Effectiveness of Topical Cyclosporin-A 0.1% Compared to Combined Topical Cyclosporin-A 0.1% with Topical Sodium Hyaluronate on Interleukin-6 Levels in the Tears of Patients with Dry Eye Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyclosporin-A
2.2. Combination of Cyclosporin-A and Sodium Hyaluronate
2.3. Clinical Severity of DED
2.4. OSDI Score
2.5. Schirmer Test 1
2.6. TBUT
2.7. Fluorescein Test
2.8. Clinical Examination Procedure
2.9. IL-6 Cytokine Analysis of Tear Samples
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry Eye as a Mucosal Autoimmune Disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear Cytokine Profiles in Dysfunctional Tear Syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Han, S.J.; Nam, S.M.; Yoon, S.C.; Ahn, J.M.; Kim, T.-I.; Kim, E.K.; Seo, K.Y. Analysis of Tear Cytokines and Clinical Correlations in Sjögren Syndrome Dry Eye Patients and Non–Sjögren Syndrome Dry Eye Patients. Am. J. Ophthalmol. 2013, 156, 247–253.e1. [Google Scholar] [CrossRef]
- Heidari, M.; Noorizadeh, F.; Wu, K.; Inomata, T.; Mashaghi, A. Dry Eye Disease: Emerging Approaches to Disease Analysis and Therapy. J. Clin. Med. 2019, 8, 1439. [Google Scholar] [CrossRef]
- Liu, R.; Gao, C.; Chen, H.; Li, Y.; Jin, Y.; Qi, H. Analysis of Th17-Associated Cytokines and Clinical Correlations in Patients with Dry Eye Disease. PLoS ONE 2017, 12, e0173301. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; Dana, R. Role of Th17 Cells in the Immunopathogenesis of Dry Eye Disease. Mucosal Immunol. 2009, 2, 375–376. [Google Scholar] [CrossRef]
- Benítez-del-Castillo, J.; Labetoulle, M.; Baudouin, C.; Rolando, M.; Akova, Y.A.; Aragona, P.; Geerling, G.; Merayo-Lloves, J.; Messmer, E.M.; Boboridis, K. Visual Acuity and Quality of Life in Dry Eye Disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2017, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Tan, X.-H.; Ren, T.-L.; Wu, Z.-F.; Zhu, T.-T.; Xu, H.-Y. Clinical Analysis of Interleukin-6 Level in Tear and Serum of Dry Eye Patient. Zhonghua Shiyan Yanke Zazhi/Chin. J. Exp. Ophthalmol. 2013, 31, 186–190. [Google Scholar] [CrossRef]
- Baiula, M.; Spampinato, S. Experimental Pharmacotherapy for Dry Eye Disease: A Review. J. Exp. Pharmacol. 2021, 13, 345–358. [Google Scholar] [CrossRef]
- Turner, K.; Pflugfelder, S.C.; Ji, Z.; Feuer, W.J.; Stern, M.; Reis, B.L. Interleukin-6 Levels in the Conjunctival Epithelium of Patients with Dry Eye Disease Treated with Cyclosporine Ophthalmic Emulsion. Cornea 2000, 19, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Kim, T.; Kwon, S.M.; Seo, K.Y.; Kim, S.W.; Kim, E.K.; Park, W.C. Efficacy of Combined 0.05% Cyclosporine and 1% Methylprednisolone Treatment for Chronic Dry Eye. Cornea 2012, 31, 509–513. [Google Scholar] [CrossRef]
- Kang, M.-J.; Kim, Y.-H.; Chou, M.; Hwang, J.; Cheon, E.-J.; Lee, H.-J.; Chung, S.-H. Evaluation of the Efficacy and Safety of A Novel 0.05% Cyclosporin A Topical Nanoemulsion in Primary Sjögren’s Syndrome Dry Eye. Ocul. Immunol. Inflamm. 2020, 28, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.C.H.; Sng, J.J.; Wang, P.X.H.; Htoon, H.M.; Tong, L.H.T. Sodium Hyaluronate in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 9013. [Google Scholar] [CrossRef] [PubMed]
- Tuan, H.-I.; Chi, S.-C.; Kang, Y.-N. An Updated Systematic Review With Meta-Analysis Of Randomized Trials On Topical Cyclosporin A For Dry-Eye Disease. Drug Des. Devel. Ther. 2020, 14, 265–274. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkeç, M.; Benítez del Castillo, J.; Geerling, G.; Labetoulle, M.; Bonini, S.; ODISSEY European Consensus Group members. Diagnosing the Severity of Dry Eye: A Clear and Practical Algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef]
- Mrugacz, M.; Ostrowska, L.; Bryl, A.; Szulc, A.; Zelazowska-Rutkowska, B.; Mrugacz, G. Pro-Inflammatory Cytokines Associated with Clinical Severity of Dry Eye Disease of Patients with Depression. Adv. Med. Sci. 2017, 62, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Cocho, L.; Fernández, I.; Calonge, M.; Martínez, V.; González-García, M.J.; Caballero, D.; López-Corral, L.; García-Vázquez, C.; Vázquez, L.; Stern, M.E.; et al. Biomarkers in Ocular Chronic Graft Versus Host Disease: Tear Cytokine- and Chemokine-Based Predictive Model. Investig. Opthalmol. Vis. Sci. 2016, 57, 746. [Google Scholar] [CrossRef]
- Matossian, C.; McDonald, M.; Donaldson, K.E.; Nichols, K.K.; MacIver, S.; Gupta, P.K. Dry Eye Disease: Consideration for Women’s Health. J. Women’s Health 2019, 28, 502–514. [Google Scholar] [CrossRef]
- Ayaki, M.; Kawashima, M.; Uchino, M.; Tsubota, K.; Negishi, K. Gender Differences in Adolescent Dry Eye Disease: A Health Problem in Girls. Int. J. Ophthalmol. 2018, 11, 301–307. [Google Scholar] [CrossRef]
- Tangmonkongvoragul, C.; Chokesuwattanaskul, S.; Khankaeo, C.; Punyasevee, R.; Nakkara, L.; Moolsan, S.; Unruan, O. Prevalence of Symptomatic Dry Eye Disease with Associated Risk Factors among Medical Students at Chiang Mai University Due to Increased Screen Time and Stress during COVID-19 Pandemic. PLoS ONE 2022, 17, e0265733. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, S.; Bian, A.; Hong, J.; Deng, Y.; Zhang, M.; Chen, W.; Shao, Y.; Zhao, J. Efficacy and Safety of 0.05% Cyclosporine Ophthalmic Emulsion in Treatment of Chinese Patients with Moderate to Severe Dry Eye Disease: A 12-Week, Multicenter, Randomized, Double-Masked, Placebo-Controlled Phase III Clinical Study. Medicine 2019, 98, e16710. [Google Scholar] [CrossRef]
- Im, G.; Park, S.; Yang, H.; Kim, Y.; Go, H.; Kwon, H.; Kim, M. Additive Effect of 0.05% Cyclosporine/0.3% Sodium Hyaluronate Combination Eye Drops in the Treatment of the Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 419. [Google Scholar]
- Ju, Z.; Xin-yi, W. Effects of 01% Cyclosporin Eye Drops in Combination with Sodium Hyaluronate Eye Drops in the Treatment of Dry Eye Syndrome. J. Shandong Univ. (Otolaryngol. Ophthalmol.) 2015, 29, 49–51. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, J.-E.; Oh, H.-N.; Song, J.-W.; Han, S.-Y.; Lee, J.-S. Clinical Efficacy of Combined Topical 0.05% Cyclosporine A and 0.1% Sodium Hyaluronate in the Dry Eyes with Meibomian Gland Dysfunction. Int. J. Ophthalmol. 2018, 11, 593–600. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, S.; Lee, H.K.; Chung, T.-Y.; Kim, J.Y.; Choi, C.Y.; Chung, S.H.; Kim, D.H.; Kim, K.W.; Chung, J.K.; et al. A Randomized Multicenter Evaluation of the Efficacy of 0.15% Hyaluronic Acid versus 0.05% Cyclosporine A in Dry Eye Syndrome. Sci. Rep. 2022, 12, 18737. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Lee, W.-Y.; Kim, Y.; Hong, Y. A Meta-Analysis of the Efficacy of Hyaluronic Acid Eye Drops for the Treatment of Dry Eye Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 2383. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Nagata, T.; Kudo, H.; Müller-Lierheim, W.G.K.; van Setten, G.-B.; Dogru, M.; Tsubota, K. The Effects of High Molecular Weight Hyaluronic Acid Eye Drop Application in Environmental Dry Eye Stress Model Mice. Int. J. Mol. Sci. 2020, 21, 3516. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.M. Clinical Efficacy of Sodium Hyaluronate Eye Drops Combined with Pranoprofen in the Treatment of Patients with Dry Eye. Indian J. Pharm. Sci. 2021, 83, 5. [Google Scholar] [CrossRef]
- Gulo, C.I.H.; Puruhito; Novida, H. The Effectiveness of Topical Hyaluronic Acid on Decreasing Interleukin-6 and Acceleration of Wound Healing (Push Score) in Wagner II-III Diabetic Foot Ulcer in Dr. Soetomo Hospital Surabaya. Bali Med. J. 2022, 11, 1049–1053. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic Acid in the Treatment of Dry Eye Disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Ambroziak, A.M.; Szaflik, J.; Szaflik, J.P.; Ambroziak, M.; Witkiewicz, J.; Skopiński, P. Immunomodulation on the Ocular Surface: A Review. Cent. Eur. J. Immunol. 2016, 2, 195–208. [Google Scholar] [CrossRef]
- Ames, P.; Galor, A. Cyclosporine Ophthalmic Emulsions for the Treatment of Dry Eye: A Review of the Clinical Evidence. Clin. Investig. 2015, 5, 267–285. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Tesavibul, N.; Mahawong, W. A Randomized Double-Masked Study of 0.05% Cyclosporine Ophthalmic Emulsion in the Treatment of Meibomian Gland Dysfunction. Cornea 2012, 31, 1386–1393. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Wilson, S.E. Practical Guidance for the Use of Cyclosporine Ophthalmic Solutions in the Management of Dry Eye Disease. Clin. Ophthalmol. 2019, 13, 1115–1122. [Google Scholar] [CrossRef]
- Stevenson, D.; Tauber, J.; Reis, B.L. Efficacy and Safety of Cyclosporin a Ophthalmic Emulsion in the Treatment of Moderate-to-Severe Dry Eye Disease. Ophthalmology 2000, 107, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Sall, K.; Stevenson, O.D.; Mundorf, T.K.; Reis, B.L. Two Multicenter, Randomized Studies of the Efficacy and Safety of Cyclosporine Ophthalmic Emulsion in Moderate to Severe Dry Eye Disease11Reprint Requests to: Linda Lewis, 575 Anton Blvd, Suite 900, Costa Mesa, CA 92626. Ophthalmology 2000, 107, 631–639. [Google Scholar] [CrossRef]
- Gündüz, K.; Özdemir, Ö. Topical Cyclosporin Treatment of Keratoconjunctivitis Sicca in Secondary Sjögren’s Syndrome. Acta Ophthalmol. 2009, 72, 438–442. [Google Scholar] [CrossRef]
- Jain, A.K.; Sukhija, J.; Dwedi, S.; Sood, A. Effect of Topical Cyclosporine on Tear Functions in Tear-Deficient Dry Eyes. Ann. Ophthalmol. 2007, 39, 19–25. [Google Scholar] [CrossRef]
- Roda, M.; Corazza, I.; Bacchi Reggiani, M.L.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels—A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Song, N.; Gong, L. Changes of Dry Eye Related Markers and Tear Inflammatory Cytokines After Upper Blepharoplasty. Front. Med. 2021, 8, 763611. [Google Scholar] [CrossRef] [PubMed]
- Akpek, E.K.; Wu, H.Y.; Karakus, S.; Zhang, Q.; Masli, S. Differential Diagnosis of Sjögren Versus Non-Sjögren Dry Eye Through Tear Film Biomarkers. Cornea 2020, 39, 991–997. [Google Scholar] [CrossRef]
- Cui, D.; Mathews, P.; Li, G.; VanCourt, S.; Akpek, E. Outcomes of Sjögren’s versus Non-Sjögren’s Related Dry Eye in a Longitudinal, Tertiary Clinic-Based Sample. PLoS ONE 2021, 16, e0261241. [Google Scholar] [CrossRef]
- Nair, S.; Vanathi, M.; Mahapatra, M.; Seth, T.; Kaur, J.; Velpandian, T.; Ravi, A.; Titiyal, J.S.; Tandon, R. Tear Inflammatory Mediators and Protein in Eyes of Post Allogenic Hematopoeitic Stem Cell Transplant Patients. Ocul. Surf. 2018, 16, 352–367. [Google Scholar] [CrossRef] [PubMed]
- VanDerMeid, K.R.; Su, S.P.; Ward, K.W.; Zhang, J.-Z. Correlation of Tear Inflammatory Cytokines and Matrix Metalloproteinases with Four Dry Eye Diagnostic Tests. Investig. Opthalmol. Vis. Sci. 2012, 53, 1512. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Ma, Y.; Lin, X.; Yu, X.; He, S.; Luo, C.; Xu, W. Analysis of Tear Inflammatory Molecules and Clinical Correlations in Evaporative Dry Eye Disease Caused by Meibomian Gland Dysfunction. Int. Ophthalmol. 2020, 40, 3049–3058. [Google Scholar] [CrossRef]
- Na, K.-S.; Mok, J.-W.; Kim, J.Y.; Rho, C.R.; Joo, C.-K. Correlations between Tear Cytokines, Chemokines, and Soluble Receptors and Clinical Severity of Dry Eye Disease. Investig. Opthalmol. Vis. Sci. 2012, 53, 5443. [Google Scholar] [CrossRef] [PubMed]
- Massingale, M.L.; Li, X.; Vallabhajosyula, M.; Chen, D.; Wei, Y.; Asbell, P.A. Analysis of Inflammatory Cytokines in the Tears of Dry Eye Patients. Cornea 2009, 28, 1023–1027. [Google Scholar] [CrossRef]
- Yoon, K.-C.; Jeong, I.-Y.; Park, Y.-G.; Yang, S.-Y. Interleukin-6 and Tumor Necrosis Factor-Alpha Levels in Tears of Patients with Dry Eye Syndrome. Cornea 2007, 26, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Ulhaq, Z.S.; Soraya, G.V.; Budu; Wulandari, L.R. The Role of IL-6-174 G/C Polymorphism and Intraocular IL-6 Levels in the Pathogenesis of Ocular Diseases: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 17453. [Google Scholar] [CrossRef]
- McMonnies, C.W. Dry Eye Disease Immune Responses and Topical Therapy. Eye Vis. 2019, 6, 12. [Google Scholar] [CrossRef]
- Boujnah, Y.; Mouchel, R.; El-Chehab, H.; Dot, C.; Burillon, C.; Kocaba, V. Prospective, monocentric, uncontrolled study of efficacy, tolerance and adherence of cyclosporin 0.1% for severe dry eye syndrome. J. Fr. Ophtalmol. 2018, 41, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Straub, M.; Bron, A.M.; Muselier-Mathieu, A.; Creuzot-Garcher, C. Long-Term Outcome after Topical Ciclosporin in Severe Dry Eye Disease with a 10-Year Follow-Up. Br. J. Ophthalmol. 2016, 100, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Van Setten, G.; Amrane, M.; Ismail, D.; Garrigue, J.-S.; Figueiredo, F.C.; Baudouin, C. Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease: A Multicenter Randomized Trial. Eur. J. Ophthalmol. 2016, 26, 287–296. [Google Scholar] [CrossRef]
- Baudouin, C.; Figueiredo, F.C.; Messmer, E.M.; Ismail, D.; Amrane, M.; Garrigue, J.-S.; Bonini, S.; Leonardi, A. A Randomized Study of the Efficacy and Safety of 0.1% Cyclosporine A Cationic Emulsion in Treatment of Moderate to Severe Dry Eye. Eur. J. Ophthalmol. 2017, 27, 520–530. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group A n (%) | Group B n (%) | p-Value |
|---|---|---|---|
| Age (years) | 41.90 ± 12.02 | 33.00 ± 18.00 # | 0.539 * |
| Gender (%) | |||
| Male | 6 (60) | 0 (0) | 0.011 ** |
| Female | 4 (40) | 9 (100) | |
| Comorbidities (%) | |||
| Yes | 1 (10) | 1 (11.1) | 1.000 ** |
| No | 9 (90) | 8 (88.9) | |
| OSDI score | 41.79 ± 7.13 | 50.26 ± 16.24 | 0.307 *** |
| TBUT value (seconds) | 5.00 ± 2.00 | 4.33 ± 1.00 | 0.483 * |
| Ocular staining (n, %) | |||
| Grade 1 | 10 (50) | 11 (61.1) | 1.000 ** |
| Grade 2 | 10 (50) | 3 (16.7) | |
| Grade 3 | - | 4 (22.2) | |
| Schirmer values without anesthesia (mm) | 13.00 ± 12.50 | 18.89 ± 10.82 | 0.671 * |
| Group | OSDI Score (Mean ± SD) | Percentage of Decrease (%) | p-Value | |
|---|---|---|---|---|
| Before | After | |||
| A | 41.79 ± 7.13 $ | 9.42 ± 4.37 | 77.46 | 0.005 * |
| B | 50.26 ± 16.24 | 8.40 ± 2.84 | 83.29 | 0.000 ** |
| p-value | 0.307 # | 0.586 ## | ||
| Group | TBUT score (mean ± SD) | Percentage of increase (%) | p-value | |
| Before (second) | After (second) | |||
| A | 5.00 ± 2.00 $ | 7.56 ± 2.79 | 51.20 | 0.000 * |
| B | 4.33 ± 1.00 | 8.11 ± 2.42 | 87.30 | 0.000 ** |
| p-value | 0.483 # | 0.807 ## | ||
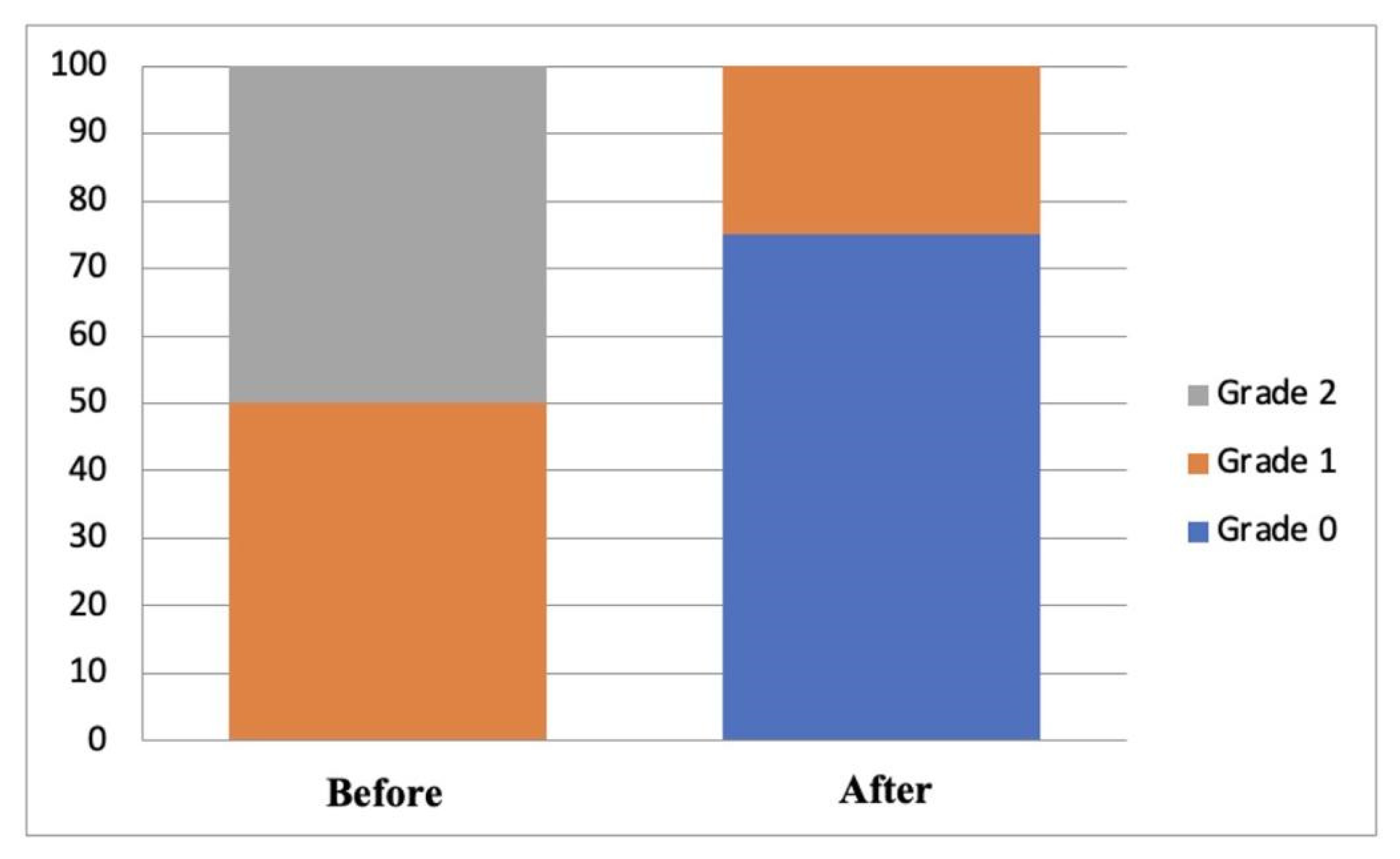
| Group | Degree of Ocular Staining (n, %) | p-Value * | |
|---|---|---|---|
| Before | After | ||
| A | |||
| Grade 0 | - | 15 (75) | 0.000 |
| Grade 1 | 10 (50) | 5 (25) | |
| Grade 2 | 10 (50) | - | |
| Grade 3 | - | - | |
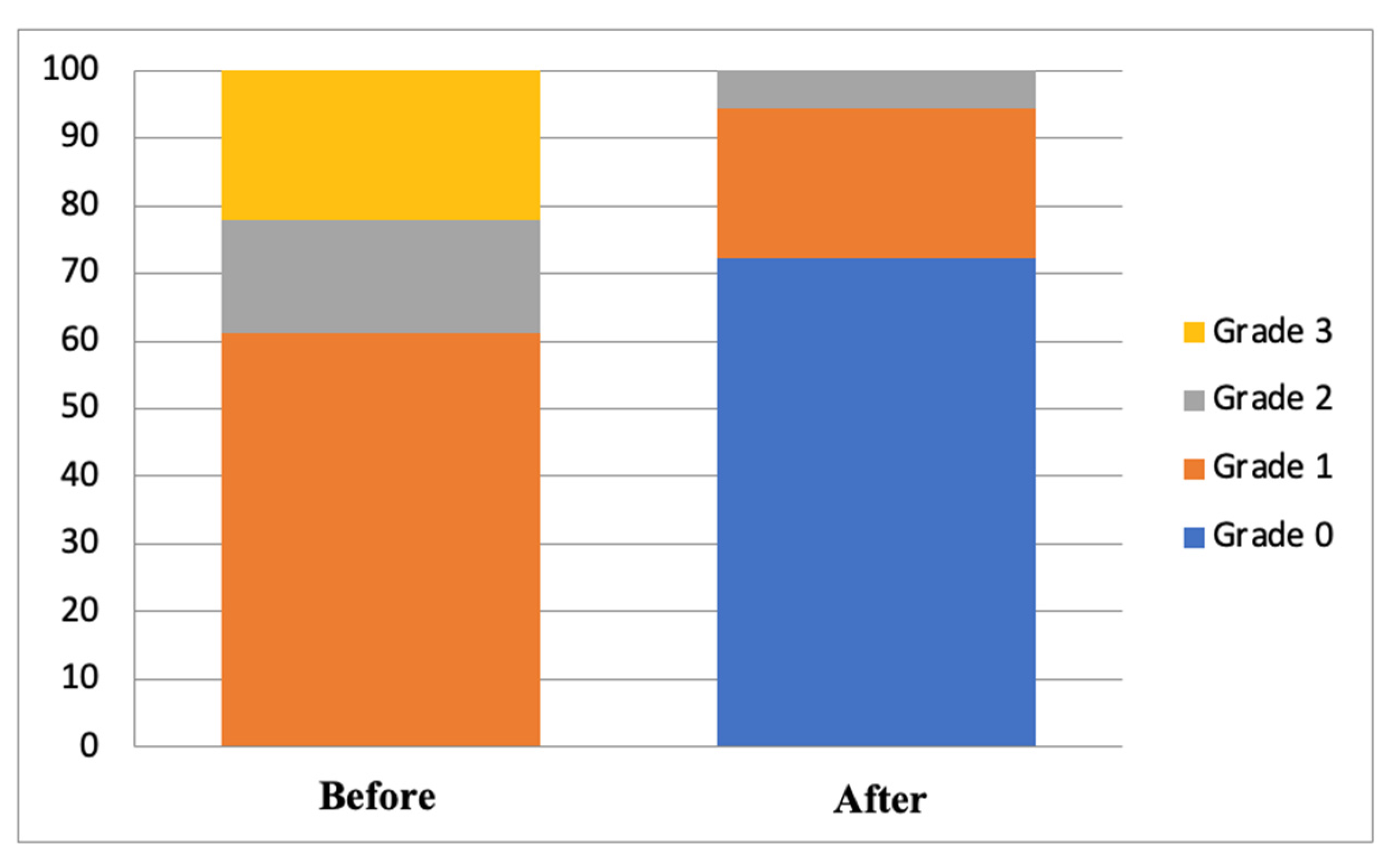
| B | |||
| Grade 0 | - | 13 (72.2) | 0.000 |
| Grade 1 | 11 (61.1) | 4 (22.2) | |
| Grade 2 | 3 (16.7) | 1 (5.6) | |
| Grade 3 | 4 (22.2) | - | |
| p-value # | 1.000 | 0.828 | |
| Group | Schirmer Scores without Anesthesia (Mean ± SD) | Percentage of Change (%) | p-Value | |
|---|---|---|---|---|
| Before (mm) | After (mm) | |||
| A | 13.00 ±12.50 $ | 17.33 ± 8.07 | 33.30 | 0.589 * |
| B | 18.89 ± 10.82 | 17.00 ± 4.24 | −10.00 | 0.740 ** |
| p-value | 0.671 # | 0.657 ## | ||
| Group | IL-6 levels (mean ± SD) | Percentage of decrease (%) | p-value ** | |
| Before (pg/mL) | After (pg/mL) | |||
| A | 82.64 ± 19.96 | 69.71 ± 21.72 | 15.66 | 0.034 |
| B | 97.65 ± 28.35 | 73.38 ± 13.97 | 24.85 | 0.002 |
| p-value ## | 0.065 | 0.545 | ||
| Group | OSDI Scores (n, %) | p-Value * | |
|---|---|---|---|
| Before | After | ||
| A | |||
| Normal | - | 7 (70) | 0.004 |
| Mild | - | 3 (30) | |
| Moderate | - | - | |
| Severe | 10 (100) | - | |
| B | |||
| Normal | - | 9 (100) | 0.004 |
| Mild | - | - | |
| Moderate | 1 (11.1) | - | |
| Severe | 8 (88.9) | - | |
| p-value # | 0.292 | 0.081 | |
| Group | TBUT values (n, %) | p-value * | |
| Before (second) | After (second) | ||
| A | |||
| Normal | - | 1 (5) | 0.000 |
| Mild | - | 11 (55) | |
| Moderate | 8 (40) | 8 (40) | |
| Severe | 12 (60) | - | |
| B | |||
| Normal | - | 6 (33.3) | 0.000 |
| Mild | - | 9 (50) | |
| Moderate | 16 (88.9) | 3 (16.7) | |
| Severe | 2 (11.1) | - | |
| p-value # | 0.002 | 0.023 | |
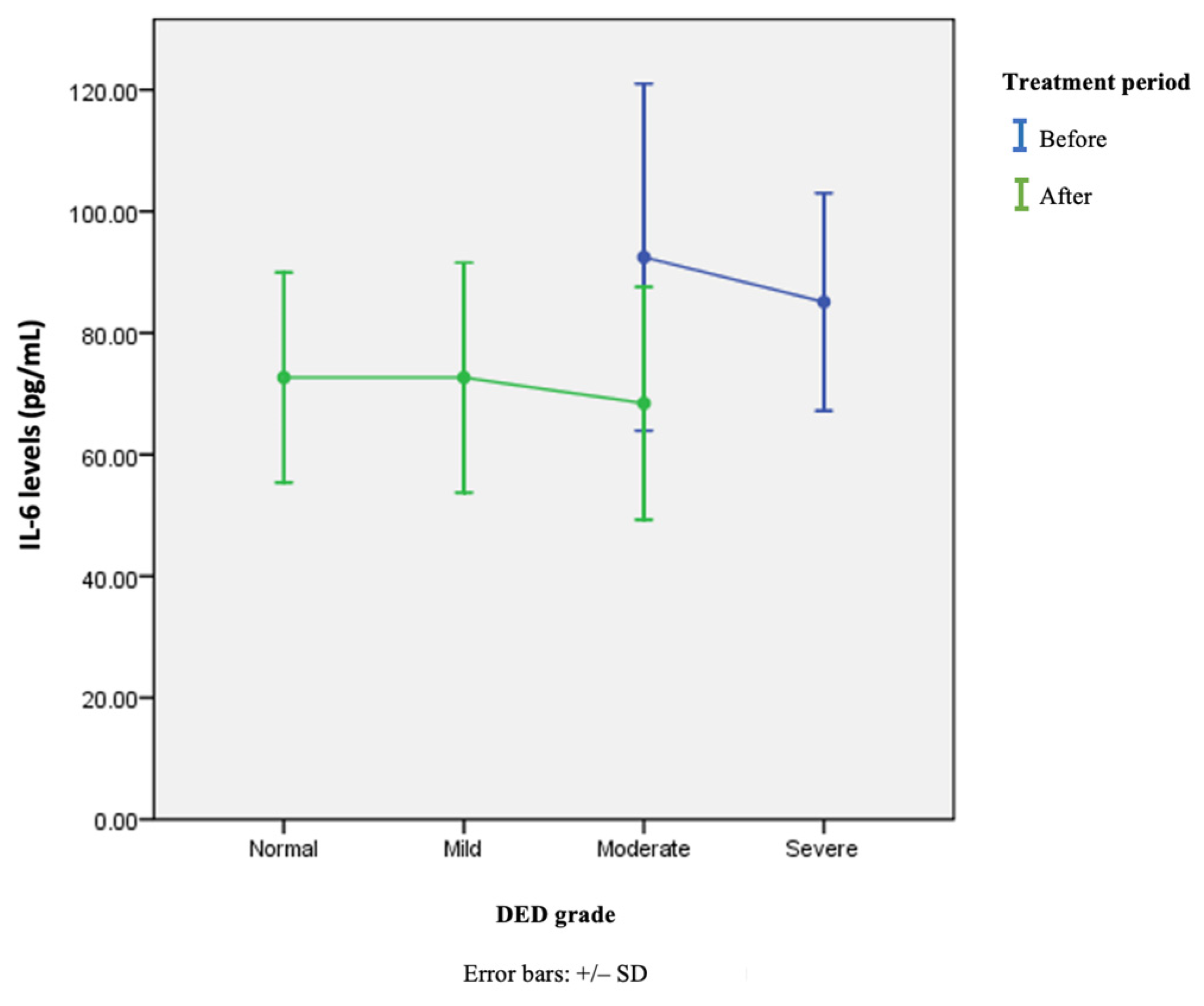
| Variable | IL-6 Levels in pg/mL (Mean ± SD) | p-Value |
|---|---|---|
| Before | ||
| Normal (n = 0) | 0 | 0.391 * |
| Mild (n = 0) | 0 | |
| Moderate (n = 24) | 92.46 ± 28.54 | |
| Severe (n = 14) | 85.10 ± 17.88 | |
| After | ||
| Normal (n = 7) | 72.68 ± 17.28 | 0.819 ** |
| Mild (n = 20) | 72.67 ± 18.92 | |
| Moderate (n = 11) | 68.44 ± 18.31 | |
| Severe (n = 0) | 0 | |
| Combined before and after treatment | ||
| Normal (n = 7) | 72.68 ± 17.28 | 0.198 ** |
| Mild (n = 20) | 72.67 ± 18.92 | |
| Moderate (n = 35) | 84.91 ± 28.05 | |
| Severe (n = 14) | 85.10 ± 17.88 |
| IL-6 (pg/mL) | ||||
|---|---|---|---|---|
| Variable | Immediate Measurement Results | Degree of Severity | ||
| p-Value | p-Value | p-Value | p-Value | |
| OSDI scores | 0.104 | 0.535 | 0.305 | 0.062 |
| TBUT value | −0.132 | 0.257 | 0.259 | 0.024 |
| Schirmer Scores | 0.156 | 0.177 | −0.098 | 0.400 |
| Ocular staining | 0.239 | 0.038 | 0.239 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priani, D.; Muhiddin, H.S.; Sirajuddin, J.; Eka, H.B.; Bahar, B.; Bukhari, A. Effectiveness of Topical Cyclosporin-A 0.1% Compared to Combined Topical Cyclosporin-A 0.1% with Topical Sodium Hyaluronate on Interleukin-6 Levels in the Tears of Patients with Dry Eye Disease. Vision 2023, 7, 31. https://doi.org/10.3390/vision7020031
Priani D, Muhiddin HS, Sirajuddin J, Eka HB, Bahar B, Bukhari A. Effectiveness of Topical Cyclosporin-A 0.1% Compared to Combined Topical Cyclosporin-A 0.1% with Topical Sodium Hyaluronate on Interleukin-6 Levels in the Tears of Patients with Dry Eye Disease. Vision. 2023; 7(2):31. https://doi.org/10.3390/vision7020031
Chicago/Turabian StylePriani, Desti, Habibah S. Muhiddin, Junaedi Sirajuddin, Hasnah B. Eka, Burhanuddin Bahar, and Agussalim Bukhari. 2023. "Effectiveness of Topical Cyclosporin-A 0.1% Compared to Combined Topical Cyclosporin-A 0.1% with Topical Sodium Hyaluronate on Interleukin-6 Levels in the Tears of Patients with Dry Eye Disease" Vision 7, no. 2: 31. https://doi.org/10.3390/vision7020031
APA StylePriani, D., Muhiddin, H. S., Sirajuddin, J., Eka, H. B., Bahar, B., & Bukhari, A. (2023). Effectiveness of Topical Cyclosporin-A 0.1% Compared to Combined Topical Cyclosporin-A 0.1% with Topical Sodium Hyaluronate on Interleukin-6 Levels in the Tears of Patients with Dry Eye Disease. Vision, 7(2), 31. https://doi.org/10.3390/vision7020031





