; Coleman, L.; Shipman, P.; Walwyn, T.; Williams, M.; Sullivan, M.; Campbell, M.; Bhatia, K.; et al. Use of Bevacizumab as a Single Agent or in Adjunct with Traditional Chemotherapy Regimens in Children with Unresectable or Progressive Low-Grade Glioma. Cancer Med. 2019, 8, 40–50. [Google Scholar] [CrossRef]
- Green, K.; Panagopoulou, P.; D’Arco, F.; O’Hare, P.; Bowman, R.; Walters, B.; Dahl, C.; Jorgensen, M.; Patel, P.; Slater, O.; et al. A Nationwide Evaluation of Bevacizumab-Based Treatments in Pediatric Low-Grade Glioma in the UK: Safety, Efficacy, Visual Morbidity, and Outcomes. Neuro-Oncology 2023, 25, 774–785. [Google Scholar] [CrossRef]
- Yamasaki, F.; Takano, M.; Yonezawa, U.; Taguchi, A.; Kolakshyapati, M.; Okumichi, H.; Kiuchi, Y.; Kurisu, K. Bevacizumab for Optic Pathway Glioma with Worsening Visual Field in Absence of Imaging Progression: 2 Case Reports and Literature Review. Childs Nerv. Syst. 2020, 36, 635–639. [Google Scholar] [CrossRef]
- Fangusaro, J.; Onar-Thomas, A.; Young Poussaint, T.; Wu, S.; Ligon, A.H.; Lindeman, N.; Banerjee, A.; Packer, R.J.; Kilburn, L.B.; Goldman, S.; et al. Selumetinib in Paediatric Patients with BRAF-Aberrant or Neurofibromatosis Type 1-Associated Recurrent, Refractory, or Progressive Low-Grade Glioma: A Multicentre, Phase 2 Trial. Lancet Oncol. 2019, 20, 1011–1022. [Google Scholar] [CrossRef]
- Walsh, K.S.; Wolters, P.L.; Widemann, B.C.; Del Castillo, A.; Sady, M.D.; Inker, T.; Roderick, M.C.; Martin, S.; Toledo-Tamula, M.A.; Struemph, K.; et al. Impact of MEK Inhibitor Therapy on Neurocognitive Functioning in NF1. Neurol. Genet. 2021, 7, e616. [Google Scholar] [CrossRef]
- Harder, A. MEK Inhibitors-Novel Targeted Therapies of Neurofibromatosis Associated Benign and Malignant Lesions. Biomark. Res. 2021, 9, 26. [Google Scholar] [CrossRef]
- Pillay-Smiley, N.; Fletcher, J.S.; de Blank, P.; Ratner, N. Shedding New Light: Novel Therapies for Common Disorders in Children with Neurofibromatosis Type I. Pediatr. Clin. N. Am. 2023, 70, 937–950. [Google Scholar] [CrossRef]
- Del Bufalo, F.; Ceglie, G.; Cacchione, A.; Alessi, I.; Colafati, G.S.; Carai, A.; Diomedi-Camassei, F.; De Billy, E.; Agolini, E.; Mastronuzzi, A.; et al. BRAF V600E Inhibitor (Vemurafenib) for BRAF V600E Mutated Low Grade Gliomas. Front. Oncol. 2018, 8, 526. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Prabhu, S.P.; Packer, R.J.; Goldman, S.; Robison, N.J.; Allen, J.C.; Viskochil, D.H.; Gutmann, D.H.; Perentesis, J.P.; Korf, B.R.; et al. Visual Outcomes Following Everolimus Targeted Therapy for Neurofibromatosis Type 1-Associated Optic Pathway Gliomas in Children. Pediatr. Blood Cancer 2021, 68, e28833. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Prabhu, S.P.; Reddy, A.T.; Fisher, M.J.; Packer, R.; Goldman, S.; Robison, N.J.; Gutmann, D.H.; Viskochil, D.H.; Allen, J.C.; et al. A Phase II Study of Continuous Oral MTOR Inhibitor Everolimus for Recurrent, Radiographic-Progressive Neurofibromatosis Type 1-Associated Pediatric Low-Grade Glioma: A Neurofibromatosis Clinical Trials Consortium Study. Neuro-Oncology 2020, 22, 1527–1535. [Google Scholar] [CrossRef]
- Falsini, B.; Chiaretti, A.; Rizzo, D.; Piccardi, M.; Ruggiero, A.; Manni, L.; Soligo, M.; Dickmann, A.; Federici, M.; Salerni, A.; et al. Nerve Growth Factor Improves Visual Loss in Childhood Optic Gliomas: A Randomized, Double-Blind, Phase II Clinical Trial. Brain 2016, 139, 404–414. [Google Scholar] [CrossRef]
- Brannan, C.I.; Perkins, A.S.; Vogel, K.S.; Ratner, N.; Nordlund, M.L.; Reid, S.W.; Buchberg, A.M.; Jenkins, N.A.; Parada, L.F.; Copeland, N.G. Targeted Disruption of the Neurofibromatosis Type-1 Gene Leads to Developmental Abnormalities in Heart and Various Neural Crest-Derived Tissues. Genes Dev. 1994, 8, 1019–1029. [Google Scholar] [CrossRef]
- Jacks, T.; Shih, T.S.; Schmitt, E.M.; Bronson, R.T.; Bernards, A.; Weinberg, R.A. Tumour Predisposition in Mice Heterozygous for a Targeted Mutation in Nf1. Nat. Genet. 1994, 7, 353–361. [Google Scholar] [CrossRef]
- Toonen, J.A.; Anastasaki, C.; Smithson, L.J.; Gianino, S.M.; Li, K.; Kesterson, R.A.; Gutmann, D.H. NF1 Germline Mutation Differentially Dictates Optic Glioma Formation and Growth in Neurofibromatosis-1. Hum. Mol. Genet. 2016, 25, 1703–1713. [Google Scholar] [CrossRef]
- Zhu, Y.; Romero, M.I.; Ghosh, P.; Ye, Z.; Charnay, P.; Rushing, E.J.; Marth, J.D.; Parada, L.F. Ablation of NF1 Function in Neurons Induces Abnormal Development of Cerebral Cortex and Reactive Gliosis in the Brain. Genes Dev. 2001, 15, 859–876. [Google Scholar] [CrossRef]
- Bajenaru, M.L.; Hernandez, M.R.; Perry, A.; Zhu, Y.; Parada, L.F.; Garbow, J.R.; Gutmann, D.H. Optic Nerve Glioma in Mice Requires Astrocyte Nf1 Gene Inactivation and Nf1 Brain Heterozygosity. Cancer Res. 2003, 63, 8573–8577. [Google Scholar]
- Zhu, Y.; Harada, T.; Liu, L.; Lush, M.E.; Guignard, F.; Harada, C.; Burns, D.K.; Bajenaru, M.L.; Gutmann, D.H.; Parada, L.F. Inactivation of NF1 in CNS Causes Increased Glial Progenitor Proliferation and Optic Glioma Formation. Development 2005, 132, 5577–5588. [Google Scholar] [CrossRef]
- Yvone, G.M.; Breunig, J.J. Pediatric Low-Grade Glioma Models: Advances and Ongoing Challenges. Front. Oncol. 2023, 13, 1346949. [Google Scholar] [CrossRef]
- Dasgupta, B.; Li, W.; Perry, A.; Gutmann, D.H. Glioma Formation in Neurofibromatosis 1 Reflects Preferential Activation of K-RAS in Astrocytes. Cancer Res. 2005, 65, 236–245. [Google Scholar] [CrossRef]
- Hegedus, B.; Dasgupta, B.; Shin, J.E.; Emnett, R.J.; Hart-Mahon, E.K.; Elghazi, L.; Bernal-Mizrachi, E.; Gutmann, D.H. Neurofibromatosis-1 Regulates Neuronal and Glial Cell Differentiation from Neuroglial Progenitors in Vivo by Both CAMP- and Ras-Dependent Mechanisms. Cell Stem Cell 2007, 1, 443–457. [Google Scholar] [CrossRef]
- Solga, A.C.; Toonen, J.A.; Pan, Y.; Cimino, P.J.; Ma, Y.; Castillon, G.A.; Gianino, S.M.; Ellisman, M.H.; Lee, D.Y.; Gutmann, D.H. The Cell of Origin Dictates the Temporal Course of Neurofibromatosis-1 (Nf1) Low-Grade Glioma Formation. Oncotarget 2017, 8, 47206–47215. [Google Scholar] [CrossRef]
- Chatterjee, J.; Koleske, J.P.; Chao, A.; Sauerbeck, A.D.; Chen, J.-K.; Qi, X.; Ouyang, M.; Boggs, L.G.; Idate, R.; Marco Y Marquez, L.I.; et al. Brain Injury Drives Optic Glioma Formation through Neuron-Glia Signaling. Acta Neuropathol. Commun. 2024, 12, 21. [Google Scholar] [CrossRef]
- Banerjee, S.; Crouse, N.R.; Emnett, R.J.; Gianino, S.M.; Gutmann, D.H. Neurofibromatosis-1 Regulates MTOR-Mediated Astrocyte Growth and Glioma Formation in a TSC/Rheb-Independent Manner. Proc. Natl. Acad. Sci. USA 2011, 108, 15996–16001. [Google Scholar] [CrossRef]
- Daginakatte, G.C.; Gianino, S.M.; Zhao, N.W.; Parsadanian, A.S.; Gutmann, D.H. Increased C-Jun-NH2-Kinase Signaling in Neurofibromatosis-1 Heterozygous Microglia Drives Microglia Activation and Promotes Optic Glioma Proliferation. Cancer Res. 2008, 68, 10358–10366. [Google Scholar] [CrossRef]
- Pong, W.W.; Higer, S.B.; Gianino, S.M.; Emnett, R.J.; Gutmann, D.H. Reduced Microglial CX3CR1 Expression Delays Neurofibromatosis-1 Glioma Formation. Ann. Neurol. 2013, 73, 303–308. [Google Scholar] [CrossRef]
- Logiacco, F.; Grzegorzek, L.C.; Cordell, E.C.; Popp, O.; Mertins, P.; Gutmann, D.H.; Kettenmann, H.; Semtner, M. Neurofibromatosis Type 1-Dependent Alterations in Mouse Microglia Function Are Not Cell-Intrinsic. Acta Neuropathol. Commun. 2023, 11, 36. [Google Scholar] [CrossRef]
- Guo, X.; Pan, Y.; Xiong, M.; Sanapala, S.; Anastasaki, C.; Cobb, O.; Dahiya, S.; Gutmann, D.H. Midkine Activation of CD8+ T Cells Establishes a Neuron-Immune-Cancer Axis Responsible for Low-Grade Glioma Growth. Nat. Commun. 2020, 11, 2177. [Google Scholar] [CrossRef]
- Lee, D.Y.; Gianino, S.M.; Gutmann, D.H. Innate Neural Stem Cell Heterogeneity Determines the Patterning of Glioma Formation in Children. Cancer Cell 2012, 22, 131–138. [Google Scholar] [CrossRef]
- Chen, Y.-H.; McGowan, L.D.; Cimino, P.J.; Dahiya, S.; Leonard, J.R.; Lee, D.Y.; Gutmann, D.H. Mouse Low-Grade Gliomas Contain Cancer Stem Cells with Unique Molecular and Functional Properties. Cell Rep. 2015, 10, 1899–1912. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, E.; Wang, X.; Novitch, B.G.; Yoshikawa, K.; Chang, L.-S.; Zhu, Y. ERK Inhibition Rescues Defects in Fate Specification of Nf1-Deficient Neural Progenitors and Brain Abnormalities. Cell 2012, 150, 816–830. [Google Scholar] [CrossRef]
- Jecrois, E.S.; Zheng, W.; Bornhorst, M.; Li, Y.; Treisman, D.M.; Muguyo, D.; Huynh, S.; Andrew, S.F.; Wang, Y.; Jiang, J.; et al. Treatment during a Developmental Window Prevents NF1-Associated Optic Pathway Gliomas by Targeting Erk-Dependent Migrating Glial Progenitors. Dev. Cell 2021, 56, 2871–2885.e6. [Google Scholar] [CrossRef]
- Tang, Y.; Chatterjee, J.; Wagoner, N.; Bozeman, S.; Gutmann, D.H. Estrogen-Induced Glial IL-1β Mediates Extrinsic Retinal Ganglion Cell Vulnerability in Murine Nf1 Optic Glioma. Ann. Clin. Transl. Neurol. 2024, 11, 812–818. [Google Scholar] [CrossRef]
- Osum, S.H.; Watson, A.L.; Largaespada, D.A. Spontaneous and Engineered Large Animal Models of Neurofibromatosis Type 1. Int. J. Mol. Sci. 2021, 22, 1954. [Google Scholar] [CrossRef]
- Métin, C.; Irons, W.A.; Frost, D.O. Retinal Ganglion Cells in Normal Hamsters and Hamsters with Novel Retinal Projections. I. Number, Distribution, and Size. J. Comp. Neurol. 1995, 353, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Salinas-Navarro, M.; Sobrado-Calvo, P.; Alburquerque-Béjar, J.J.; Vidal-Sanz, M.; Agudo-Barriuso, M. Whole Number, Distribution and Co-Expression of Brn3 Transcription Factors in Retinal Ganglion Cells of Adult Albino and Pigmented Rats. PLoS ONE 2012, 7, e49830. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Salinas-Navarro, M.; Jiménez-López, M.; Sobrado-Calvo, P.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Displaced Retinal Ganglion Cells in Albino and Pigmented Rats. Front. Neuroanat. 2014, 8, 99. [Google Scholar] [CrossRef]
- Isakson, S.H.; Rizzardi, A.E.; Coutts, A.W.; Carlson, D.F.; Kirstein, M.N.; Fisher, J.; Vitte, J.; Williams, K.B.; Pluhar, G.E.; Dahiya, S.; et al. Genetically Engineered Minipigs Model the Major Clinical Features of Human Neurofibromatosis Type 1. Commun. Biol. 2018, 1, 158. [Google Scholar] [CrossRef]
- White, K.A.; Swier, V.J.; Cain, J.T.; Kohlmeyer, J.L.; Meyerholz, D.K.; Tanas, M.R.; Uthoff, J.; Hammond, E.; Li, H.; Rohret, F.A.; et al. A Porcine Model of Neurofibromatosis Type 1 That Mimics the Human Disease. JCI Insight 2018, 3, e120402. [Google Scholar] [CrossRef]
- Monlux, A.W.; Davis, C.L. Multiple Schwannomas of Cattle (Nerve Sheath Tumors; Multiple Neurilemmomas; Neurofibromatosis). Am. J. Vet. Res. 1953, 14, 499–509. [Google Scholar]
- Schöniger, S.; Summers, B.A. Localized, Plexiform, Diffuse, and Other Variants of Neurofibroma in 12 Dogs, 2 Horses, and a Chicken. Vet. Pathol. 2009, 46, 904–915. [Google Scholar] [CrossRef]
- Miettinen, M.M.; Antonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; et al. Histopathologic Evaluation of Atypical Neurofibromatous Tumors and Their Transformation into Malignant Peripheral Nerve Sheath Tumor in Patients with Neurofibromatosis 1-a Consensus Overview. Hum. Pathol. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Shin, J.; Padmanabhan, A.; de Groh, E.D.; Lee, J.-S.; Haidar, S.; Dahlberg, S.; Guo, F.; He, S.; Wolman, M.A.; Granato, M.; et al. Zebrafish Neurofibromatosis Type 1 Genes Have Redundant Functions in Tumorigenesis and Embryonic Development. Dis. Model Mech. 2012, 5, 881–894. [Google Scholar] [CrossRef]
- He, S.; Mansour, M.R.; Zimmerman, M.W.; Ki, D.H.; Layden, H.M.; Akahane, K.; Gjini, E.; de Groh, E.D.; Perez-Atayde, A.R.; Zhu, S.; et al. Synergy between Loss of NF1 and Overexpression of MYCN in Neuroblastoma Is Mediated by the GAP-Related Domain. eLife 2016, 5, e14713. [Google Scholar] [CrossRef]
- King, L.B.; Boto, T.; Botero, V.; Aviles, A.M.; Jomsky, B.M.; Joseph, C.; Walker, J.A.; Tomchik, S.M. Developmental Loss of Neurofibromin across Distributed Neuronal Circuits Drives Excessive Grooming in Drosophila. PLoS Genet. 2020, 16, e1008920. [Google Scholar] [CrossRef] [PubMed]
- The, I.; Hannigan, G.E.; Cowley, G.S.; Reginald, S.; Zhong, Y.; Gusella, J.F.; Hariharan, I.K.; Bernards, A. Rescue of a Drosophila NF1 Mutant Phenotype by Protein Kinase A. Science 1997, 276, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; White, D.; Resar, L.; Bar, E.; Groves, M.; Cohen, A.; Jackson, E.; Bynum, J.; Rubens, J.; Mumm, J.; et al. Conditional Reprogramming Culture Conditions Facilitate Growth of Lower-Grade Glioma Models. Neuro-Oncology 2021, 23, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Boia, R.; Ruzafa, N.; Aires, I.D.; Pereiro, X.; Ambrósio, A.F.; Vecino, E.; Santiago, A.R. Neuroprotective Strategies for Retinal Ganglion Cell Degeneration: Current Status and Challenges Ahead. Int. J. Mol. Sci. 2020, 21, 2262. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Smith, A.J.; Ali, R.R. The Future Looks Brighter After 25 Years of Retinal Gene Therapy. Hum. Gene Ther. 2017, 28, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Chang, K.-C.; Wu, S.; Sun, C.; Xia, X.; Nahmou, M.; Bian, M.; Wen, R.R.; Zhu, Y.; Shah, S.; et al. Directly Induced Human Retinal Ganglion Cells Mimic Fetal RGCs and Are Neuroprotective after Transplantation in Vivo. Stem Cell Rep. 2022, 17, 2690–2703. [Google Scholar] [CrossRef]
- Millán-Rivero, J.E.; Nadal-Nicolás, F.M.; García-Bernal, D.; Sobrado-Calvo, P.; Blanquer, M.; Moraleda, J.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Human Wharton’s Jelly Mesenchymal Stem Cells Protect Axotomized Rat Retinal Ganglion Cells via Secretion of Anti-Inflammatory and Neurotrophic Factors. Sci. Rep. 2018, 8, 16299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyagishima, K.J.; Qiao, F.; Stasheff, S.F.; Nadal-Nicolás, F.M. Visual Deficits and Diagnostic and Therapeutic Strategies for Neurofibromatosis Type 1: Bridging Science and Patient-Centered Care. Vision 2024, 8, 31. https://doi.org/10.3390/vision8020031
Miyagishima KJ, Qiao F, Stasheff SF, Nadal-Nicolás FM. Visual Deficits and Diagnostic and Therapeutic Strategies for Neurofibromatosis Type 1: Bridging Science and Patient-Centered Care. Vision. 2024; 8(2):31. https://doi.org/10.3390/vision8020031
Chicago/Turabian StyleMiyagishima, Kiyoharu J., Fengyu Qiao, Steven F. Stasheff, and Francisco M. Nadal-Nicolás. 2024. "Visual Deficits and Diagnostic and Therapeutic Strategies for Neurofibromatosis Type 1: Bridging Science and Patient-Centered Care" Vision 8, no. 2: 31. https://doi.org/10.3390/vision8020031
APA StyleMiyagishima, K. J., Qiao, F., Stasheff, S. F., & Nadal-Nicolás, F. M. (2024). Visual Deficits and Diagnostic and Therapeutic Strategies for Neurofibromatosis Type 1: Bridging Science and Patient-Centered Care. Vision, 8(2), 31. https://doi.org/10.3390/vision8020031




