An Update on Multimodal Ophthalmological Imaging of Diffuse Choroidal Hemangioma in Sturge–Weber Syndrome
Abstract
:1. Introduction
2. Discussion
2.1. Histopathological Findings and Clinical Characteristics of Choroidal Hemangiomas in Sturge-Weber Disease
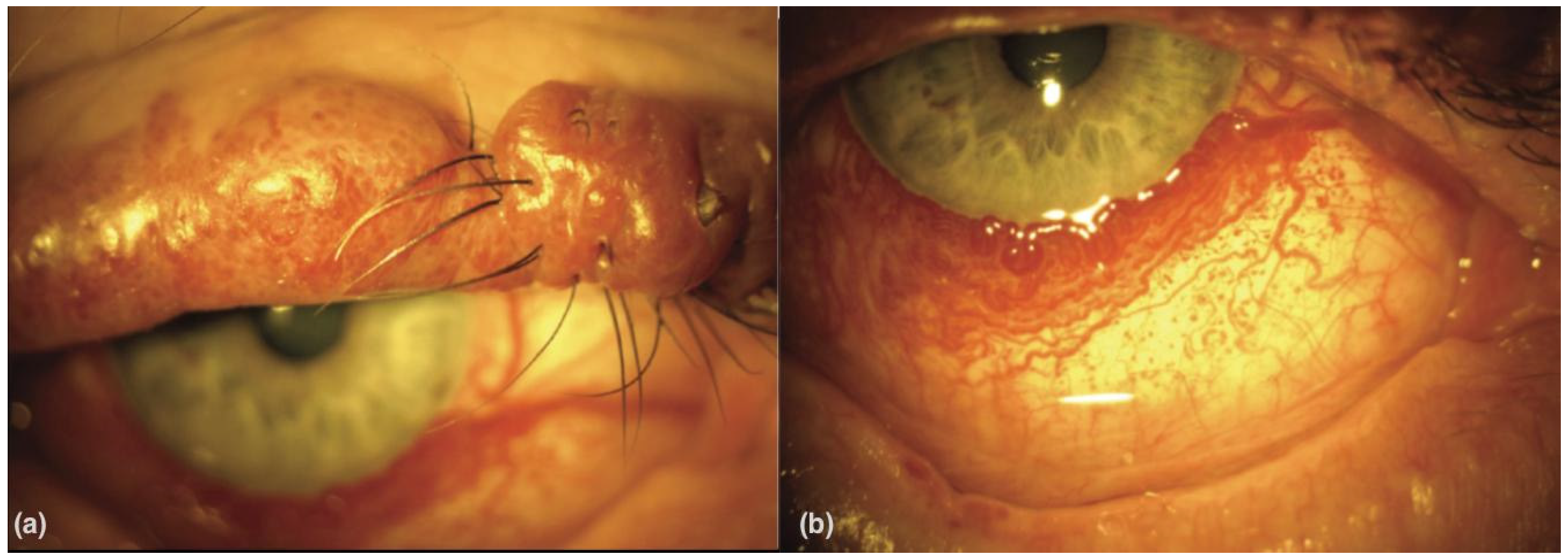
2.2. Fundus Photography
2.3. Fundus Autofluorescence (FA)
2.4. Fluorescein Angiography (FFA)
2.5. Indocyanine Green Angiography (ICGA)
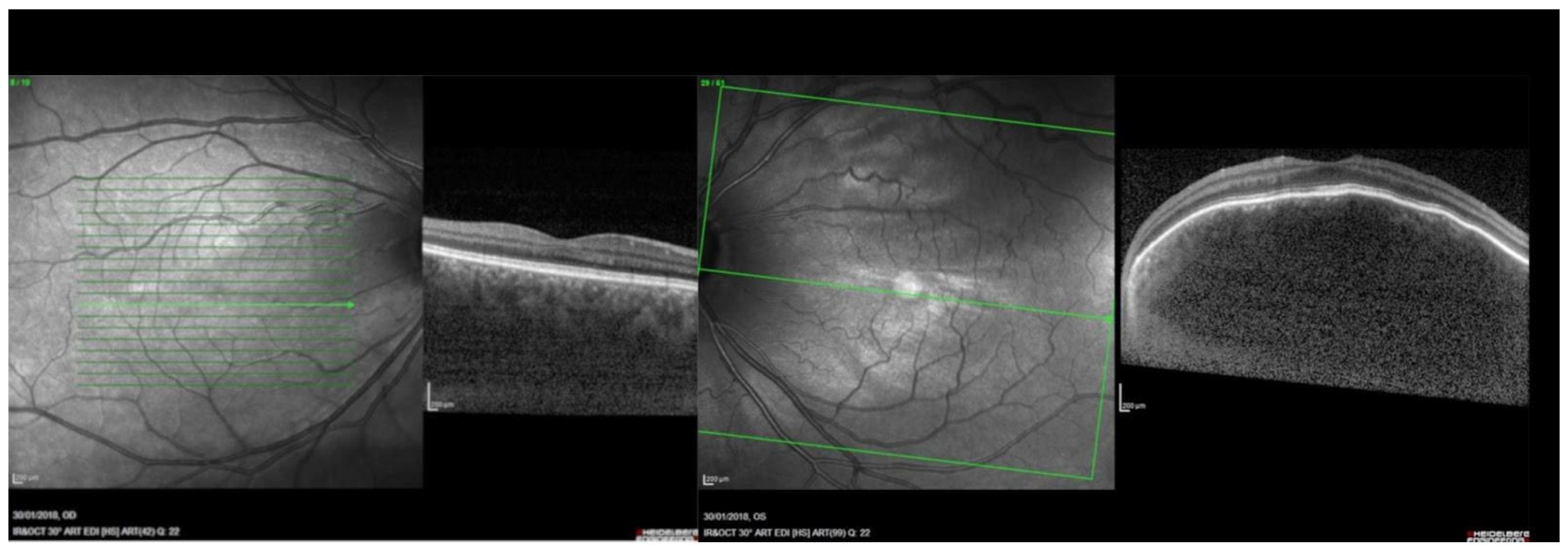
2.6. Spectral Domain Optical Coherence Tomography (SDOCT)
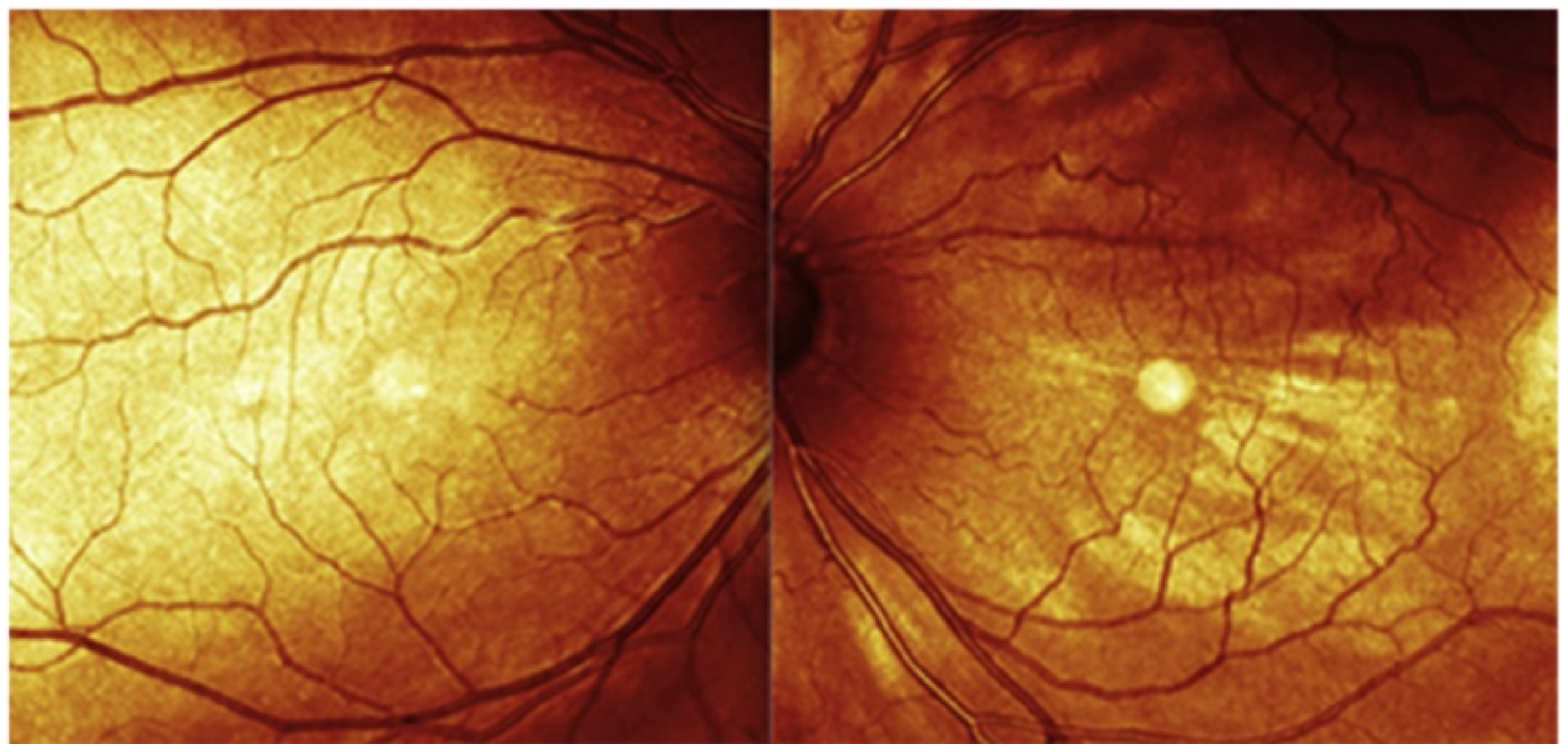
2.7. Near-Infrared Reflectance (NIR)
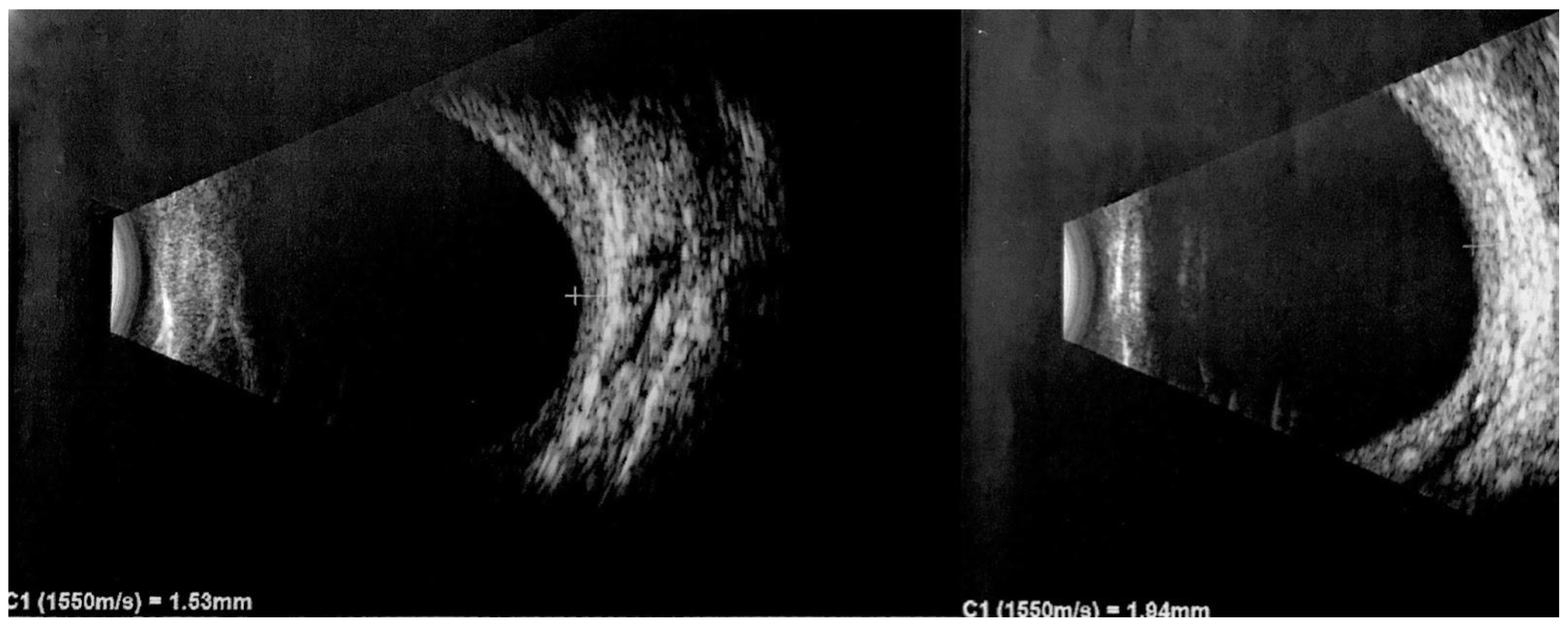
2.8. Ultrasound Imaging
2.9. Treatment Strategies for DCHs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çelebí, S.; Alagöz, G.; Aykan, Ü. Ocular Findings in Sturge-Weber Syndrome. Eur. J. Ophthalmol. 2000, 10, 239–243. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Pugi, D.M.; De Paula, A.; Scuderi, G. Ocular Manifestations in Phakomatosis Pigmentovascularis: Current Concepts on Pathogenesis, Diagnosis, and Management. Surv. Ophthalmol. 2021, 66, 482–492. [Google Scholar] [CrossRef]
- Roach, E.S. Neurocutaneous Syndromes. Pediatr. Clin. N. Am. 1992, 39, 591–620. [Google Scholar] [CrossRef]
- Hildebrand, M.S.; Harvey, A.S.; Malone, S.; Damiano, J.A.; Do, H.; Ye, Z.; McQuillan, L.; Maixner, W.; Kalnins, R.; Nolan, B.; et al. Somatic GNAQ Mutation in the Forme Fruste of Sturge-Weber Syndrome. Neurol. Genet. 2018, 4, e236. [Google Scholar] [CrossRef]
- Maslin, J.S.; Dorairaj, S.K.; Ritch, R. Sturge-Weber Syndrome (Encephalotrigeminal Angiomatosis): Recent Advances and Future Challenges. Asia-Pac. J. Ophthalmol. 2014, 3, 361–367. [Google Scholar] [CrossRef]
- Formisano, M.; Abdolrahimzadeh, B.; Mollo, R.; Bruni, P.; Malagola, R.; Abdolrahimzadeh, S. Bilateral Diffuse Choroidal Hemangioma in Sturge Weber Syndrome: A Case Report Highlighting the Role of Multimodal Imaging and a Brief Review of the Literature. J. Curr. Ophthalmol. 2019, 31, 242–249. [Google Scholar] [CrossRef]
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge–Weber Syndrome and Port-Wine Stains Caused by Somatic Mutation in GNAQ. N. Engl. J. Med. 2013, 368, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, J.; Xu, M.; Chen, N.; Yang, Y. Sturge-Weber Syndrome. Ann. Dermatol. 2011, 23, 551. [Google Scholar] [CrossRef] [PubMed]
- Waelchli, R.; Aylett, S.E.; Robinson, K.; Chong, W.K.; Martinez, A.E.; Kinsler, V.A. New Vascular Classification of Port-wine Stains: Improving Prediction of Sturge–Weber Risk. Br. J. Dermatol. 2014, 171, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Sugano, H.; Iimura, Y.; Igarashi, A.; Nakazawa, M.; Suzuki, H.; Mitsuhashi, T.; Nakajima, M.; Higo, T.; Ueda, T.; Nakanishi, H.; et al. Extent of Leptomeningeal Capillary Malformation Is Associated with Severity of Epilepsy in Sturge-Weber Syndrome. Pediatr. Neurol. 2021, 117, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pilli, V.K.; Behen, M.E.; Hu, J.; Xuan, Y.; Janisse, J.; Chugani, H.T.; Juhász, C. Clinical and Metabolic Correlates of Cerebral Calcifications in Sturge-Weber Syndrome. Dev. Med. Child Neurol. 2017, 59, 952–958. [Google Scholar] [CrossRef]
- Sabeti, S.; Ball, K.L.; Bhattacharya, S.K.; Bitrian, E.; Blieden, L.S.; Brandt, J.D.; Burkhart, C.; Chugani, H.T.; Falchek, S.J.; Jain, B.G.; et al. Consensus Statement for the Management and Treatment of Sturge-Weber Syndrome: Neurology, Neuroimaging, and Ophthalmology Recommendations. Pediatr. Neurol. 2021, 121, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, K.; Nourinia, R.; Gerami, E.; Mahmoudi, G.; Esfandiari, H. Ocular Manifestations of the Sturge–Weber Syndrome. J. Ophthalmic Vis. Res. 2021, 16, 415. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cao, J.; Choi, E.Y.; Li, Y. Progressive Retinal Vessel Malformation in a Premature Infant with Sturge-Weber Syndrome: A Case Report and a Literature Review of Ocular Manifestations in Sturge-Weber Syndrome. BMC Ophthalmol. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Abdolrahimzadeh, S.; Scavella, V.; Felli, L.; Cruciani, F.; Contestabile, M.T.; Recupero, S.M. Ophthalmic Alterations in the Sturge-Weber Syndrome, Klippel-Trenaunay Syndrome, and the Phakomatosis Pigmentovascularis: An Independent Group of Conditions? BioMed Res. Int. 2015, 2015, 786519. [Google Scholar] [CrossRef] [PubMed]
- Sujansky, E.; Conradi, S. Outcome of Sturge–Weber syndrome in 52 adults. Am. J. Med. Genet. 1995, 57, 35–45. [Google Scholar] [CrossRef]
- Sujansky, E.; Conradi, S. Sturge–Weber syndrome: Age of onset of seizures and glaucoma and the prognosis for affected children. J. Child Neurol. 1995, 10, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Mantelli, F.; Mannino, G.; Recupero, S.M. An Unusual Case of Acute Glaucoma in Sturge-Weber Syndrome. Eur. J. Ophthalmol. 2015, 25, 103–105. [Google Scholar] [CrossRef]
- Yan, H.; Hu, M.; Cui, Y.; Li, L.; Liang, T. Clinical Characteristics of Infants with Port-Wine Stain and Glaucoma Secondary to Sturge–Weber Syndrome. BMC Ophthalmol. 2022, 22, 260. [Google Scholar] [CrossRef]
- Sullivan, T.; Clarke, M.; Morin, J. The ocular manifestations of the Sturge–Weber syndrome. J. Pediatr. Ophthalmol. Strabismus 1992, 29, 349–356. [Google Scholar] [CrossRef]
- Weiss, D. Dual origin of glaucoma in encephalotrigeminal haemangiomatosis. Trans. Ophthalmol. Soc. 1973, 93, 477. [Google Scholar]
- Phelps, C.D. The pathogenesis of glaucoma in Sturge-Weber syndrome. Ophthalmology 1978, 85, 276–286. [Google Scholar] [CrossRef]
- Basler, L.; Sowka, J. Sturge-Weber syndrome and glaucoma. Optom.-J. Am. Optom. Assoc. 2011, 82, 306–309. [Google Scholar] [CrossRef]
- Maruyama, I.; Ohguro, H.; Nakazawa, M. A case of acute angle-closure glaucoma secondary to posterior scleritis in patient with Sturge–Weber syndrome. Jpn. J. Ophthalmol. 2002, 46, 74–77. [Google Scholar] [CrossRef]
- Ikeda, H.; Ishigooka, H.; Muto, T. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch. Ophthalmol. 2004, 122, 1122–1128. [Google Scholar] [CrossRef]
- Cruciani, F.; Lorenzatti, M.; Nazzarro, V.; Abdolrahimzadeh, S. Bilateral acute angle closure glaucoma and myopia induced by topiramate. Clin. Ter. 2009, 160, 215–216. [Google Scholar] [PubMed]
- Agarwal, H.C.; Sandramouli, S.; Sihota, R.; Sood, N. Sturge-Weber syndrome: Management of glaucoma with combined trabeculotomy-trabeculectomy. Ophthalmic Surg. 1993, 24, 399–402. [Google Scholar] [CrossRef]
- Barbosa, G.; Susanna, B.; Okuno, R.; Junior, R. Serous Retinal Detachment Resolution Following Trabeculectomy in a Patient with Sturge–Weber Syndrome. J. Curr. Ophthalmol. 2021, 33, 209. [Google Scholar] [CrossRef]
- Junttila, T.L.; Alberto, N.; Winkels, M.; Greenwood, M.D. Successful Reduction of Intraocular Pressure in a Patient with Glaucoma Secondary to Sturge-Weber Syndrome Using a Suprachoroidal Shunt. J. Curr. Glaucoma Pract. 2020, 14, 43–46. [Google Scholar] [CrossRef]
- Amini, H.; Razeghinejad, M.R.; Esfandiarpour, B. Primary single-plate Molteno tube implantation for management of glaucoma in children with Sturge-Weber syndrome. Int. Ophthalmol. 2007, 27, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.K.D.; Malek, M.I.A.; Mannaf, S.M.A.; Iftekhar, Q.S.; Mahatma, M.; Sarkar, M.K.; Rahman, M. Outcome of Trabeculectomy versus Ahmed Glaucoma Valve Implantation in the Surgical Management of Glaucoma in Patients with Sturge–Weber Syndrome. Br. J. Ophthalmol. 2021, 105, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Rihani, H.T.; Dalvin, L.A.; Hodge, D.O.; Pulido, J.S. Incidence of Sturge–Weber Syndrome and Associated Ocular Involvement in Olmsted County, Minnesota, United States. Ophthalmic Genet. 2020, 41, 108–124. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; De Potter, P. Patterns of Indocyanine Green Videoangiography of Choroidal Tumours. Br. J. Ophthalmol. 1995, 79, 237–245. [Google Scholar] [CrossRef]
- Peer, J.; Sancho, C.; Cantu, J.; Eilam, S.; Barzel, I.; Shulman, M.; Blumenthal, E.Z. Measurement of choroidal melanoma basal diameter by wide-angle digital fundus camera: A comparison with ultrasound measurement. Ophthalmologica 2006, 220, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Kaiserman, I. Long Term Ultrasonic Follow up of Choroidal Naevi and Their Transformation to Melanomas. Br. J. Ophthalmol. 2006, 90, 994–998. [Google Scholar] [CrossRef]
- Yu, Y.-Y. Ruthenium-106 Plaque Brachytherapy for the Treatment of Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome. Int. J. Ophthalmol. 2020, 13, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Formisano, M.; Di Pippo, M.C.; Scuderi, L.; Abdolrahimzadeh, S. Current Concepts on Diffuse Choroidal Hemangioma in Sturge Weber Syndrome. Ophthalmic Genet. 2021, 42, 375–382. [Google Scholar] [CrossRef]
- Shields, C.L.; Honavar, S.G.; Shields, J.A.; Cater, J.; Demirci, H. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology 2001, 108, 2237–2248. [Google Scholar] [CrossRef]
- Shields, C.L.; Atalay, H.T.; Wuthisiri, W.; Levin, A.V.; Lally, S.E.; Shields, J.A. Sector Iris Hemangioma in Association with Diffuse Choroidal Hemangioma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2015, 19, 83–86. [Google Scholar] [CrossRef]
- Witschel, H.; Font, R.L. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv. Ophthalmol. 1976, 20, 415–431. [Google Scholar] [CrossRef]
- Helmi, H.A.; Alkatan, H.M.; Al-Essa, R.S.; Aljudi, T.W.; Maktabi, A.M.Y.; Eberhart, C.G. Choroidal Hemangioma in Sturge Weber Syndrome: Case Series with Confirmed Tissue Diagnosis. Int. J. Surg. Case Rep. 2021, 89, 106626. [Google Scholar] [CrossRef] [PubMed]
- Abdolrahimzadeh, S.; Parisi, F.; Mantelli, F.; Perdicchi, A.; Scuderi, G. Retinal Pigment Epithelium–Photoreceptor Layer Alterations in a Patient with Sturge–Weber Syndrome with Diffuse Choroidal Hemangioma. Ophthalmic Genet. 2017, 38, 567–569. [Google Scholar] [CrossRef]
- Dave, T.; Dave, V.P.; Shah, G.; Pappuru, R.R. Diffuse Choroidal Hemangioma Masquerading as Central Serous Chorioretinopathy Treated with Oral Propanolol. Retin. Cases Brief Rep. 2016, 10, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Parolini, B.; Cardillo, D.; Baldi, A.; Di Salvatore, A.; Finzi, A.; Pinackatt, S.J.; Frisina, R.; Besozzi, G. Partial Thickness Sclerectomy to Treat Exudative Retinal Detachment Secondary a Submacular Choroidal Hemangioma in a Sturge–Weber Syndrome. Int. Ophthalmol. 2019, 39, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kubicka-Trząska, A.; Karska-Basta, I.; Oleksy, P.; Romanowska-Dixon, B. Management of Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome with Ruthenium-106 Plaque Radiotherapy. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2015–2019. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Shields, C.L.; Harmon, S.A.; Shields, J. Autofluorescense of Choroidal Hemangioma in 34 Consecutive Eyes. Retina 2010, 30, 16–22. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Ciancimino, C.; Grassi, F.; Sordi, E.; Fragiotta, S.; Scuderi, G. Near-Infrared Reflectance Imaging in Retinal Diseases Affecting Young Patients. J. Ophthalmol. 2021, 2021, 5581851. [Google Scholar] [CrossRef]
- Cavallerano, A.A. Ophthalmic fluorescein angiography. Optom. Clin. 1996, 5, 1–23. [Google Scholar]
- Callaway, N.F.; Mruthyunjaya, P. Widefield Imaging of Retinal and Choroidal Tumors. Int. J. Retin. Vitr. 2019, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Wu, D. Indocyanine Green Angiographic Findings in Diffuse Choroidal Hemangioma Associated with Sturge-Weber Syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 625–627. [Google Scholar] [CrossRef]
- Schalenbourg, A.; Piguet, B.; Zografos, L. Indocyanine Green Angiographic Findings in Choroidal Hemangiomas: A Study of 75 Cases. Ophthalmologica 2000, 214, 246–252. [Google Scholar] [CrossRef]
- Bach, A.; Gold, A.S.; Villegas, V.M.; Wildner, A.C.; Ehlies, F.J.; Murray, T.G. Spontaneous Exudative Retinal Detachment in a Patient with Sturge-Weber Syndrome after Taking Arginine, a Supplement for Erectile Dysfunction. Eye Vis. 2014, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, A.; Canavesi, C.; Hayes, A.; Tankam, P.; Duma, V.-F.; Santhanam, A.; Thompson, K.P.; Rolland, J.P. MEMS-Based Handheld Scanning Probe with Pre-Shaped Input Signals for Distortion-Free Images in Gabor-Domain Optical Coherence Microscopy. Opt. Express 2016, 24, 13365. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical Coherence Tomography Today: Speed, Contrast, and Multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical Coherence Tomography. Science 2015, 254, 1178–1181. [Google Scholar] [CrossRef]
- Cacciamani, A.; Scarinci, F.; Parravano, M.; Giorno, P.; Varano, M. Choroidal Thickness Changes with Photodynamic Therapy for a Diffuse Choroidal Hemangioma in Sturge–Weber Syndrome. Int. Ophthalmol. 2014, 34, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Slakter, J.S.; Yannuzzi, L.A.; Guyer, D.R.; Sorenson, J.A.; Orlock, D.A. Indocyanine-green angiography. Curr. Opin. Ophthalmol. 1995, 6, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.; Spaide, R.F. A Pilot Study of Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Normal Eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015, 133, 45. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moreno, I.; Ruiz-Medrano, J.; Duker. J.S.; Ruiz-Moreno, J.M. The relationship between retinal and choroidal thickness and visual acuity in highly myopic eyes. Br. J. Ophthalmol. 2013, 97, 1010–1013. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Parisi, F.; Plateroti, A.M.; Evangelista, F.; Fenicia, V.; Scuderi, G.; Recupero, S.M. Visual acuity, and macular and peripapillary thickness in high myopia. Curr. Eye Res. 2017, 42, 1468–1473. [Google Scholar] [CrossRef]
- Arora, K.S.; Quigley, H.A.; Comi, A.M.; Miller, R.B.; Jampel, H.D. Increased Choroidal Thickness in Patients with Sturge-Weber Syndrome. JAMA Ophthalmol. 2013, 131, 1216. [Google Scholar] [CrossRef] [PubMed]
- Sobol, E.K.; Francis, J.H.; Abramson, D.H.; Freund, K.B.; Spaide, R.F.; Barbazetto, I. Subfoveal choroidal thickness and vascular architecture in fellow eyes of patients with circumscribed choroidal hemangioma. Retina 2020, 40, 758–764. [Google Scholar] [CrossRef] [PubMed]
- García Caride, S.; Fernández-Vigo, J.I.; Valverde-Megías, A. Update on the Diagnosis and Treatment of Choroidal Hemangioma. Arch. Soc. Española Oftalmol. 2023, 98, 281–291. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Scavella, V.; Battaglia, D.; Recupero, S.M. Spectral Domain Optical Coherence Tomography of Choroidal and Outer Retinal Layer Thickness in the Sturge Weber Syndrome. Curr. Eye Res. 2016, 41, 1614–1617. [Google Scholar] [CrossRef]
- Filloy, A.; Caminal, J.M.; Arias, L.; Jordán, S.; Català, J. Swept Source Optical Coherence Tomography Imaging of a Series of Choroidal Tumours. Can. J. Ophthalmol. 2015, 50, 242–248. [Google Scholar] [CrossRef]
- Scuderi, G.; Ciancimino, C.; D’Apolito, F.; Maurizi Enrici, M.; Guglielmelli, F.; Scuderi, L.; Abdolrahimzadeh, S. Short-Term Effects of Dark Chocolate on Retinal and Choriocapillaris Perfusion in Young, Healthy Subjects Using Optical Coherence Tomography Angiography. Nutrients 2020, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Di Pippo, M.; Santia, C.; Rullo, D.; Ciancimino, C.; Grassi, F.; Abdolrahimzadeh, S. The Choroidal Vascularity Index Versus Optical Coherence Tomography Angiography in the Evaluation of the Choroid with a Focus on Age-Related Macular Degeneration. Tomography 2023, 9, 1456–1470. [Google Scholar] [CrossRef] [PubMed]
- Konana, V.; Shanmugam, P.m.; Ramanjulu, R.; Mishra, K.C.; Sagar, P. Optical Coherence Tomography Angiography Features of Choroidal Hemangioma. Indian J. Ophthalmol. 2018, 66, 581. [Google Scholar]
- Takkar, B.; Azad, S.; Shakrawal, J.; Gaur, N.; Venkatesh, P. Blood Flow Pattern in a Choroidal Hemangioma Imaged on Swept-Source-Optical Coherence Tomography Angiography. Indian J. Ophthalmol. 2017, 65, 1240. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Rossi, C.; Breve, M.A.; Velotti, N.; Farella, A.; Liuzzi, R.; Cennamo, G. Evaluation of Vascular Changes with Optical Coherence Tomography Angiography after Ruthenium-106 Brachytherapy of Circumscribed Choroidal Hemangioma. Eye 2018, 32, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Tripathy, K.; Sharma, A.; Vohra, R. Swept Source Optical Coherence Tomography-Angiography of Choroid in Choroidal Hemangioma before and after Laser Photocoagulation. Indian J. Ophthalmol. 2017, 65, 751. [Google Scholar] [PubMed]
- Amirikia, A.; Scott, I.U.; Murray, T.G. Bilateral Diffuse Choroidal Hemangiomas with Unilateral Facial Nevus Flammeus in Sturge–Weber Syndrome. Am. J. Ophthalmol. 2000, 130, 362–364. [Google Scholar] [CrossRef]
- Kadakia, A.; Zhang, J.; Yao, X.; Zhou, Q.; Heiferman, M.J. Ultrasound in ocular oncology: Technical advances, clinical applications, and limitations. Exp. Biol. Med. 2023, 248, 371–379. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Hodes, B.L. Ultrasonographic diagnosis of choroidal malignant melanoma. Surv. Ophthalmol. 1977, 22, 29–40. [Google Scholar] [PubMed]
- Singh, N.; Fonkeu, Y.; Lorek, B.H.; Singh, A.D. Diagnostic A-Scan of Choroidal Tumors: Comparison of Quantified Parameters. Ocul. Oncol. Pathol. 2019, 5, 358–368. [Google Scholar] [CrossRef]
- Toslak, D.; Son, T.; Erol, M.K.; Kim, H.; Kim, T.-H.; Chan, R.V.P.; Yao, X. Portable Ultra-Widefield Fundus Camera for Multispectral Imaging of the Retina and Choroid. Biomed. Opt. Express 2020, 11, 6281. [Google Scholar] [CrossRef]
- Frampton, G.K.; Kalita, N.; Payne, L.; Colquitt, J.L.; Loveman, E.; Downes, S.M.; Lotery, A.J. Fundus Autofluorescence Imaging: Systematic Review of Test Accuracy for the Diagnosis and Monitoring of Retinal Conditions. Eye 2017, 31, 995–1007. [Google Scholar] [CrossRef]
- Ly, A.; Nivison-Smith, L.; Assaad, N.; Kalloniatis, M. Fundus Autofluorescence in Age-Related Macular Degeneration. Optom. Vis. Sci. 2017, 94, 246–259. [Google Scholar] [CrossRef]
- Rabiolo, A.; Parravano, M.; Querques, L.; Cicinelli, M.V.; Carnevali, A.; Sacconi, R.; Centoducati, T.; Vujosevic, S.; Bandello, F.; Querques, G. Ultra-Wide-Field Fluorescein Angiography in Diabetic Retinopathy: A Narrative Review. Clin. Ophthalmol. 2017, 11, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Lee, S.Y. Multifocal Photodynamic Therapy for Diffuse Choroidal Hemangioma. Clin. Ophthalmol. 2012, 6, 1467–1469. [Google Scholar] [CrossRef]
- Anaya-Pava, E.J.; Saenz-Bocanegra, C.H.; Flores-Trejo, A.; Castro-Santana, N.A. Diffuse Choroidal Hemangioma Associated with Exudative Retinal Detachment in a Sturge–Weber Syndrome Case: Photodynamic Therapy and Intravitreous Bevacizumab. Photodiagn. Photodyn. Ther. 2015, 12, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Anand, R. Photodynamic Therapy for Diffuse Choroidal Hemangioma Associated with Sturge Weber Syndrome. Am. J. Ophthalmol. 2003, 136, 758–760. [Google Scholar] [CrossRef]
- Monteiro, S.; Casal, I.; Santos, M.; Meireles, A. Photodynamic Therapy for Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome. Case Rep. Med. 2014, 2014, 452372. [Google Scholar] [CrossRef]
- Arevalo, J.F. Oral Propranolol for Exudative Retinal Detachment in Diffuse Choroidal Hemangioma. Arch. Ophthalmol. 2011, 129, 1373. [Google Scholar] [CrossRef]
- Gambrelle, J.; Kivelä, T.; Grange, J.-D. Sturge-Weber Syndrome: Decrease in Intraocular Pressure after Transpupillary Thermotherapy for Diffuse Choroidal Haemangioma. Acta Ophthalmol. 2011, 89, 190–193. [Google Scholar] [CrossRef]
- Huiskamp, E.A.; Müskens, R.P.H.M.; Ballast, A.; Hooymans, J.M.M. Diffuse Choroidal Haemangioma in Sturge–Weber Syndrome Treated with Photodynamic Therapy under General Anaesthesia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Abdolrahimzadeh, S.; Fragiotta, S.; Ciacimino, C.; Di Pippo, M.; Scuderi, G. Choroidal Vascularity Index in Adult-Onset Foveomacular Vitelliform Dystrophy: A Pilot Study. Appl. Sci. 2021, 11, 10487. [Google Scholar] [CrossRef]
| Imaging Modality Technical Characteristics | Advantages | Disadvantages | DCH Appearance |
|---|---|---|---|
| Fundus Photography WL 490–615 nm [78] FOV 45°–200° [78] | Non-invasive; ease of capturing images; both eyes can be assessed simultaneously. | The extent or thickness of the hemangioma is not assessable. Shallow DCH does not significantly alter the fundus appearance. | DCH’s subtle color differences known as “tomato ketchup” appearance. Additional characteristics such as exudative retinal detachments, venous congestion, or hyperplastic RPE changes can be monitored. |
| FA WL 488–514 nm [79] FOV 30°–200° [80] | Efficient; non-invasive; useful in evaluating the presence of subretinal fluid or retinal detachment. | The extent or thickness of the hemangioma is not assessable. | Untreated DCH exhibits intrinsic iso-FA or hypo-FA patterns; treated DCH often displays a higher prevalence of hypo-FA traits. Extrinsic DCH findings including RPE hyperplasia, atrophy, and fibrous metaplasia. |
| NIR WL 790 nm [79] FOV 30–200° [80] | Efficient; non-invasive. | Further studies for its implication in DCH’s diagnosis and monitoring are necessary. | Greater tissue penetration of NIR allows rapid evaluation of the EPR. A rare case of hyperreflective dots was reported. |
| FFA WL 465–530 nm [81] FOV 55°–200° [81] | FFA can be particularly informative in cases where DCH is associated with retinal detachment. | It requires dye administration which rarely can give rise to allergic reactions. It can interfere with renal and hepatic metabolisms. Requires substantial patient collaboration. | FFA has been shown to depict early hyperfluorescence and subsequent late-phase leakage consistent with exudative retinal detachment linked to DCH. |
| ICGA WL 790–835 nm [57] FOV 55°–105° [81] | ICGA offers a detailed dynamic visualization of DCH; valuable insights into the intricate choroidal circulation within DCH. | Requires dye administration, which rarely can give rise to allergic reactions. It can interfere with renal and hepatic metabolisms. Requires substantial patient collaboration. | Early phase: vascular network within the hemangioma is quickly filled. Intermediate phase: complete dye filling yields intense hyperfluorescence with a mix of hyper- and hypofluorescent dots on an already luminescent background. Late phase: pronounced hypofluorescence of the tumor when compared to the adjacent choroid emerges as a signature feature. |
| SDOCT WL 840–870 nm [56,57,58,59]. | Efficient; non-invasive; allows for the identification of retinal layers and assessment of retinal thickness. | In cases of thickened choroid SDOCT falls short in evaluating the choroidal-scleral junction. | It can reveal the breakdown of Bruch’s membrane and RPE, intraretinal fluid, and subretinal fluid. |
| EDI-SDOCT WL 840–870 nm [56,57,58,59]. | Efficient; non-invasive; allows for a better visualization of the choroidal-scleral junction which renders manual choroidal thickness measurement easier. | Not always available in hospital settings. | Choroidal vasculature is visualized on EDI-SDOCT cross sectional images. Profile alterations of the retina and choroid are well visible. |
| SSOCT WL 1055–1300 nm [65,66,67]. | Efficient; non-invasive; allows for a better visualization of the choroidal-scleral junction which renders manual choroidal thickness measurement easier. | Expensive technology, rarely available in hospital settings. | Consistent accuracy of SSOCT in measuring hemangioma thickness and effectively distinguishing the lesion from surrounding normal choroid. Profile alterations of the retina and choroid are better evaluated. |
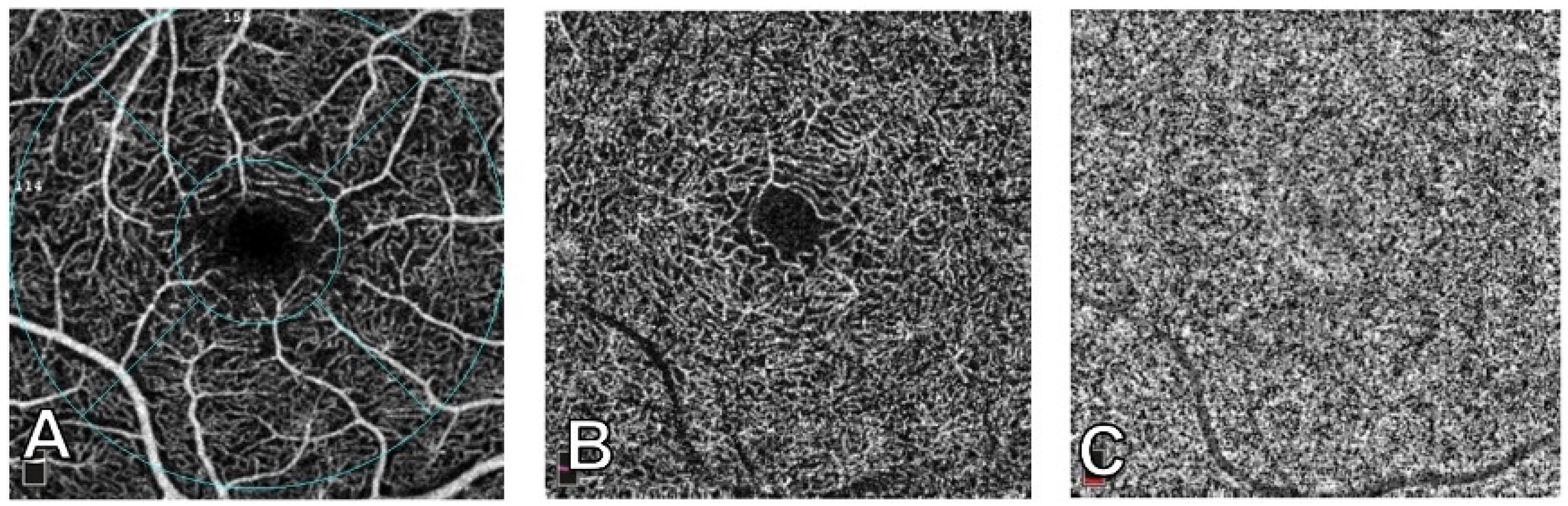
| OCTA WL 840–870 nm [56,57,58,59]. | Efficient; non-invasive; it provides insights into lesion characteristics by analyzing density, flow, and vascular patterns. | Further studies for its implication in DCH diagnosis and monitoring are necessary. | Differentiated vascular patterns, like worm-like, spaghetti-shaped, and club-shaped, have been identified within CCH using OCTA. |
| US Frequency: A-scan 7–10 MHz B-scan 10–20 MHz [75] | Cost effective, efficient. Helps in distinguishing DCH from malignant choroidal tumors such as melanomas. | It provides two-dimensional images and may not capture the full extent of complex hemangiomas or small lesions. | Benign tumors reveal a thickened choroid with pronounced internal reflectivity. DCH demonstrates a regular internal structure and lacks significant vascularization. DCH appears as a solid, dome-shaped lesion with hyperechogenicity and lacks a posterior shadow. Detects presence of associated features like retinal detachment and superficial calcifications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciancimino, C.; Di Pippo, M.; Rullo, D.; Ruggeri, F.; Grassi, F.; Scuderi, G.; Abdolrahimzadeh, S. An Update on Multimodal Ophthalmological Imaging of Diffuse Choroidal Hemangioma in Sturge–Weber Syndrome. Vision 2023, 7, 64. https://doi.org/10.3390/vision7040064
Ciancimino C, Di Pippo M, Rullo D, Ruggeri F, Grassi F, Scuderi G, Abdolrahimzadeh S. An Update on Multimodal Ophthalmological Imaging of Diffuse Choroidal Hemangioma in Sturge–Weber Syndrome. Vision. 2023; 7(4):64. https://doi.org/10.3390/vision7040064
Chicago/Turabian StyleCiancimino, Chiara, Mariachiara Di Pippo, Daria Rullo, Francesco Ruggeri, Flaminia Grassi, Gianluca Scuderi, and Solmaz Abdolrahimzadeh. 2023. "An Update on Multimodal Ophthalmological Imaging of Diffuse Choroidal Hemangioma in Sturge–Weber Syndrome" Vision 7, no. 4: 64. https://doi.org/10.3390/vision7040064
APA StyleCiancimino, C., Di Pippo, M., Rullo, D., Ruggeri, F., Grassi, F., Scuderi, G., & Abdolrahimzadeh, S. (2023). An Update on Multimodal Ophthalmological Imaging of Diffuse Choroidal Hemangioma in Sturge–Weber Syndrome. Vision, 7(4), 64. https://doi.org/10.3390/vision7040064





