Flash Electroretinography as a Measure of Retinal Function in Myopia and Hyperopia: A Systematic Review
Abstract
1. Introduction
1.1. Mechanisms Controlling Eye Growth and the Development of Refractive Errors
1.2. Electrophysiology as a Technique for Understanding the Role of Retinal Cells in Human Refractive Errors
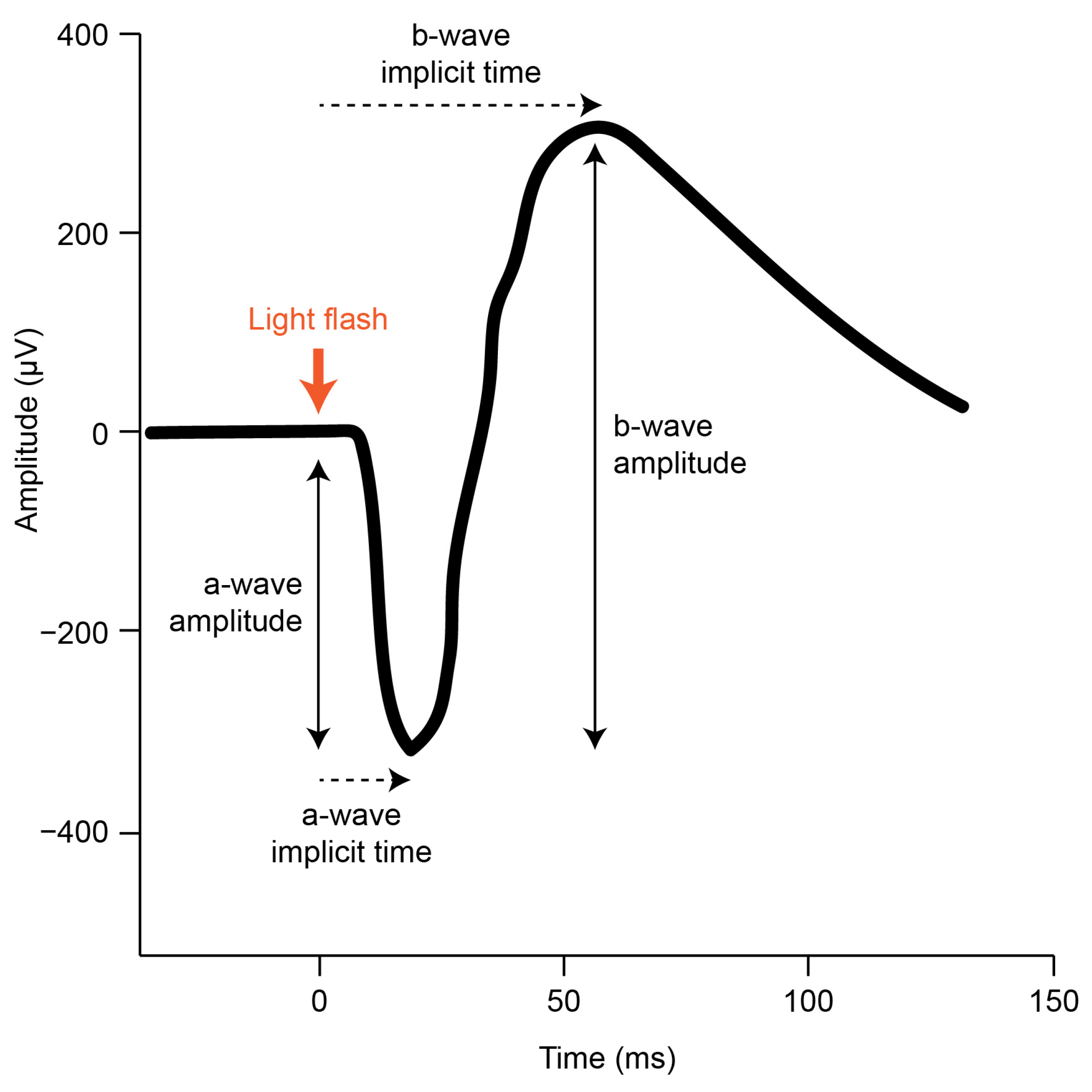
1.3. Using the gfERG to Functionally Dissect Retinal Activity
1.4. Rationale and Aim of the Current Systematic Review
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias Assessment
3. Results
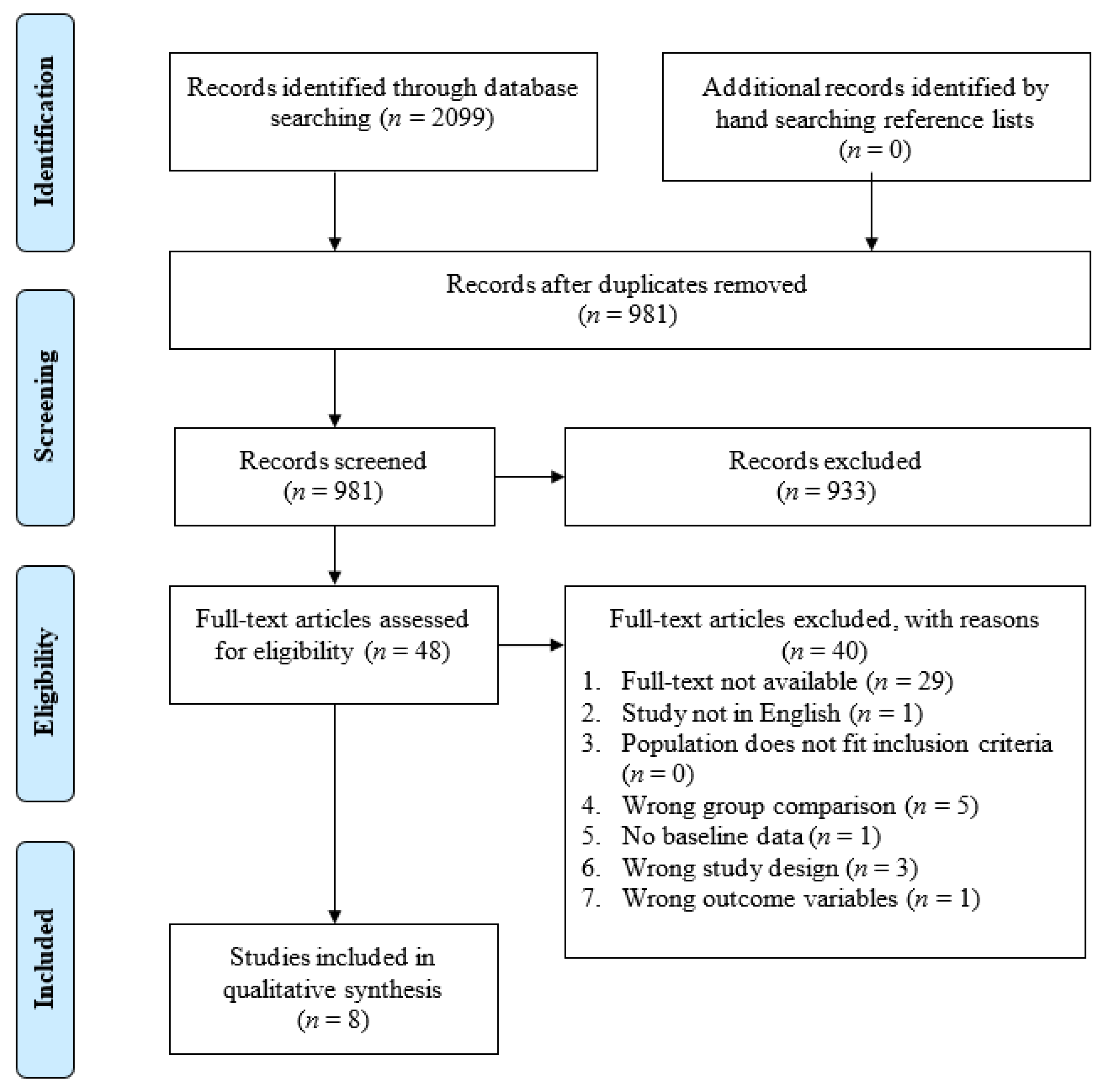
3.1. Study Selection
3.2. Risk of Bias Assessment
3.3. Study Characteristics
3.4. Effect of Refractive Errors on the gfERG Waveform
3.5. Effect of Myopia on the gfERG
3.6. Effect of Hyperopia on the ERG
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baird, P.N.; Saw, S.M.; Lanca, C.; Guggenheim, J.A.; Smith Iii, E.L.; Zhou, X.; Matsui, K.O.; Wu, P.C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Salim, A.T.; Praveen, A.; Fonseka, D.; Ting, D.S.W.; He, M.G.; Bourne, R.R.; Crowston, J.; Wong, T.Y.; Dirani, M. Association between digital smart device use and myopia: A systematic review and meta-analysis. Lancet Digit. Health 2021, 3, e806–e818. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, B.S.; Abbott, R.L.; Fong, D.S.; Lum, F.; Tan, D.; Ang, M.; Chiarito, S.; Cotter, S.A.; Fernandez, A.M.; Grzybowski, A. Reducing the global burden of myopia by delaying the onset of myopia and reducing myopic progression in children: The Academy’s Task Force on Myopia. Ophthalmology 2021, 128, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Luensmann, D. The prevalence and impact of high myopia. Eye Contact Lens 2012, 38, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog. Retin. Eye Res. 2012, 31, 622–660. [Google Scholar] [CrossRef]
- Arden, G.B.; Vaegan; Hogg, C.R. Clinical and experimental evidence that the pattern electroretinogram (PERG) is generated in more proximal retinal layers than the focal electroretinogram (FERG). Ann. N. Y. Acad. Sci. 1982, 388, 580–607. [Google Scholar] [CrossRef]
- Rose, K.A.; French, A.N.; Morgan, I.G. Environmental factors and myopia: Paradoxes and prospects for prevention. Asia-Pac. J. Ophthalmol. 2016, 5, 403–410. [Google Scholar] [CrossRef]
- Lanca, C.; Yam, J.C.; Jiang, W.J.; Tham, Y.C.; Hassan Emamian, M.; Tan, C.S.; Guo, Y.; Liu, H.; Zhong, H.; Zhu, D. Near work, screen time, outdoor time and myopia in schoolchildren in the Sunflower Myopia AEEC Consortium. Acta Ophthalmol. 2022, 100, 302–311. [Google Scholar] [CrossRef]
- Seet, B.; Wong, T.Y.; Tan, D.T.; Saw, S.M.; Balakrishnan, V.; Lee, L.K.; Lim, A.S. Myopia in Singapore: Taking a public health approach. Br. J. Ophthalmol. 2001, 85, 521–526. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Lo, C.-T.; Sheu, S.-J.; Lin, J.L. What factors are associated with myopia in young adults? A survey study in Taiwan Military Conscripts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Wildsoet, C.F.; Chia, A.; Cho, P.; Guggenheim, J.A.; Polling, J.R.; Read, S.; Sankaridurg, P.; Saw, S.-M.; Trier, K.; Walline, J.J. IMI–interventions for controlling myopia onset and progression report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M106–M131. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Ang, M.; Cho, P.; Guggenheim, J.A.; He, M.G.; Jong, M.; Logan, N.S.; Liu, M.; Morgan, I.; Ohno-Matsui, K. IMI prevention of myopia and its progression. Investig. Ophthalmol. Vis. Sci. 2021, 62, 6. [Google Scholar] [CrossRef] [PubMed]
- Wildsoet, C.F. Neural pathways subserving negative lens-induced emmetropization in chicks–insights from selective lesions of the optic nerve and ciliary nerve. Curr. Eye Res. 2003, 27, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Troilo, D.; Gottlieb, M.D.; Wallman, J. Visual deprivation causes myopia in chicks with optic nerve section. Curr. Eye Res. 1987, 6, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Troilo, D.; Smith, E.L.; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.-S. IMI–Report on experimental models of emmetropization and myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, Z.; Soorma, T.; Tariq, A.; Bhatti, T.; Baneke, A.J.; Pontikos, N.; Leo, S.M.; Webster, A.R.; Williams, K.M. Electrical responses from human retinal cone pathways associate with a common genetic polymorphism implicated in myopia. Proc. Natl. Acad. Sci. USA 2022, 119, e2119675119. [Google Scholar] [CrossRef]
- Crewther, D.P. The role of photoreceptors in the control of refractive state. Prog. Retin. Eye Res. 2000, 19, 421–457. [Google Scholar] [CrossRef]
- Crewther, S.G.; Liang, H.; Junghans, B.M.; Crewther, D.P. Ionic control of ocular growth and refractive change. Proc. Natl. Acad. Sci. USA 2006, 103, 15663–15668. [Google Scholar] [CrossRef]
- Crewther, D.P.; Crewther, S.G.; Xie, R.Z. Changes in eye growth produced by drugs which affect retinal ON or OFF responses to light. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 1996, 12, 193–208. [Google Scholar] [CrossRef]
- Crewther, S.G.; Crewther, D.P. Inhibition of retinal ON/OFF systems differentially affects refractive compensation to defocus. Neuroreport 2003, 14, 1233–1237. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef]
- Barrington, M.; Sattayasai, J.; Zappia, J.; Ehrlich, D. Excitatory amino acids interfere with normal eye growth in posthatch chick. Curr. Eye Res. 1989, 8, 781–792. [Google Scholar] [CrossRef]
- Ehrlich, D.; Sattayasai, J.; Zappia, J.; Barrington, M. Effects of selective neurotoxins on eye growth in the young chick. Ciba Found. Symp. 1990, 155, 63–84; discussion 84–68. [Google Scholar]
- McBrien, N.A.; Moghaddam, H.O.; Cottriall, C.L.; Leech, E.M.; Cornell, L.M. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vision Res. 1995, 35, 1141–1152. [Google Scholar] [CrossRef]
- Norton, T.T.; Essinger, J.A.; McBrien, N.A. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis. Neurosci. 1994, 11, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Wildsoet, C.F.; Pettigrew, J.D. Kainic acid-induced eye enlargement in chickens: Differential effects on anterior and posterior segments. Investig. Ophthalmol. Vis. Sci. 1988, 29, 311–319. [Google Scholar]
- Murphy, M.; Crewther, S. Ouabain inhibition of Na/K-ATPase across the retina prevents signed refractive compensation to lens-induced defocus, but not default ocular growth in young chicks. F1000 Res. 2013, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Tedja, M.S.; Wojciechowski, R.; Hysi, P.G.; Eriksson, N.; Furlotte, N.A.; Verhoeven, V.J.; Iglesias, A.I.; Meester-Smoor, M.A.; Tompson, S.W.; Fan, Q. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat. Genet. 2018, 50, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Eggenberger, E.; Kaufman, D. Current electrophysiology in ophthalmology: A review. Curr. Opin. Ophthalmol. 2012, 23, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.G.; Frishman, L.J.; Grigg, J.; Hamilton, R.; Jeffrey, B.G.; Kondo, M.; Li, S.; McCulloch, D.L. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc. Ophthalmol. 2022, 144, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, J.; Shen, M.; Yao, A.; Xue, A.; Fan, Y.; Huang, S.; Wang, J.; Lu, F.; Shao, Y. Photoreceptor degeneration is correlated with the deterioration of macular retinal sensitivity in high myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Heckenlively, J.R.; Arden, G.B. Principles and Practice of Clinical Electrophysiology of Vision; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Creel, D.J. Electroretinograms. Handb. Clin. Neurol. 2019, 160, 481–493. [Google Scholar] [PubMed]
- Wachtmeister, L. Oscillatory potentials in the retina: What do they reveal. Prog. Retin. Eye Res. 1998, 17, 485–521. [Google Scholar] [CrossRef]
- Fujikado, T.; Hosohata, J.; Omoto, T. ERG of form deprivation myopia and drug induced ametropia in chicks. Curr. Eye Res. 1996, 15, 79–86. [Google Scholar] [CrossRef]
- Fujikado, T.; Kawasaki, Y.; Suzuki, A.; Ohmi, G.; Tano, Y. Retinal function with lens-induced myopia compared with form-deprivation myopia in chicks. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 320–324. [Google Scholar] [CrossRef]
- Riddell, N.; Murphy, M.J.; Crewther, S.G. Electroretinography and gene expression measures implicate phototransduction and metabolic shifts in chick myopia and hyperopia models. Life 2021, 11, 501. [Google Scholar] [CrossRef]
- Westbrook, A.M.; Crewther, D.P.; Crewther, S.G. Cone receptor sensitivity is altered in form deprivation myopia in the chicken. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 1999, 76, 326–338. [Google Scholar] [CrossRef]
- Wan, W.; Chen, Z.; Lei, B. Increase in electroretinogram rod-driven peak frequency of oscillatory potentials and dark-adapted responses in a cohort of myopia patients. Doc. Ophthalmol. 2020, 140, 189–199. [Google Scholar] [CrossRef]
- Westall, C.A.; Dhaliwal, H.S.; Panton, C.M.; Sigesmund, D.; Levin, A.V.; Nischal, K.K.; Héon, E. Values of electroretinogram responses according to axial length. Doc. Ophthalmol. 2001, 102, 115–130. [Google Scholar] [CrossRef]
- Perlman, I.; Meyer, E.; Haim, T.; Zonis, S. Retinal function in high refractive error assessed electroretinographically. Br. J. Ophthalmol. 1984, 68, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Blach, R.; Jay, B.; Kolb, H. Electrical activity of the eye in high myopia. Br. J. Ophthalmol. 1966, 50, 629. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.; Adams, G.; Robson, A.; Holder, G. Retinal dysfunction and refractive errors: An electrophysiological study of children. Br. J. Ophthalmol. 2005, 89, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Crowther, M.; Lim, W.; Crowther, M.A. Systematic review and meta-analysis methodology. Blood J. Am. Soc. Hematol. 2010, 116, 3140–3146. [Google Scholar] [CrossRef]
- Office of Health Assessment and Translation. OHAT Risk of Bias Tool for Human and Animal Studies. Available online: http://ntp.niehs.nih.gov/go/38673 (accessed on 1 July 2022).
- NHMRC. Guidelines for Guidelines: Assessing Risk of Bias. Available online: https://nhmrc.gov.au/guidelinesforguidelines/develop/assessing-risk-bias (accessed on 1 July 2022).
- Kennet, R.; Meyer, E.; Perlman, I. Visual function in hypermetropia. Doc. Ophthalmol. 1993, 84, 47–59. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nitta, K.; Kamiyama, M. Cone electroretinogram to chromatic stimuli in myopic eyes. Vision Res. 1997, 37, 2157–2159. [Google Scholar] [CrossRef]
- Sachidanandam, R.; Ravi, P.; Sen, P. Effect of axial length on full-field and multifocal electroretinograms. Clin. Exp. Optom. 2017, 100, 668–675. [Google Scholar] [CrossRef]
- Perlman, I. Relationship between the amplitudes of the b wave and the a wave as a useful index for evaluating the electroretinogram. Br. J. Ophthalmol. 1983, 67, 443–448. [Google Scholar] [CrossRef]
- Perlman, I. The Electroretinogram: ERG. In Webvision The Organization of the Retina and Visual System; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2007. [Google Scholar]
- Sundin, O.H.; Leppert, G.S.; Silva, E.D.; Yang, J.-M.; Dharmaraj, S.; Maumenee, I.H.; Santos, L.C.; Parsa, C.F.; Traboulsi, E.I.; Broman, K.W. Extreme hyperopia is the result of null mutations in MFRP, which encodes a Frizzled-related protein. Proc. Natl. Acad. Sci. USA 2005, 102, 9553–9558. [Google Scholar] [CrossRef] [PubMed]
- Castagno, V.D.; Fassa, A.G.; Carret, M.L.V.; Vilela, M.A.P.; Meucci, R.D. Hyperopia: A meta-analysis of prevalence and a review of associated factors among school-aged children. BMC Ophthalmol. 2014, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Na Park, H.; Hanif, A.M.; Sidhu, C.S.; Iuvone, P.M.; Pardue, M.T. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp. Eye Res. 2015, 137, 79–83. [Google Scholar] [CrossRef]
- Beresford, J.A.; Crewther, S.G.; Kiely, P.M.; Crewther, D.P. Comparison of refractive state and circumferential morphology of retina, choroid, and sclera in chick models of experimentally induced ametropia. Optom. Vis. Sci. 2001, 78, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Crewther, D.; Crewther, S.G.; Barila, A. A role for photoreceptor outer segments in the induction of deprivation myopia. Vision Res. 1995, 35, 1217–1225. [Google Scholar] [CrossRef]
- Zhong, X.; Ge, J.; Chen, X.; Nie, H.; Huang, J. Comparison of retinal morphology and ultrastructure in defocus-induced myopia and form-deprivation myopia in rhesus monkeys. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2005, 41, 625–630. [Google Scholar]
- Smith, E.L., 3rd; Fox, D.A.; Duncan, G.C. Refractive-error changes in kitten eyes produced by chronic on-channel blockade. Vision Res. 1991, 31, 833–844. [Google Scholar] [CrossRef]
- Kergoat, H.; Kergoat, M.J.; Justino, L. Age-Related Changes in the Flash Electroretinogram and Oscillatory Potentials in Individuals Age 75 and Older. J. Am. Geriatr. Soc. 2001, 49, 1212–1217. [Google Scholar] [CrossRef]
- Weleber, R.G. The effect of age on human cone and rod ganzfeld electroretinograms. Investig. Ophthalmol. Vis. Sci. 1981, 20, 392–399. [Google Scholar]
- Hrynchak, P.K.; Mittelstaedt, A.; Machan, C.M.; Bunn, C.; Irving, E.L. Increase in myopia prevalence in clinic-based populations across a century. Optom. Vis. Sci. 2013, 90, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
| Search Terms | Search Results |
|---|---|
| 1. myopi* OR short-sightedness OR near-sightedness OR hyperopi* OR long-sightedness OR far-sightedness OR emmetrop* OR “refractive error” OR “refractive adaptation” OR “refractive compensation” OR “refractive status” OR “refractive state” | 37,353 |
| 2. “retinal functioning” OR “retinal neurophysiology” OR electroretinog* OR ERG OR electrophysiology | 139,612 |
| 1 AND 2 | 754 |
| Order | Reason for Exclusion |
|---|---|
| 1 | Full text not available (e.g., conference abstract) |
| 2 | Study not in English |
| 3 | Population does not fit inclusion criteria (e.g., non-human animals, or congenital stationary night blindness) |
| 4 | Wrong group comparison (i.e., does not compare ERG measures between different refractive error groups) |
| 5 | Intervention study with no baseline data |
| 6 | Wrong study design (e.g., case studies) |
| 7 | Wrong outcomes variables |
| Categories | Variables Extracted |
|---|---|
| Demographics | Sample size (N), sex (F, M), and age (M, SD, range) |
| Refractive error characteristics | Refractive error (M, SD, range) and presence/absence of secondary pathology |
| gfERG characteristics | Study design, stimulus (i.e., ISCEV photopic and scotopic flash), adaptation state (duration (mins) of dark-adaptation and light-adaptation), and changes in the amplitude and implicit time of all reported waves |
| No. | Questions Assessing Risk of Bias |
|---|---|
| 1 | Was administration dose or exposure level adequately randomized? |
| 2 | Was allocation to study adequately concealed? |
| 3 | Did selection of study participants result in appropriate comparison groups? |
| 4 | Did the study design or analysis account for important confounding and modifying variables? |
| 5 | Were experimental conditions identical across study groups? |
| 6 | Were the research personnel and human subjects blinded to the study group during the study? |
| 7 | Were outcome data complete without attrition or exclusion from analysis? |
| 8 | Can we be confident in the exposure characterization? |
| 9 | Can we be confident in the outcome assessment? |
| 10 | Were all measured outcomes reported? |
| 11 | Were there no other potential threats to internal validity? |
| Citation | Q3 | Q4 | Q6 | Q7 | Q8 | Q9 | Q10 |
|---|---|---|---|---|---|---|---|
| Blach et al., 1966 [43] |  |  |  |  |  |  |  |
| Flitcroft et al., 2005 [44] |  |  |  |  |  |  |  |
| Kennet et al., 1993 [50] |  |  |  |  |  |  |  |
| Perlman et al., 1984 [42] |  |  |  |  |  |  |  |
| Sachidanandam et al., 2017 [52] |  |  |  |  |  |  |  |
| Wan et al., 2020 [40] |  |  |  |  |  |  |  |
| Westall et al., 2001 [41] |  |  |  |  |  |  |  |
| Yamamoto et al., 1997 [51] |  |  |  |  |  |  |  |
 definitely low
definitely low  probably low
probably low  probably high
probably high  definitely high.
definitely high. | Study | Demographics | Refractive Error Characteristics | ERG Characteristics | ||||
|---|---|---|---|---|---|---|---|
| Sample Sizes: N (Sex: M, F) | Age: M (SD), Range | Refractive Error: M (SD), Range | Secondary Pathology | Study Design | Stimulus | Adaptation State | |
| Blach et al., 1966 [43] | My: 35 Em: 25 | My: >10 y | My: −2 to −27 D | My: all show chorioretinal degeneration | Between groups | Photopic and scotopic square-wave flash | DA: 20 min |
| Flitcroft et al., 2005 [44] | Total: 123 (74 M, 49 F) High My: 15 Low My: 19 Em: 35 Low Hy: 44 High Hy: 10 | 7.1(4.4) y | High My: ≤−6D Low My: >−6D and ≤−0.75D Em: >−0.75 and <1.5D Low Hy: ≥1.5D and <6D High Hy: ≥6D | All patients had reduced vision of unknown cause | Between groups | ISCEV standard photopic and scotopic flash and 30 Hz flicker | DA: 5 min |
| Kennet et al., 1993 [50] | Hy: 25 (15 M, 10 F) Em: 10 | 15–23 y | Hy: 6.60(1.7)D, 5D to 11.5D Em: 0 to −2D | No retinal abnormalities | Within group and correlational | Photopic and scotopic flash | DA: 25 min |
| Perlman et al., 1984 [42] | High My: 7 Em: 26 Aphakia: 7 Hy: 31 | High My: 15–50 y Aphakia: 15–50 y Hy: 7–25 y | High My: <−6D Em: Normal (not otherwise defined) Hy: >+5D | No retinal abnormalities except myopic crescents in eyes with high myopia | Correlational | Photopic and scotopic flash | DA: 25 min |
| Sachidanandam et al., 2017 [52] | Total: 100 (44 M, 56 F) | 22.01(5.6) y | +0.5 to −18D | No pathology (including myopic retinopathy) | Correlational | ISCEV standard photopic and scotopic flash | LA: 20 min DA: 10 min |
| Wan et al., 2020 [40] | High My: 10 (4 M, 5 F) Moderate My: 11 (5 M, 6 F) Low My: 11 (4 M, 6 F) Em: 10 (4 M, 6 F) | High My: 26.0(2.2) y Moderate My: 26.9(2.4) y Low My: 25.3(2.5) y Em: 25.5(2.2) y | High My: −7.2(0.7), <−6.25D Moderate My: −4.5(0.8), −3.25 to −6D Low My: −2.4(0.6), >−3.00D Em: 0.1(0.1) | Participants with ophthalmological disease excluded | Between groups and correlational | ISCEV standard photopic and scotopic flash | LA: 20 min DA: 30 min |
| Westall et al., 2001 [41] | High My: 33 Low My: 8 Small RE (control): 19 | High My: 31, 13–37 y Low My: 28, 24–37 y Small RE (control): 27, 20–36 y | High My: −8.78D, −6.00D to −14.50DLow My: −3.75D, −3.00D to −5.00D Small RE (control): −0.13D, +0.75D to −2.75D | All groups: No myopic retinopathy High my: N = 4 partial posterior vitreous detachment Low My: N = 2 lattice (no holes) | Between groups and correlational | ISCEV standard photopic and scotopic flash | LA: 10 min DA: 30 min |
| Yamamoto et al., 1997 [51] | High My: 12 Low My: 19 Em: 22 | High My: 26.7(8.1) y Low My: 26.6(5.1) y Em: 25.5(4.6) y | High My: >−6.25D Low My: −3D to −6D Em: +2.5D to −2.5D (median −0.5D) | High my: No lattice or staphyloma | Between groups | Photopic chromatic flash ERGs to isolate cone responses | |
| Study | Amplitude | Implicit Time * | Note | |||
|---|---|---|---|---|---|---|
| A-Wave | B-Wave | A/B-Ratio | OPs | |||
| Blach et al., 1966 [43] | ↑ | ↓ | ↑ | a-wave amplitude increases were larger in early and moderate (relative to advanced) pathology groups. B-wave amplitude decreases were larger in myopes with advanced pathology. | ||
| Flitcroft et al., 2005 [44] | Abnormal in subset | Abnormal in subset | Abnormal in subset | An increased proportion of abnormal ERG results were obtained in patients with high ametropia (<−6D). The most common abnormalities for myopes were combined rod/cone defects and abnormal b-wave to a-wave amplitude ratios or abnormal on-off pathway responses. Quantitative data regarding wave amplitudes or implicit times were not reported. | ||
| Perlman et al., 1984 [42] | ↓ (photopic and scotopic) | ↓ (photopic and scotopic) | ↑ (see note) | No change (b-wave photopic) | a/b ratio displayed no significant change in between-group comparisons but was inversely related to refraction in myopic patients. | |
| Sachidanandam et al., 2017 [52] | ↓ (photopic and scotopic) | ↓ (photopic and scotopic) | No change | No change | Scotopic amplitudes were more affected than photopic amplitudes. | |
| Wan et al., 2020 [40] | ↑ (scotopic), No change (photopic) | ↑ (scotopic), No change (photopic) | No change | ↑ (scotopic), No change (photopic) | No change (photopic and scotopic a- and b-waves and OPs) | |
| Westall et al., 2001 [41] | ↓ (scotopic and photopic) | ↓ (scotopic and photopic) | No change | ↓ (photopic) | No change | Amplitude decrease was greater for later (versus earlier) photopic OPs. |
| Yamamoto et al., 1997 [51] | ↓ (s-cone ERG and L,M-cone ERG) | No change | ||||
| Study | Amplitude | Implicit Time * | Notes | ||
|---|---|---|---|---|---|
| A-Wave | B-Wave | A/B-Ratio | |||
| Flitcroft et al., 2005 [44] | Abnormal in subset | Abnormal in subset | Abnormal in subset | An increased proportion of abnormal ERG results were obtained in patients with high ametropia (>+6D). The most common abnormalities for hyperopes were combined rod/cone defects. Quantitative data regarding wave amplitudes or implicit times were not reported. | |
| Kennet et al., 1993 [50] | No association with degree of RE | No association with degree of RE | No change (photopic b-wave) | Study reports reassessment of a subset of patients from Perlman et al. (1984). No significant changes in wave amplitudes were observed, suggesting that the subgroups identified 8 years earlier by Perlman et al. represented stable ERG profiles. | |
| Perlman et al., 1984 [42] | ↑ (36%), normal (45%), | ↓ (36%), normal (45%), ↑ (19%) | Three subgroups were identified: Group 1 (N = 11) displayed increased a-wave and decreased b-wave amplitudes. Group 2 (N = 14) displayed normal ERG responses. Group 3 (N = 6) displayed increased b-wave amplitudes. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, S.; Murphy, M.J.; Crewther, S.G.; Riddell, N. Flash Electroretinography as a Measure of Retinal Function in Myopia and Hyperopia: A Systematic Review. Vision 2023, 7, 15. https://doi.org/10.3390/vision7010015
Zahra S, Murphy MJ, Crewther SG, Riddell N. Flash Electroretinography as a Measure of Retinal Function in Myopia and Hyperopia: A Systematic Review. Vision. 2023; 7(1):15. https://doi.org/10.3390/vision7010015
Chicago/Turabian StyleZahra, Sania, Melanie J. Murphy, Sheila G. Crewther, and Nina Riddell. 2023. "Flash Electroretinography as a Measure of Retinal Function in Myopia and Hyperopia: A Systematic Review" Vision 7, no. 1: 15. https://doi.org/10.3390/vision7010015
APA StyleZahra, S., Murphy, M. J., Crewther, S. G., & Riddell, N. (2023). Flash Electroretinography as a Measure of Retinal Function in Myopia and Hyperopia: A Systematic Review. Vision, 7(1), 15. https://doi.org/10.3390/vision7010015






