Central and Peripheral Ocular High-Order Aberrations and Their Relationship with Accommodation and Refractive Error: A Review
Abstract
1. Introduction
2. Central HOAs and Accommodation
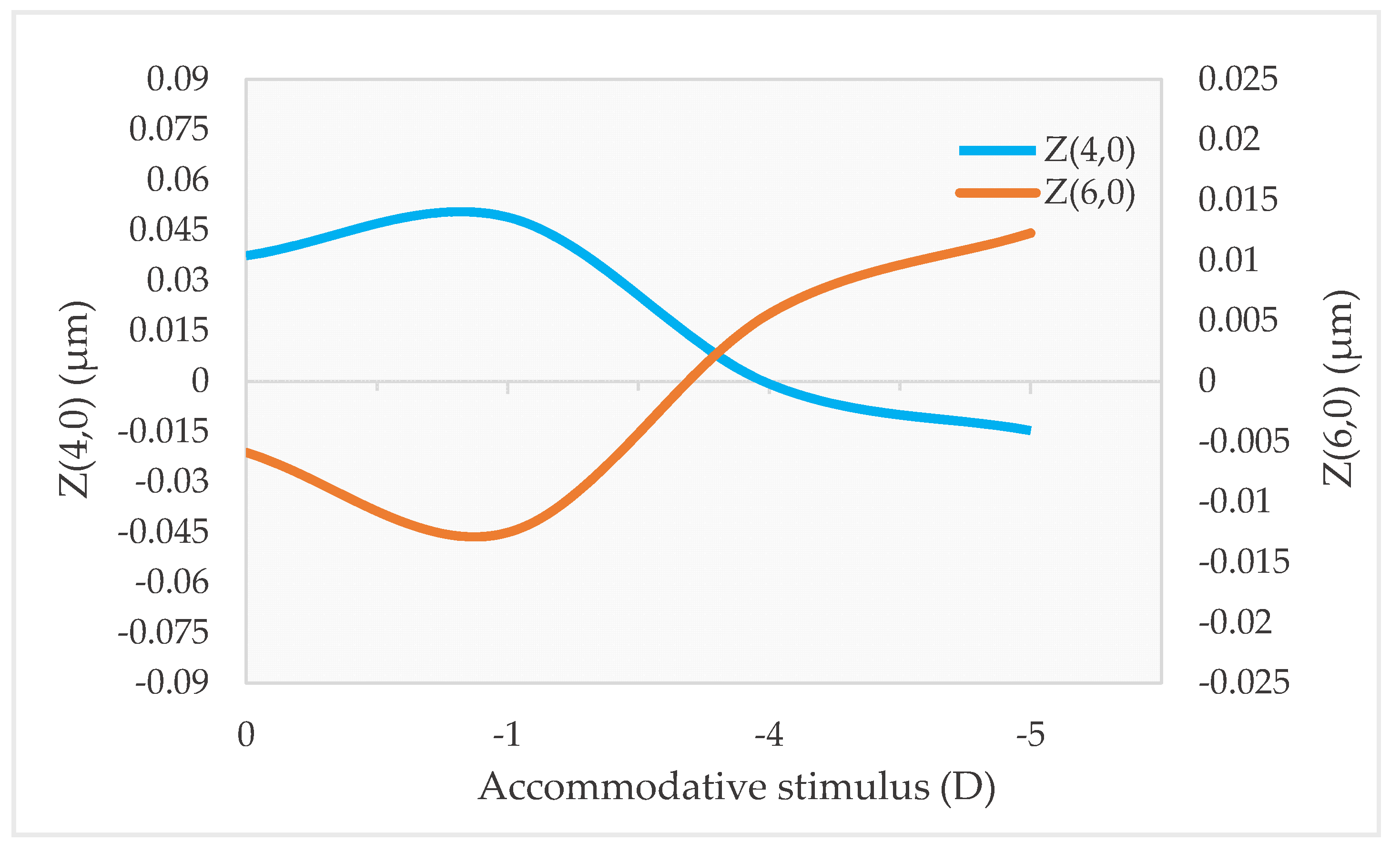
2.1. Z(4,0), Z(6,0) and Accommodation
2.2. Other Zernike Terms and Accommodation
2.3. Z(4,0), Z(6,0) and Accommodative Lag
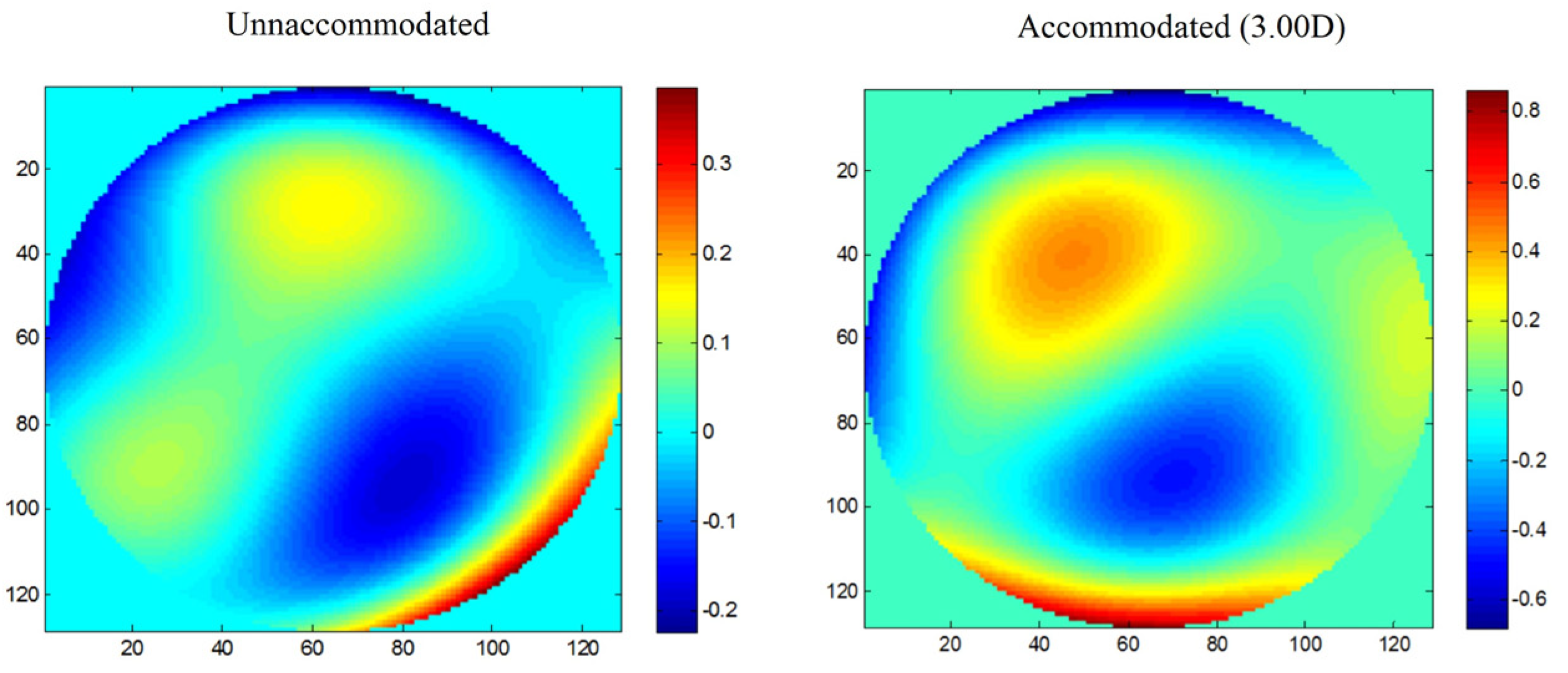
2.4. Total, Corneal, and Internal HOAs during Accommodation
3. Central HOAs and Refractive Error
3.1. Ocular HOAs and Astigmatism
3.2. Corneal and Internal HOAs and Refractive Error
3.3. HOAs in Myopic Children
3.4. HOAs, Axial Length (AL), and Myopia Progression
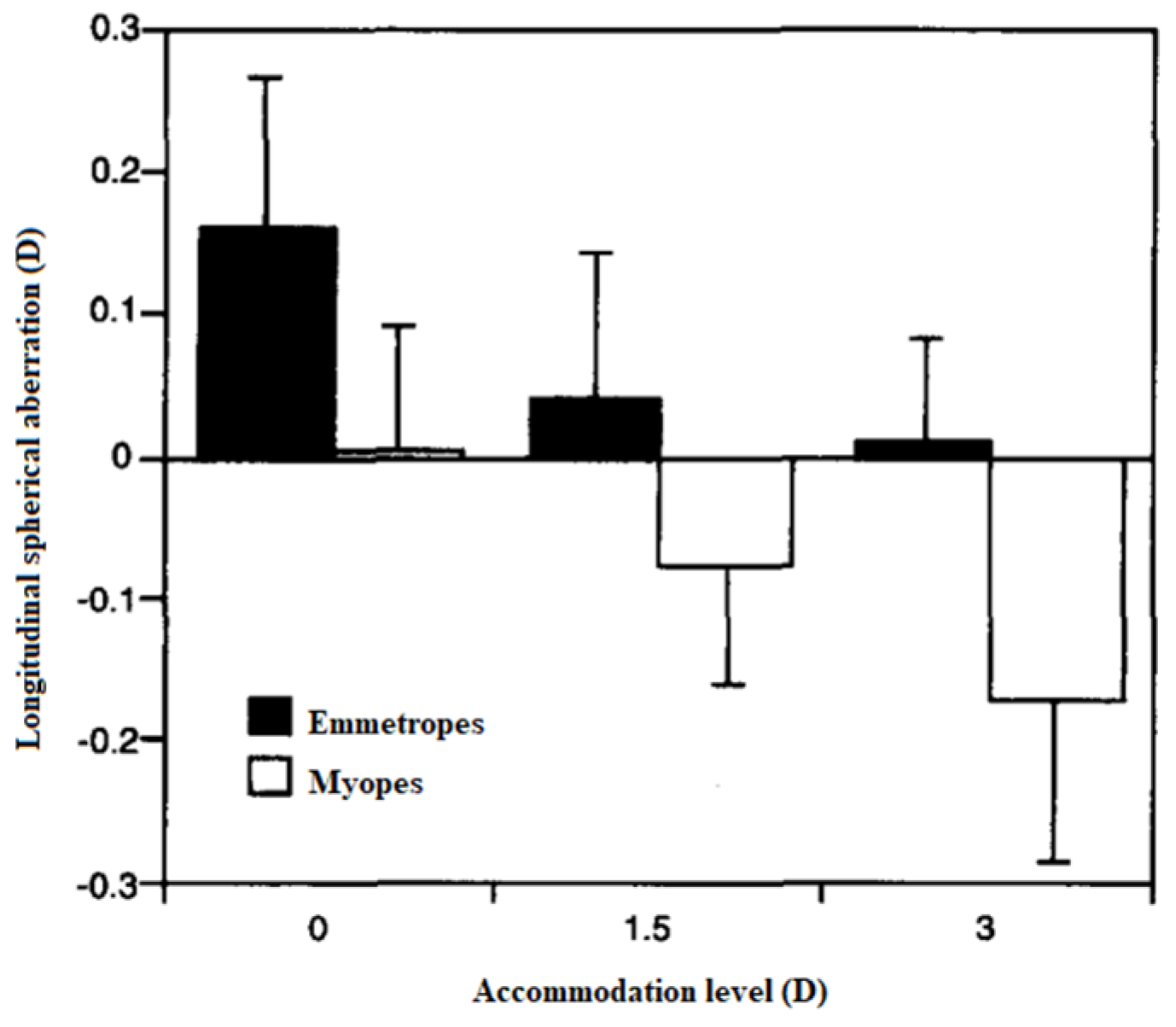
3.5. HOAs’ Changes during Accommodation and Refractive Error
4. Peripheral HOAs, Accommodation, and Refractive Error
4.1. Peripheral HOAs and Accommodation
4.2. Peripheral HOAs and Accommodation
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, M.J.; Wildsoet, C.F. Monochromatic aberrations and myopia. Vis. Res. 1995, 35, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, T.; Kakita, T.; Okamoto, F.; Oshika, T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology 2015, 122, 93–100. [Google Scholar] [CrossRef]
- Osuagwu, U.L.; Suheimat, M.; Atchison, D.A. Peripheral aberrations in adult hyperopes, emmetropes and myopes. Ophthalmic Physiol. Opt. 2017, 37, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Valentina, B.S.; Ramona, B.; Speranta, S.; Calin, T. The influence of optical aberrations in refractive surgery. Rom. J. Ophthalmol. 2015, 59, 217–222. [Google Scholar] [PubMed]
- López-Gil, N.; Rucker, F.J.; Stark, L.R.; Badar, M.; Borgovan, T.; Burke, S. and Kruger, Effect of third order aberrations on dynamic accommodation. Vis. Res. 2007, 47, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Plainis, S.; Ginis, H.S.; Pallikaris, A. The effect of ocular aberrations on steady-state errors of accommodative response. J. Vis. 2005, 5, 7. [Google Scholar] [CrossRef]
- Li, Y.J.; Choi, J.A.; Kim, H.; Yu, S.Y.; Joo, C.K. Changes in ocular wavefront aberrations and retinal image quality with objective accommodation. J. Cataract Refract. Surg. 2011, 37, 835–841. [Google Scholar] [CrossRef]
- He, J.C.; Burns, S.A. Monochromatic aberrations in the accommodated human eye. Vis. Res. 2000, 40, 41–48. [Google Scholar] [CrossRef]
- Cheng, H.; Barnett, J.K.; Vilupuru, A.S.; Marsck, J.D.; Kasthurirangan, S.; Applegate, R.A.; Roorda, A. A population study on changes in wave aberrations with accomodation. J. Vis. 2004, 4, 272–280. [Google Scholar] [CrossRef]
- Buehren, T.; Collins, M.J. Accommodation stimulus-response function and retinal image quality. Vis. Res. 2006, 46, 1633–1645. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Yuan, Y. Simultaneously measuring ocular aberration and anterior segment biometry during accommodation. J. Innov. Opt. Health Sci. 2015, 8, 1550005. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, L.; Zhou, X.T.; Yu, Z.Q. Wavefront aberration changes caused by a gradient of increasing accommodation stimuli. Eye 2015, 29, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Atchison, D.A.; Collins, M.J.; Wildsoet, C.F.; Christensen, J.; Waterworth, M.D. Measurement of monochromatic ocular aberrations of human eyes as a function of accommodation by the howland aberroscope technique. Vis. Res. 1995, 35, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Fujikado, T.; Kuroda, T.; Maeda, N.; Tano, Y.; Oshika, T.; Hirohara, Y.; Mihashi, T. Changes of ocular aberration with accommodation. Am. J. Ophthalmol. 2002, 134, 924–926. [Google Scholar] [CrossRef]
- He, J.C.; Gwiazda, J.; Thorn, F.; Held, R.; Huang, W. Change in corneal shape and corneal wave-front aberrations with accommodation. J. Vis. 2003, 3, 456–463. [Google Scholar] [CrossRef]
- Hazel, C.A.; Cox, M.J.; Strang, N.C. Wavefront aberration and its relationship to the accommodative stimulus-response function in myopic subjects. Optom. Vis. Sci. 2003, 80, 151–158. [Google Scholar] [CrossRef]
- Gicquel, J.J.; Nguyen-Khoa, J.L.; Lopez-Gil, N.; Legras, R.; Dighiero, P.; Lebuisson, D.A.; Gargasson, J.F. Optical Aberrations Variations of the Human Eye During Accommodation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1993. [Google Scholar]
- Wang, Y.; Wang, Z.Q.; Guo, H.Q.; Wang, Y.; Zuo, T. Wavefront aberrations in the accommodated human eye based on individual eye model. Optik 2007, 118, 271–277. [Google Scholar] [CrossRef]
- López-Gil, N.; Fernández-Sánchez, V.; Legras, R.; Montés-Micó, R.; Lara, F.; Nguyen-Khoa, J.L. Accommodation-related changes in monochromatic aberrations of the human eye as a function of age. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1736–1743. [Google Scholar] [CrossRef]
- López-Gil, N.; Fernández-Sánchez, V. The change of spherical aberration during accommodation and its effect on the accommodation response. J. Vis. 2010, 10, 12. [Google Scholar] [CrossRef]
- Fritzsch, M.; Dawczynski, J.; Vollandt, R.; Strobel, J. Aberrationen höherer Ordnung bei Akkommodation. Ophthalmologe 2011, 108, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shao, Y.; Tao, A.; Shen, M.; Wang, J.; Shi, G.; Chen, Q.; Zhu, D.; Lian, Y.; Qu, J.; et al. Ocular Anterior Segment Biometry and High-Order Wavefront Aberrations During Accommodation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 10. [Google Scholar] [CrossRef]
- Moreno-Barriuso, E.; Merayo Lloves, J.; Marcos, S.; Navarro, R.; Llorente, L.; Barbero, S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1396–1403. [Google Scholar]
- Theagarayan, B.; Radhakrishnan, H.; Allen, P.M.; Calver, R.I.; Rae, S.M.; O’Leary, D.J. The effect of altering spherical aberration on the static accommodative response. Ophthalmic Physiol. Opt. 2009, 29, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, Y.; Yuan, Y.; Wei, L.; Lv, F.; Zhang, Y. Measurement of ocular anterior segment dimension and wavefront aberration simultaneously during accommodation. J. Biomed. Opt. 2012, 17, 120501. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Benito, A.; Alcón, A.; Artal, P. Mechanism of compensation of aberrations in the human eye. J. Opt. Soc. Am. A 2007, 24, 3274. [Google Scholar] [CrossRef]
- Franco, S.; Gomes, J.; Gonçalves, A. Compensation effect between corneal and internal ocular aberrations during a computer task. In Proceedings of the Fourth International Conference on Applications of Optics and Photonics, Lisbon, Portugal, 31 May–4 June 2019; p. 11207. [Google Scholar]
- Khan, V.; Humayun, S.; Fawad, A.; Ishaq, M.; Arzoo, S.; Mashhadi, F. Comparison of higher order aberrations in patients with various refractive errors. Pakistan J. Med. Sci. 2015, 31, 812–815. [Google Scholar] [CrossRef]
- Martinez, A.A.; Sankaridurg, P.R.; Naduvilath, T.J.; Mitchell, P. Monochromatic aberrations in hyperopic and emmetropic children. J. Vis. 2009, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Fleck, A.; Lakshminarayanan, V.; Bobier, W.R. Ocular wavefront aberration and refractive error in pre-school children. J. Mod. Opt. 2011, 58, 1681–1689. [Google Scholar] [CrossRef]
- Yazar, S.; Hewitt, A.; Forward, H.; McKnight, C.; Tan, A.; Mountain, J.; Mackey, D. Comparison of monochromatic aberrations in young adults with different visual acuity and refractive errors. J. Cataract Refract. Surg. 2014, 40, 441–449. [Google Scholar] [CrossRef]
- Paquin, M.; Hamam, H.; Julius, F. Objective Measurement of Optical Aberrations. Optom. Vis. Sci. 2002, 79, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Karimian, F.; Feizi, S.; Doozande, A. Higher-order aberrations in myopic eyes. J. Ophthalmic Vis. Res. 2010, 5, 3–9. [Google Scholar] [PubMed]
- Li, T.; Zhou, X.; Chen, Z.; Zhou, X.; Chu, R.; Hoffman, M.R. Relationship between ocular wavefront aberrations and refractive error in Chinese school children. Clin. Exp. Optom. 2012, 95, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Little, J.A.; McCullough, S.J.; Breslin, K.M.M.; Saunders, K.J. Higher order ocular aberrations and their relation to refractive error and ocular biometry in children. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4791–4800. [Google Scholar] [CrossRef]
- Cheng, X.; Bradley, A.; Hong, X.; Thibos, L.N. Relationship between refractive error and monochromatic aberrations of the eye. Optom. Vis. Sci. 2003, 80, 43–49. [Google Scholar] [CrossRef]
- Nayak, B. Editorial Understanding the relevance of sample size calculation. Indian J. Ophthalmol. 2010, 58, 469–470. [Google Scholar] [CrossRef]
- Lim, K.L.; Hons, B.; Fam, H.B. Ethnic differences in higher-order aberrations: Spherical aberration in the South East Asian Chinese eye. J. Cart. Refract. Surg. 2009, 35, 2144–2148. [Google Scholar] [CrossRef]
- Ip, J.M.; Huynh, S.C.; Robaei, D.; Kifley, A.; Rose, K.A. Ethnic differences in refraction and ocular biometry in a year-old Australian children. Eye 2006, 22, 649–656. [Google Scholar] [CrossRef]
- Shahidi, Z.R.; Blair, N.P.; Mori, M. Optical section retinal imaging and wavefront sensing in diabetes. Optom. Vis. Sci. 2004, 81, 778–784. [Google Scholar] [CrossRef]
- Wiemer, N.G.; Dubbelman, M.; Ringens, P.J. Measuring the refractive properties of the diabetic eye during blurred vision and hyperglycaemia using aberrometry and Scheimpflug imaging. Acta Ophthalmol. 2009, 87, 176–182. [Google Scholar] [CrossRef]
- Publishers, M.; All, L. Optical quality of the diabetic eye: A review. Eye 2014, 28, 1271–1280. [Google Scholar]
- Adnan, X.; Suheimat, M.; Mathur, A.; Efron, N. Straylight, lens yellowing and aberrations of eyes in Type 1 diabetes. Biomed. Opt. Express 2015, 6, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Access, O. Refractive errors in patients with newly diagnosed diabetes mellitus. Pak. J. Med. Sci. 2015, 31, 1481–1484. [Google Scholar]
- Anbar, M.; Mohamed Mostafa, E.; Elhawary, A.M.; Awny, I.; Farouk, M.M.; Mounir, A. Evaluation of Corneal Higher-Order Aberrations by Scheimpflug-Placido Topography in Patients with Different Refractive Errors: A Retrospective Observational Study. J. Ophthalmol. 2019, 2019, 5640356. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, M.; Heidari, Z.; Mohammad-Rabei, H.; Jafarzadehpur, E.; Jabbarvand, M.; Hashemi, H.; Khabazkhoob, M. Correlation of higher order aberrations and components of astigmatism in myopic refractive surgery candidates. J Curr. Ophthalmol. 2016, 28, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Llorente, L.; Barbero, S.; Cano, D.; Dorronsoro, C.; Marcos, S. Myopic versus hyperopic eyes: Axial length, corneal shape and optical aberrations. J. Vis. 2004, 4, 288–298. [Google Scholar] [CrossRef]
- Philip, K.; Martinez, A.; Ho, A.; Conrad, F.; Ale, J.; Mitchell, P.; Sankaridurg, P. Total ocular, anterior corneal and lenticular higher order aberrations in hyperopic, myopic and emmetropic eyes. Vis. Res. 2012, 52, 31–37. [Google Scholar] [CrossRef]
- Marcos, S.; Barbero, S.; Llorente, L. The Sources Of Optical Aberrations In Myopic Eyes. ARVO Meet. Abstr. 2002, 43, 1510. [Google Scholar]
- Kirwan, C.; O’Keefe, M.; Soeldner, H. Higher-order aberrations in children. Am. J. Ophthalmol. 2006, 141, 67–70. [Google Scholar] [CrossRef]
- He, J.C.; Sun, P.; Held, R.; Thorn, F.; Sun, X.; Gwiazda, J.E. Wavefront aberrations in eyes of emmetropic and moderately myopic school children and young adults. Vis. Res. 2002, 42, 1063–1070. [Google Scholar] [CrossRef]
- Murali, M.; Vidhya, C. Original Article Pattern of wavefront aberrations in Indian children with ametropia. J. Clin. Ophthalmol. Res. 2018, 6, 117–120. [Google Scholar]
- Kwan, W.C.K.; Yip, S.P.; Yap, M.K.H. Monochromatic aberrations of the human eye and myopia. Clin. Exp. Optom. 2009, 92, 304–312. [Google Scholar] [CrossRef]
- Carkeet, A.; Luo, D.; Tong, L.; Saw, S.M.; Tan, D.T.H. Refractive error and monochromatic aberrations in Singaporean children. Vis. Res. 2002, 42, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Coletta, N.J.; Marcos, S.; Troilo, D. Wavefront Aberrations of the Eye during the Development of Refractive Error. Front. Opt. 2014, 4, 2. [Google Scholar]
- Zhang, N.; Yang, X.B.; Zhang, W.Q.; Liu, L.-Q.; Dong, G.-J.; Chen, T.-W.; Liao, M.; Liao, X. Relationship between higher-order aberrations and myopia progression in schoolchildren: A retrospective study. Int. J. Ophthalmol. 2013, 6, 295–299. [Google Scholar] [PubMed]
- Philip, K.; Sankaridurg, P.; Holden, B.; Ho, A.; Mitchell, P. Influence of higher order aberrations and retinal image quality in myopisation of emmetropic eyes. Vis. Res. 2014, 105, 233–243. [Google Scholar] [CrossRef]
- Lau, J.K.; Vincent, S.J.; Collins, M.J.; Cheung, S.W.; Cho, P. Ocular higher-order aberrations and axial eye growth in young Hong Kong children. Sci. Rep. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Hiraoka, T.; Kotsuka, J.; Kakita, T.; Okamoto, F.; Oshika, T. Relationship between higher-order wavefront aberrations and natural progression of myopia in schoolchildren. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Collins, M.J.; Buehren, T.; Iskander, D.R. Iskander. Retinal image quality, reading and myopia. Vis. Res. 2006, 46, 196–215. [Google Scholar] [CrossRef]
- Lundström, L.; Rosén, R.; Baskaran, K.; Jaeken, B.; Gustafsson, J.; Artal, P.; Unsbo, P. Symmetries in peripheral ocular aberrations. J. Mod. Opt. 2011, 58, 1690–1695. [Google Scholar] [CrossRef]
- Navarro, R.; Moreno, E.; Dorronsoro, C. Monochromatic aberrations and point-spread functions of the human eye across the visual field. J. Opt. Soc. Am. A 1998, 15, 2522. [Google Scholar] [CrossRef]
- Atchison, D.A.; Scott, D.H. Monochromatic aberrations of human eyes in the horizontal visual field. J. Opt. Soc. Am. 2002, 19, 2180–2184. [Google Scholar] [CrossRef]
- Atchison, D.A. Higher order aberrations across the horizontal visual field. J. Biomed. Opt. 2006, 11, 034026. [Google Scholar] [CrossRef]
- Lundström, L.; Gustafsson, J.; Unsbo, P. Population distribution of wavefront aberrations in the peripheral human eye. J. Opt. Soc. Am. A 2009, 26, 2192–2198. [Google Scholar] [CrossRef]
- Atchison, D.A.; Mathur, A.; Read, S.A.; Walker, M.I.; Newman, A.R.; Tanos, P.P.; McLennan, R.T.; Tran, A.H. Peripheral ocular aberrations in mild and moderate keratoconus. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6850–6857. [Google Scholar] [CrossRef]
- Ma, L.; Atchison, D.; Charman, W.N. Off-axis refraction and aberrations following conventional laser in situ keratomileusis. J. Cataract Refract. Surg. 2005, 31, 489–498. [Google Scholar] [CrossRef]
- Mathur, A.; Atchison, D. Influence of spherical intraocular lens implantation and conventional laser in situ keratomileusis on peripheral ocular aberrations. J. Cataract Refract. Surg. 2010, 36, 1127–1134. [Google Scholar] [CrossRef]
- Romashchenko, D.; Papadogiannis, P.; Unsbo, P.; Lundström, L. Simultaneous measurements of foveal and peripheral aberrations with accommodation in myopic and emmetropic eyes. Biomed. Opt. Express 2021, 12, 7422. [Google Scholar] [CrossRef]
- Mathur, A.; Atchison, D.A.; Charman, W.N. Effect of accommodation on peripheral ocular aberrations. J. Vis. 2009, 9, 20. [Google Scholar] [CrossRef]
- Lundström, L.; Mira-Agudelo, A.; Artal, P. Peripheral optical errors and their change with accommodation differ between emmetropic and myopic eyes. J. Vis. 2009, 9, 17. [Google Scholar] [CrossRef]
- Sapkota, K.; Gomes, J.; Franco, S. Effect of Accommodation on Peripheral Higher Order Aberrations. Photonics 2021, 9, 64. [Google Scholar] [CrossRef]
- Mathur, A.; Atchison, D.A.; Charman, W.N. Charman. Myopia and peripheral ocular aberrations. J. Vis. 2009, 9, 15. [Google Scholar]
- Fedtke, C.; Ehrmann, K.; Falk, D.; Bakaraju, R.C.; Holden, B.A. The BHVI-eyemapper: Peripheral refraction and aberration profiles. Optom. Vis. Sci. 2014, 91, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Philip, K.; Sankaridurg, P.R.; Ale, J.B.; Naduvilath, T.J.; Mitchell, P. Profile of off-axis higher order aberrations and its variation with time among various refractive error groups. Vis. Res. 2018, 153, 111–123. [Google Scholar] [CrossRef]



| Author (Year) | Eyes | Age (Years) | Refractive State (D) | Accommodative Stimulus (D) | Accommodation Stimulation Method | Measurement Method |
|---|---|---|---|---|---|---|
| Atchison et al. (1995) [13] | 15 | 0D, 1.5D and 3.00D | Aberroscope | |||
| He et al. (2000) [8] | 8 | 24–38 | SEQ: −2.00D–−5.56D | Between 0 and 6.00D in steps of 1.00D | Spatially resolved refractometer | |
| Ninomiya et al. (2002) [14] | 33 | 28.7 ± 4.4 | 0 and 3.00D | TDV | S-H | |
| He et al. (2003) [15] | 12 | 23–32 | SEQ: 0–−3.00D; Cil ≤ 0.25D | 0.25D and 5.00D | TDV | CTS |
| Hazel et al. (2003) [16] | 30 | 18–27 | SEQ: +0.50D–−6.00D; Cil < 0.50D | Between 0 and 4.00D in steps of 1.00D | NSL | S-H |
| Cheng et al. (2004) [9] | 76 | 21–40 | Sphere: +1.25D–−8.25D; Cil: −0.25D–−2.75D | 0, 3.00D and 6.00D | TDV | S-H |
| Gicquel et al. (2005) [17] | 28 | 20–25 | SEQ: −2.00D–+1.00D | Between 1.00D and 5.00D in steps of 0.50D | TDV | S-H |
| Plainis et al. (2005) [6] | 7 | 23–33 | Emmetropes; Myopes with sphere: −2.00D–−2.50D | Between 0D and 8.00D in steps of 1.00D | TDV | S-H |
| Buehren et al. (2006) [10] | 10 | 22–36 | Sphere: emmetropes +0.05D ± 0.19D; myopes −2.25D ± 0.85D Cil for both: −0.30D ± 0.45D | 0.17D, 1.00D, 2.00D, 3.00D, 4.00D and 5.00D | TDV | S-H |
| Wang et. al (2007) [18] | 20 | 18–32 | Sphere: −6.00D–+3.00D | Between 0 and −4.00D in steps of 1.00D | TDV | S-H |
| López-Gil et al. (2008) [19] | 24 | 19–29 | Sphere: −3.00D–+3.00D; Cil <1.00D | Between 0 and 5.00D in steps of 0.50D | TDV | S-H |
| López-Gil et al. (2010) [20] | 15 | 20–38 | Sphere: 0.38D–−3.06D; Cil: −0.38D ± 0.25D | Between 0.50D and 9.50D in steps of 0.50D | Badal | S-H |
| Fritzsch et. al (2011) [21] | 25 | 15–27 | Emmetropes | 0.22D and 5.00D | TDV | S-H |
| Yuan et al. (2013) [22] | 35 | 20–33 | SEQ: +0.50D–−2.38D; Cil <0.75D | 0.25D and 3.00D | TDV | S-H |
| Zhou et al. (2015) [12] | 22 | 18–28 | Sphere: 0D–−1.00D; Cil: −0.75D and 0D | Between 1.00D and 6.00D in steps of 1.00D | NSL | S-H |
| Wang et al. (2015) [11] | 10 | 0 and 3.00D | TDV | S-H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, J.; Sapkota, K.; Franco, S. Central and Peripheral Ocular High-Order Aberrations and Their Relationship with Accommodation and Refractive Error: A Review. Vision 2023, 7, 19. https://doi.org/10.3390/vision7010019
Gomes J, Sapkota K, Franco S. Central and Peripheral Ocular High-Order Aberrations and Their Relationship with Accommodation and Refractive Error: A Review. Vision. 2023; 7(1):19. https://doi.org/10.3390/vision7010019
Chicago/Turabian StyleGomes, Jessica, Kishor Sapkota, and Sandra Franco. 2023. "Central and Peripheral Ocular High-Order Aberrations and Their Relationship with Accommodation and Refractive Error: A Review" Vision 7, no. 1: 19. https://doi.org/10.3390/vision7010019
APA StyleGomes, J., Sapkota, K., & Franco, S. (2023). Central and Peripheral Ocular High-Order Aberrations and Their Relationship with Accommodation and Refractive Error: A Review. Vision, 7(1), 19. https://doi.org/10.3390/vision7010019






