Uncovering the Role of the Early Visual Cortex in Visual Mental Imagery
Abstract
1. Introduction
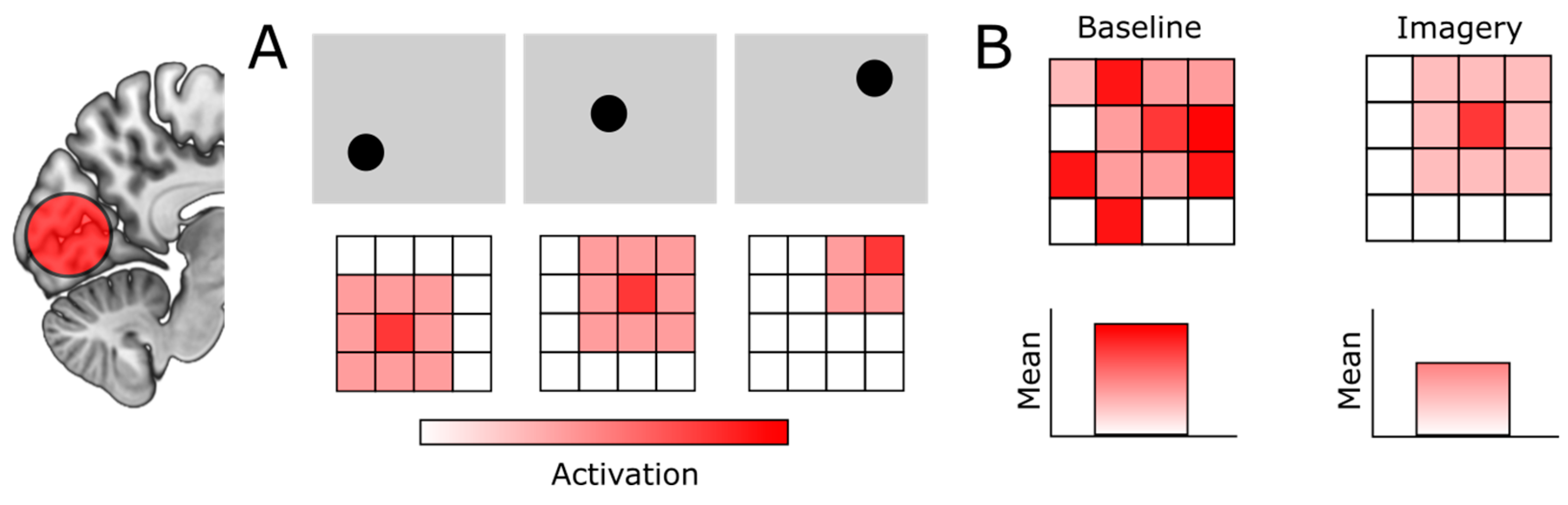
2. Organizational Principles of the Early Visual Cortex
3. The Challenges of Studying the Early Visual Cortex during Mental Imagery
4. The Role of the Early Visual Cortex in Visual Mental Imagery
5. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Nanay, B. Mental Imagery. In The Stanford Encyclopedia of Philosophy; Winter 2021; Zalta, E.N., Ed.; Metaphysics Research Lab, Stanford University: Stanford, CA, USA, 2021; Available online: https://plato.stanford.edu/archives/win2021/entries/mental-imagery/ (accessed on 27 July 2023).
- Sterelny, K. The Imagery Debate. Philos. Sci. 1986, 53, 560–583. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Hajhajate, D.; Liu, J.; Spagna, A. Assessing the causal role of early visual areas in visual mental imagery. Nat. Rev. Neurosci. 2020, 21, 517. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, N.; Bosch, S.E.; van Gerven, M.A.J. Shared Neural Mechanisms of Visual Perception and Imagery. Trends Cogn. Sci. 2019, 23, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J. The human imagination: The cognitive neuroscience of visual mental imagery. Nat. Rev. Neurosci. 2019, 20, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Spagna, A.; Hajhajate, D.; Liu, J.; Bartolomeo, P. Visual mental imagery engages the left fusiform gyrus, but not the early visual cortex: A meta-analysis of neuroimaging evidence. Neurosci. Biobehav. Rev. 2021, 122, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Receptive Fields and Functional Architecture of monkey striate cortex. J. Physiol. 1968, 195, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.J.; Fabre-Thorpe, M. Seeking categories in the brain. Science 2001, 291, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.T.; Petro, L.S.; Muckli, L. Scene representations conveyed by cortical feedback to early visual cortex can be described by line drawings. J. Neurosci. 2019, 39, 9410–9423. [Google Scholar] [CrossRef]
- Muckli, L.; De Martino, F.; Vizioli, L.; Petro, L.S.; Smith, F.W.; Ugurbil, K.; Goebel, R.; Yacoub, E. Contextual Feedback to Superficial Layers of V1. Curr. Biol. 2015, 25, 2690–2695. [Google Scholar] [CrossRef]
- Kravitz, D.J.; Kriegeskorte, N.; Baker, C.I. High-Level Visual Object Representations Are Constrained by Position. Cereb. Cortex 2010, 20, 2916–2925. [Google Scholar] [CrossRef]
- Park, S.; Serences, J.T. Relative precision of top-down attentional modulations is lower in early visual cortex compared to mid- and high-level visual areas. J. Neurophysiol. 2022, 127, 504–518. [Google Scholar] [CrossRef]
- Bigelow, E.J.; McCoy, J.P.; Ullman, T.D. Non-commitment in mental imagery. Cognition 2023, 238, 105498. [Google Scholar] [CrossRef]
- Schwarzkopf, D.S. What is the true range of mental imagery? Cortex 2024, 170, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Dubois, J.; Mangin, J.-F.; Kherif, F.; Flandin, G.; Poline, J.-B.; Denis, M.; Kosslyn, S.M.; Le Bihan, D. Retinotopic organization of visual mental images as revealed by functional magnetic resonance imaging. Brain Res. Cogn. Brain Res. 2004, 22, 26–31. [Google Scholar] [CrossRef]
- Thirion, B.; Duchesnay, E.; Hubbard, E.; Dubois, J.; Poline, J.-B.; Lebihan, D.; Dehaene, S. Inverse retinotopy: Inferring the visual content of images from brain activation patterns. NeuroImage 2006, 33, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Senden, M.; Emmerling, T.C.; van Hoof, R.; Frost, M.A.; Goebel, R. Reconstructing imagined letters from early visual cortex reveals tight topographic correspondence between visual mental imagery and perception. Brain Struct. Funct. 2019, 224, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Dentico, D.; Cheung, B.L.; Chang, J.-Y.; Guokas, J.; Boly, M.; Tononi, G.; Van Veen, B. Reversal of cortical information flow during visual imagery as compared to visual perception. NeuroImage 2014, 100, 237–243. [Google Scholar] [CrossRef]
- Dijkstra, N.; Zeidman, P.; Ondobaka, S.; van Gerven, M.A.J.; Friston, K. Distinct Top-down and Bottom-up Brain Connectivity During Visual Perception and Imagery. Sci. Rep. 2017, 7, 5677. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, A.; Price, C.J.; Friston, K.J.; Ishai, A. Where bottom-up meets top-down: Neuronal interactions during perception and imagery. Cereb. Cortex 2004, 14, 1256–1265. [Google Scholar] [CrossRef]
- Aru, J.; Siclari, F.; Phillips, W.A.; Storm, J.F. Apical drive—A cellular mechanism of dreaming? Neurosci. Biobehav. Rev. 2020, 119, 440–455. [Google Scholar] [CrossRef]
- Larkum, M. A cellular mechanism for cortical associations: An organizing principle for the cerebral cortex. Trends Neurosci. 2013, 36, 141–151. [Google Scholar] [CrossRef]
- Pace, T.; Koenig-Robert, R.; Pearson, J. Different Mechanisms for Supporting Mental Imagery and Perceptual Representations: Modulation Versus Excitation. Psychol. Sci. 2023, 34, 1229–1243. [Google Scholar] [CrossRef]
- Bartsch, M.V.; Loewe, K.; Merkel, C.; Heinze, H.-J.; Schoenfeld, M.A.; Tsotsos, J.K.; Hopf, J.-M. Attention to Color Sharpens Neural Population Tuning via Feedback Processing in the Human Visual Cortex Hierarchy. J. Neurosci. 2017, 37, 10346–10357. [Google Scholar] [CrossRef]
- Kok, P.; Jehee, J.F.M.; de Lange, F.P. Less Is More: Expectation Sharpens Representations in the Primary Visual Cortex. Neuron 2012, 75, 265–270. [Google Scholar] [CrossRef]
- Koenig-Robert, R.; Pearson, J. Why do imagery and perception look and feel so different? Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190703. [Google Scholar] [CrossRef]
- Keogh, R.; Bergmann, J.; Pearson, J. Cortical excitability controls the strength of mental imagery. eLife 2020, 9, e50232. [Google Scholar] [CrossRef]
- Winlove, C.; Milton, F.; Ranson, J.; Fulford, J.; MacKisack, M.; Macpherson, F.; Zeman, A. The neural correlates of visual imagery: A co-ordinate-based meta-analysis. Cortex 2018, 105, 4–25. [Google Scholar] [CrossRef]
- Robinson, A.K.; Quek, G.L.; Carlson, T.A. Visual Representations: Insights from Neural Decoding. Annu. Rev. Vis. Sci. 2023, 9, 313–335. [Google Scholar] [CrossRef]
- Albers, A.M.; Kok, P.; Toni, I.; Dijkerman, H.C.; De Lange, F.P. Shared representations for working memory and mental imagery in early visual cortex. Curr. Biol. CB 2013, 23, 1427–1431. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kravitz, D.J.; Baker, C.I. Disentangling visual imagery and perception of real-world objects. NeuroImage 2012, 59, 4064–4073. [Google Scholar] [CrossRef]
- Ragni, F.; Tucciarelli, R.; Andersson, P.; Lingnau, A. Decoding stimulus identity in occipital, parietal and inferotemporal cortices during visual mental imagery. Cortex 2020, 127, 371–387. [Google Scholar] [CrossRef]
- St-Yves, G.; Naselaris, T. The feature-weighted receptive field: An interpretable encoding model for complex feature spaces. NeuroImage 2018, 180, 188–202. [Google Scholar] [CrossRef]
- Guclu, U.; van Gerven, M.A.J. Deep Neural Networks Reveal a Gradient in the Complexity of Neural Representations across the Ventral Stream. J. Neurosci. 2015, 35, 10005–10014. [Google Scholar] [CrossRef]
- Naselaris, T.; Olman, C.A.; Stansbury, D.E.; Ugurbil, K.; Gallant, J.L. A voxel-wise encoding model for early visual areas decodes mental images of remembered scenes. NeuroImage 2015, 105, 215–228. [Google Scholar] [CrossRef]
- Koide-Majima, N.; Nishimoto, S.; Majima, K. Mental image reconstruction from human brain activity: Neural decoding of mental imagery via deep neural network-based Bayesian estimation. Neural Netw. 2024, 170, 349–363. [Google Scholar] [CrossRef]
- Shen, G.; Horikawa, T.; Majima, K.; Kamitani, Y. Deep image reconstruction from human brain activity. PLoS Comput. Biol. 2019, 15, e1006633. [Google Scholar] [CrossRef]
- Kosslyn, S.M.; Thompson, W.L. When is early visual cortex activated during visual mental imagery? Psychol. Bull. 2003, 129, 723–746. [Google Scholar] [CrossRef]
- Dijkstra, N.; Bosch, S.E.; van Gerven, M.A.J. Vividness of Visual Imagery Depends on the Neural Overlap with Perception in Visual Areas. J. Neurosci. 2017, 37, 1367–1373. [Google Scholar] [CrossRef]
- Dijkstra, N.; Fleming, S.M. Subjective signal strength distinguishes reality from imagination. Nat. Commun. 2023, 14, 1627. [Google Scholar] [CrossRef]
- Bartolomeo, P. The relationship between visual perception and visual mental imagery: A reappraisal of the neuropsychological evidence. Cortex 2002, 38, 357–378. [Google Scholar] [CrossRef]
- Pearson, J. Reply to: Assessing the causal role of early visual areas in visual mental imagery. Nat. Rev. Neurosci. 2020, 21, 517–518. [Google Scholar] [CrossRef]
- Cabbai, G.; Racey, C.; Simner, J.; Dance, C.; Ward, J.; Forster, S. Sensory representations in primary visual cortex are not sufficient for subjective imagery. bioRxiv 2024. in preprint. [Google Scholar] [CrossRef]
- Meng, M.; Chang, S.; Zhang, X.; Pearson, J. Imageless imagery in aphantasia: Decoding non-sensory imagery in aphantasia. 2023; in preprint. [Google Scholar] [CrossRef]
- Weber, S.; Christophe, T.B.; Gorgen, K.; Soch, J.; Haynes, J.D. Working memory and imagery in early visual cortex. Hum. Brain Mapp. 2024, 45, e26590. [Google Scholar] [CrossRef]
- Brown, R.; Lau, H.; LeDoux, J.E. Understanding the Higher-Order Approach to Consciousness. Trends Cogn. Sci. 2019, 23, 754–768. [Google Scholar] [CrossRef]
- Fleming, S.M. Awareness as inference in a higher-order state space. Neurosci. Conscious. 2020, 2020, niz020. [Google Scholar] [CrossRef]
- Lau, H.; Rosenthal, D. Empirical support for higher-order theories of conscious awareness. Trends Cogn. Sci. 2011, 15, 365–373. [Google Scholar] [CrossRef]
- Liu, J.; Bayle, D.J.; Spagna, A.; Sitt, J.D.; Bourgeois, A.; Lehongre, K.; Fernandez-Vidal, S.; Adam, C.; Lambrecq, V.; Navarro, V.; et al. Fronto-parietal networks shape human conscious report through attention gain and reorienting. Commun. Biol. 2023, 6, 730. [Google Scholar] [CrossRef]
- Iamshchinina, P.; Kaiser, D.; Yakupov, R.; Haenelt, D.; Sciarra, A.; Mattern, H.; Luesebrink, F.; Duezel, E.; Speck, O.; Weiskopf, N.; et al. Perceived and mentally rotated contents are differentially represented in cortical depth of V1. Commun. Biol. 2024, 4, 1069. [Google Scholar] [CrossRef]
- Harrison, S.A.; Tong, F. Decoding reveals the contents of visual working memory in early visual areas. Nature 2009, 458, 632–635. [Google Scholar] [CrossRef]
- Keogh, R.; Pearson, J. Attention driven phantom vision: Measuring the sensory strength of attentional templates and their relation to visual mental imagery and aphantasia. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190688. [Google Scholar] [CrossRef]
- Pounder, Z.; Jacob, J.; Jacobs, C.; Loveday, C.; Towell, T.; Silvanto, J. Mental rotation performance in aphantasia. J. Vis. 2018, 18, 1123. [Google Scholar] [CrossRef]
- Nanay, B. Unconscious mental imagery. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2021, 376, 20190689. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.N.; Barnes, G.R.; Clark, I.A.; Maguire, E.A. The neural dynamics of novel scene imagery. J. Neurosci. 2019, 39, 4375–4386. [Google Scholar] [CrossRef]
- Lawrence, S.J.D.; van Mourik, T.; Kok, P.; Koopmans, P.J.; Norris, D.G.; de Lange, F.P. Laminar Organization of Working Memory Signals in Human Visual Cortex. Curr. Biol. CB 2018, 28, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Petro, L.S.; Abbatecola, C.; Li, M.S.; Morgan, A.T.; Muckli, L. Cortical depth profiles in primary visual cortex for illusory and imaginary experiences. Nat. Commun. 2024, 15, 1002. [Google Scholar] [CrossRef]
- Bastos, A.M.; Usrey, W.M.; Adams, R.A.; Mangun, G.R.; Fries, P.; Friston, K.J. Canonical Microcircuits for Predictive Coding. Neuron 2012, 76, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.M.; Vezoli, J.; Bosman, C.A.; Schoffelen, J.-M.; Oostenveld, R.; Dowdall, J.R.; De Weerd, P.; Kennedy, H.; Fries, P. Visual Areas Exert Feedforward and Feedback Influences through Distinct Frequency Channels. Neuron 2014, 85, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.J.; Roth, M.M.; Scanziani, M. Feedback generates a second receptive field in neurons of the visual cortex. Nature 2020, 582, 545–549. [Google Scholar] [CrossRef]
- Semedo, J.D.; Jasper, A.I.; Zandvakili, A.; Krishna, A.; Aschner, A.; Machens, C.K.; Kohn, A.; Yu, B.M. Feedforward and feedback interactions between visual cortical areas use different population activity patterns. Nat. Commun. 2022, 13, 1099. [Google Scholar] [CrossRef]
- Wilming, N.; Murphy, P.R.; Meyniel, F.; Donner, T.H. Large-scale dynamics of perceptual decision information across human cortex. Nat. Commun. 2020, 11, 5109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dijkstra, N. Uncovering the Role of the Early Visual Cortex in Visual Mental Imagery. Vision 2024, 8, 29. https://doi.org/10.3390/vision8020029
Dijkstra N. Uncovering the Role of the Early Visual Cortex in Visual Mental Imagery. Vision. 2024; 8(2):29. https://doi.org/10.3390/vision8020029
Chicago/Turabian StyleDijkstra, Nadine. 2024. "Uncovering the Role of the Early Visual Cortex in Visual Mental Imagery" Vision 8, no. 2: 29. https://doi.org/10.3390/vision8020029
APA StyleDijkstra, N. (2024). Uncovering the Role of the Early Visual Cortex in Visual Mental Imagery. Vision, 8(2), 29. https://doi.org/10.3390/vision8020029



