Short-Term Morpho-Functional Changes before and after Strabismus Surgery in Children Using Structural Optical Coherence Tomography: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
2.2. Imaging Protocol
2.3. Outcomes Measured
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients Included in the Analysis
3.2. Outcome Analysis in Strabismus Group
3.3. Comparison between Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, P.; Gaur, N.; Phuljhele, S.; Saxena, R. What’s New for Us in Strabismus? Indian J. Ophthalmol. 2017, 65, 184–190. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, A.; Zhang, X.; Huang, D.; Zhu, H.; Sun, Q.; Yu, J.; Chen, J.; Zhao, X.; Li, R.; et al. Prevalence of Strabismus among Preschool Children in Eastern China and Comparison at a 5-Year Interval: A Population-Based Cross-Sectional Study. BMJ Open 2021, 11, e055112. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; Repka, M.X.; Katz, J.; Giordano, L.; Ibironke, J.; Hawse, P.; Tielsch, J.M. Prevalence of Amblyopia and Strabismus in White and African American Children Aged 6 through 71 Months. The Baltimore Pediatric Eye Disease Study. Ophthalmology 2009, 116, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Lau, Y.H.; Wang, Y.M.; Kam, K.W.; Ip, P.; Yip, W.W.; Ko, S.T.; Young, A.L.; Tham, C.C.; Pang, C.P.; et al. Prevalence of Strabismus and Its Risk Factors among School Aged Children: The Hong Kong Children Eye Study. Sci. Rep. 2021, 11, 13820. [Google Scholar] [CrossRef]
- McKean-Cowdin, R.; Cotter, S.A.; Tarczy-Hornoch, K.; Wen, G.; Kim, J.; Borchert, M.; Varma, R. Prevalence of Amblyopia or Strabismus in Asian and Non-Hispanic White Preschool Children: Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2013, 120, 2117–2124. [Google Scholar] [CrossRef]
- Kushner, B.J. The Benefits, Risks, and Efficacy of Strabismus Surgery in Adults. Optom. Vis. Sci. 2014, 91, e102–e109. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.J. The Functional Benefits of Strabismus Surgery. J. Binocul. Vis. Ocul. Motil. 2018, 68, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.E.; Kutschke, P.J. Won Ryul Lee 20th Annual Frank Costenbader Lecture--Adult Strabismus. J. Pediatr. Ophthalmol. Strabismus 1995, 32, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Mintz, H.R.; Waisbourd, M.; Kessner, R.; Stolovitch, C.; Dotan, G.; Neudorfer, M. Macular Thickness Following Strabismus Surgery as Determined by Optical Coherence Tomography. J. Pediatr. Ophthalmol. Strabismus 2016, 53, 11–15. [Google Scholar] [CrossRef]
- Nelson, L.B. Macular Changes Following Strabismus Surgery Confirmed by the Use of Optical Coherence Tomography. J. Pediatr. Ophthalmol. Strabismus 2016, 53, 10. [Google Scholar] [CrossRef]
- Turan-Vural, E.; Unlu, C.; Erdogan, G.; Aykut, A.; Bayramlar, H.; Atmaca, F. Evaluation of Macular Thickness Change after Inferior Oblique Muscle Recession Surgery. Indian J. Ophthalmol. 2014, 62, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Kasem, M.A.; Sabry, D. Detection of Macular Changes by Optical Coherence Tomography after Inferior Oblique Muscle Surgery. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2011, 15, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Andalib, D.; Javadzadeh, A.; Nabai, R.; Amizadeh, Y. Macular and Retinal Nerve Fiber Layer Thickness in Unilateral Anisometropic or Strabismic Amblyopia. J. Pediatr. Ophthalmol. Strabismus 2013, 50, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Ersan, I.; Zengin, N.; Bozkurt, B.; Özkagnici, A. Evaluation of Retinal Nerve Fiber Layer Thickness in Patients with Anisometropic and Strabismic Amblyopia Using Optical Coherence Tomography. J. Pediatr. Ophthalmol. Strabismus 2013, 50, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Tugcu, B.; Araz-Ersan, B.; Kilic, M.; Erdogan, E.T.; Yigit, U.; Karamursel, S. The Morpho-Functional Evaluation of Retina in Amblyopia. Curr. Eye Res. 2013, 38, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Bazzaz, S.; Lai, M. Optical Tomography-Measured Retinal Nerve Fiber Layer Thickness in Normal Latinos. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3369–3373. [Google Scholar] [CrossRef]
- Gonzalez Caldito, N.; Antony, B.; He, Y.; Lang, A.; Nguyen, J.; Rothman, A.; Ogbuokiri, E.; Avornu, A.; Balcer, L.; Frohman, E.; et al. Analysis of Agreement of Retinal-Layer Thickness Measures Derived from the Segmentation of Horizontal and Vertical Spectralis OCT Macular Scans. Curr. Eye Res. 2018, 43, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Dyrda, A.A.; Biarnés, M.; Gómez, A.; Martín, C.; Mora, C.; Fatti, G.; Antón, A. Diagnostic Accuracy of Spectralis SD OCT Automated Macular Layers Segmentation to Discriminate Normal from Early Glaucomatous Eyes. Ophthalmology 2017, 124, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.L.; Riesen, A.H.; Newell, F.W. Degeneration of Retinal Ganglion Cells in Infant Chimpanzees Reared in Darkness. J. Comp. Neurol. 1957, 107, 27–42. [Google Scholar] [CrossRef]
- Von Noorden, G.K.; Crawford, M.L.J.; Middleditch, P.R. Effect of Lid Suture on Retinal Ganglion Cells in Macaca Mulatta. Brain Res. 1977, 122, 437–444. [Google Scholar] [CrossRef]
- Masri, O.S.; Abiad, B.; Darwich, M.J.; Sarkis, P.A.; El Mollayess, G.M.; Nasser, Z.; Fares, Y.; Al Ahmar, E.; Estephan, E. Morphological Changes in Amblyopic Eyes in Choriocapillaris and Sattler’s Layer in Comparison to Healthy Eyes, and in Retinal Nerve Fiber Layer in Comparison to Fellow Eyes through Quantification of Mean Reflectivity: A Pilot Study. PLoS ONE 2021, 16, e0255735. [Google Scholar] [CrossRef] [PubMed]
- Rasch, E.; Swift, H.; Riesen, A.H.; Chow, K.L. Altered Structure and Composition of Retinal Cells in Darkreared Mammals. Exp. Cell Res. 1961, 25, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Fifková, E. Effect of Visual Deprivation and Light on Synapses of the Inner Plexiform Layer. Exp. Neurol. 1972, 35, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Weiskrantz, L. Sensory Deprivation and the Cat’s Optic Nervous System. Nature 1958, 181, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Cinar, F.G.; Ozkan, G. Macular Capillary System and Ganglion Cell-Layer Complex of the Amblyopic Eye with Optical Cohorence Tomography Angiography and Optical Cohorence Tomography. Int. Ophthalmol. 2021, 41, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Hooks, B.M.; Chen, C. Critical Periods in the Visual System: Changing Views for a Model of Experience-Dependent Plasticity. Neuron 2007, 56, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Purohit, R.; Patel, A.; Papageorgiou, E.; Sheth, V.; Maconachie, G.; Pilat, A.; McLean, R.J.; Proudlock, F.A.; Ottlob, I. In Vivo Foveal Development Using Optical Coherence Tomography. Invest. Ophthalmol. Vis. Sci. 2015, 56, 4537–4545. [Google Scholar] [CrossRef] [PubMed]
- Park, K.A.; Park, D.Y.; Oh, S.Y. Analysis of Spectral-Domain Optical Coherence Tomography Measurements in Amblyopia: A Pilot Study. Br. J. Ophthalmol. 2011, 95, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Chen, H.; Zheng, S. Thicknesses of Macular Inner Retinal Layers in Children with Anisometropic Amblyopia. Biomed Res. Int. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Miyake, K.; Ibaraki, N. Prostaglandins and Cystoid Macular Edema. Surv. Ophthalmol. 2002, 47, S203–S218. [Google Scholar] [CrossRef]
- Guler alis, M.; Alış, A. Influence of One or Two Horizontal Muscle Surgeries on OCT Findings. Strabismus 2021, 29, 182–188. [Google Scholar] [CrossRef] [PubMed]

| Strabismus Group | Control Group | p Value | |
|---|---|---|---|
| Number of patients, n | 21 | 14 | 0.217 |
| Number of eyes, n | 28 | 14 | 0.175 |
| Age (years) | 7.23 ± 1.3 | 7.14 ± 1.2 | 0.989 |
| Gender (M⁄F) | 18/3 | 9/5 | 0.345 |
| Initial BCVA (logMAR) | 0.1 ± 0.3 | 0.0 ± 0.0 | 0.679 |
| Before Surgical Procedure | 1 Month after Surgical Procedure | p Value | |
|---|---|---|---|
| BCVA (logMAR) | 0.1 ± 0.3 | 0.0 ± 0.2 | 0.089 |
| RNFL (μm) | 15.2 ± 1.8 | 16.1 ± 2.8 | 0.121 |
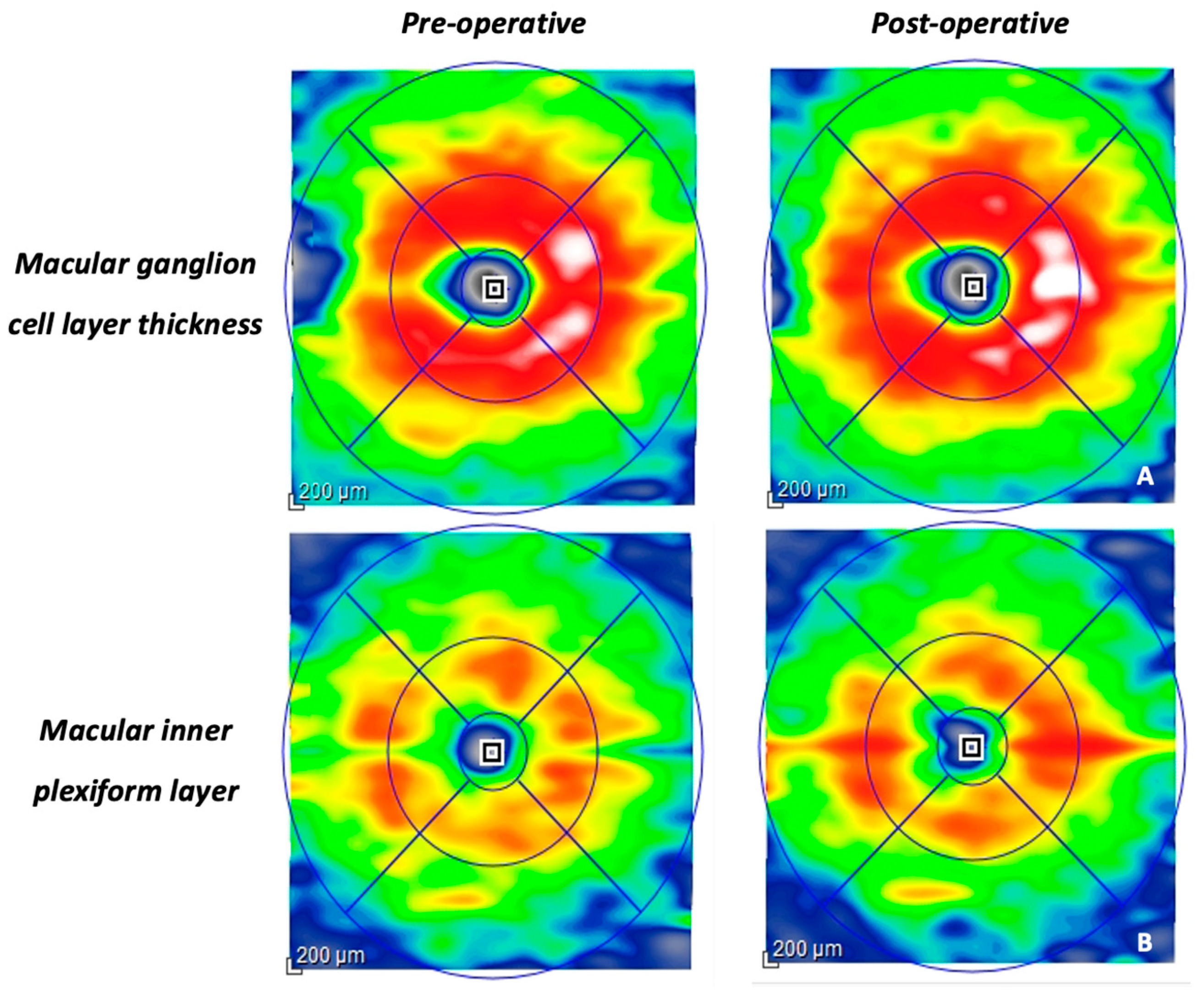
| mGCL-IPL (μm) | 60.8 ± 9.2 | 66.1 ± 13.2 | 0.026 |
| IRL (μm) | 215.1 ± 18.9 | 219.2 ± 25.3 | 0.221 |
| ORL (μm) | 84.3 ± 3.6 | 84.9 ± 2.7 | 0.669 |
| Strabismus Group at Baseline | Control Group | p Value | |
|---|---|---|---|
| BCVA (logMAR) | 0.1 ± 0.3 | 0.0 ± 0.0 | 0.679 |
| RNFL (μm) | 15.2 ± 1.8 | 15.4 ± 1.1 | 0.264 |
| mGCL-IPL (μm) | 60.8 ± 9.2 | 68.3 ± 7.2 | 0.014 |
| IRL (μm) | 215.1 ± 18.9 | 221.1 ± 11.6 | 0.095 |
| ORL (μm) | 84.3 ± 3.6 | 83.8 ± 1.5 | 0.253 |
| Strabismus Group Post-Surgery | Control Group | p Value | |
|---|---|---|---|
| BCVA (logMAR) | 0.0 ± 0.2 | 0.0 ± 0.0 | 0.893 |
| RNFL (μm) | 16.1 ± 2.8 | 15.4 ± 1.1 | 0.331 |
| mGCL-IPL (μm) | 66.1 ± 13.2 | 68.3 ± 7.2 | 0.127 |
| IRL (μm) | 219.2 ± 25.3 | 221.1 ± 11.6 | 0.164 |
| ORL (μm) | 84.9 ± 2.7 | 83.8 ± 1.5 | 0.431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viggiano, P.; Gaudiomonte, M.; Procoli, U.; Micelli Ferrari, L.; Borrelli, E.; Boscia, G.; Ferrara, A.; De Vitis, F.; Scalise, G.; Albano, V.; et al. Short-Term Morpho-Functional Changes before and after Strabismus Surgery in Children Using Structural Optical Coherence Tomography: A Pilot Study. Vision 2024, 8, 21. https://doi.org/10.3390/vision8020021
Viggiano P, Gaudiomonte M, Procoli U, Micelli Ferrari L, Borrelli E, Boscia G, Ferrara A, De Vitis F, Scalise G, Albano V, et al. Short-Term Morpho-Functional Changes before and after Strabismus Surgery in Children Using Structural Optical Coherence Tomography: A Pilot Study. Vision. 2024; 8(2):21. https://doi.org/10.3390/vision8020021
Chicago/Turabian StyleViggiano, Pasquale, Marida Gaudiomonte, Ugo Procoli, Luisa Micelli Ferrari, Enrico Borrelli, Giacomo Boscia, Andrea Ferrara, Fabio De Vitis, Gemma Scalise, Valeria Albano, and et al. 2024. "Short-Term Morpho-Functional Changes before and after Strabismus Surgery in Children Using Structural Optical Coherence Tomography: A Pilot Study" Vision 8, no. 2: 21. https://doi.org/10.3390/vision8020021
APA StyleViggiano, P., Gaudiomonte, M., Procoli, U., Micelli Ferrari, L., Borrelli, E., Boscia, G., Ferrara, A., De Vitis, F., Scalise, G., Albano, V., Alessio, G., & Boscia, F. (2024). Short-Term Morpho-Functional Changes before and after Strabismus Surgery in Children Using Structural Optical Coherence Tomography: A Pilot Study. Vision, 8(2), 21. https://doi.org/10.3390/vision8020021








