“Vision Loss” and COVID-19 Infection: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Data Extraction and Data Synthesis
2.3. Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
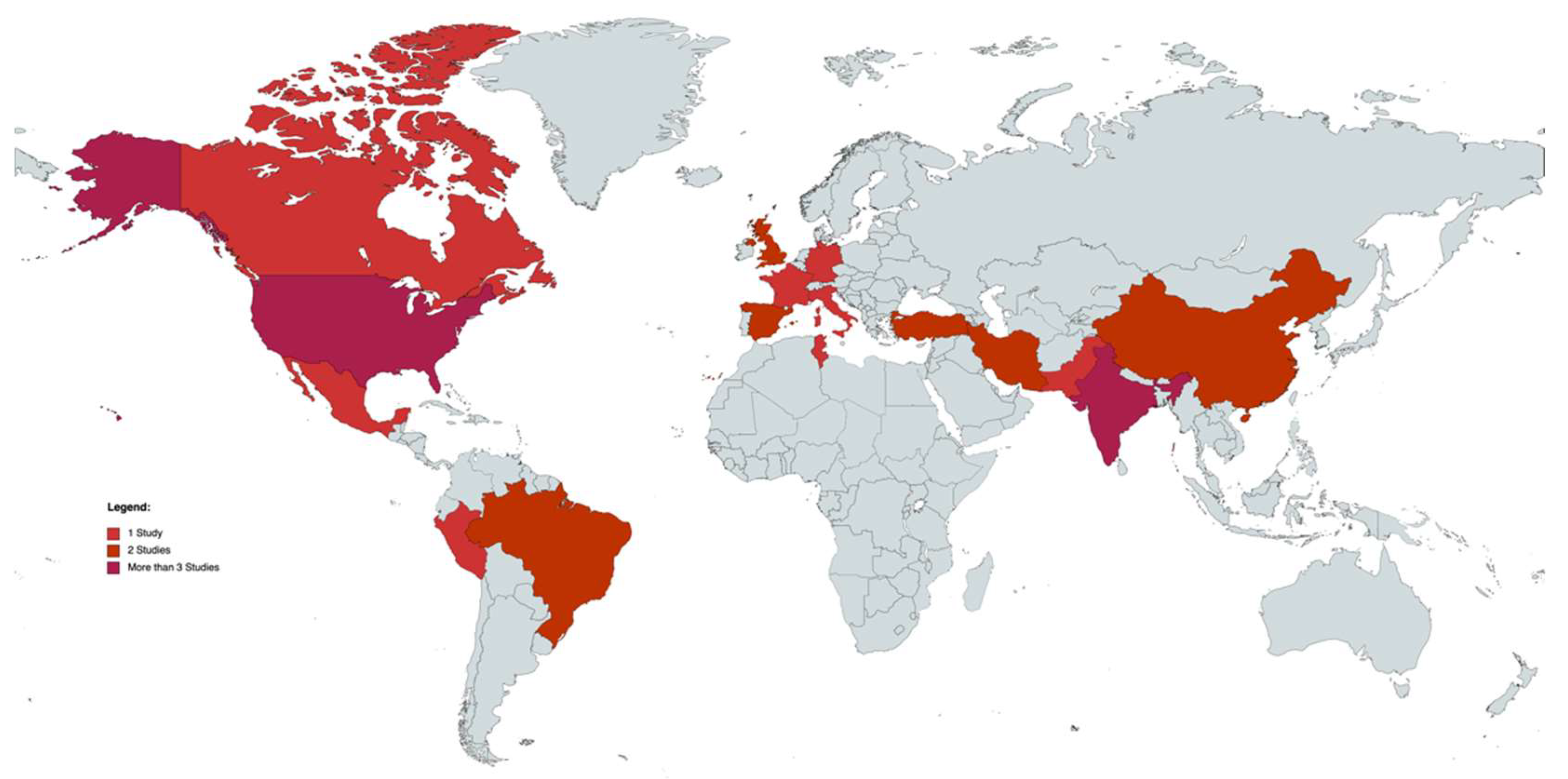
3.1. Study Selection
3.2. Study Characteristics
3.3. ”Visual Loss” Characteristics
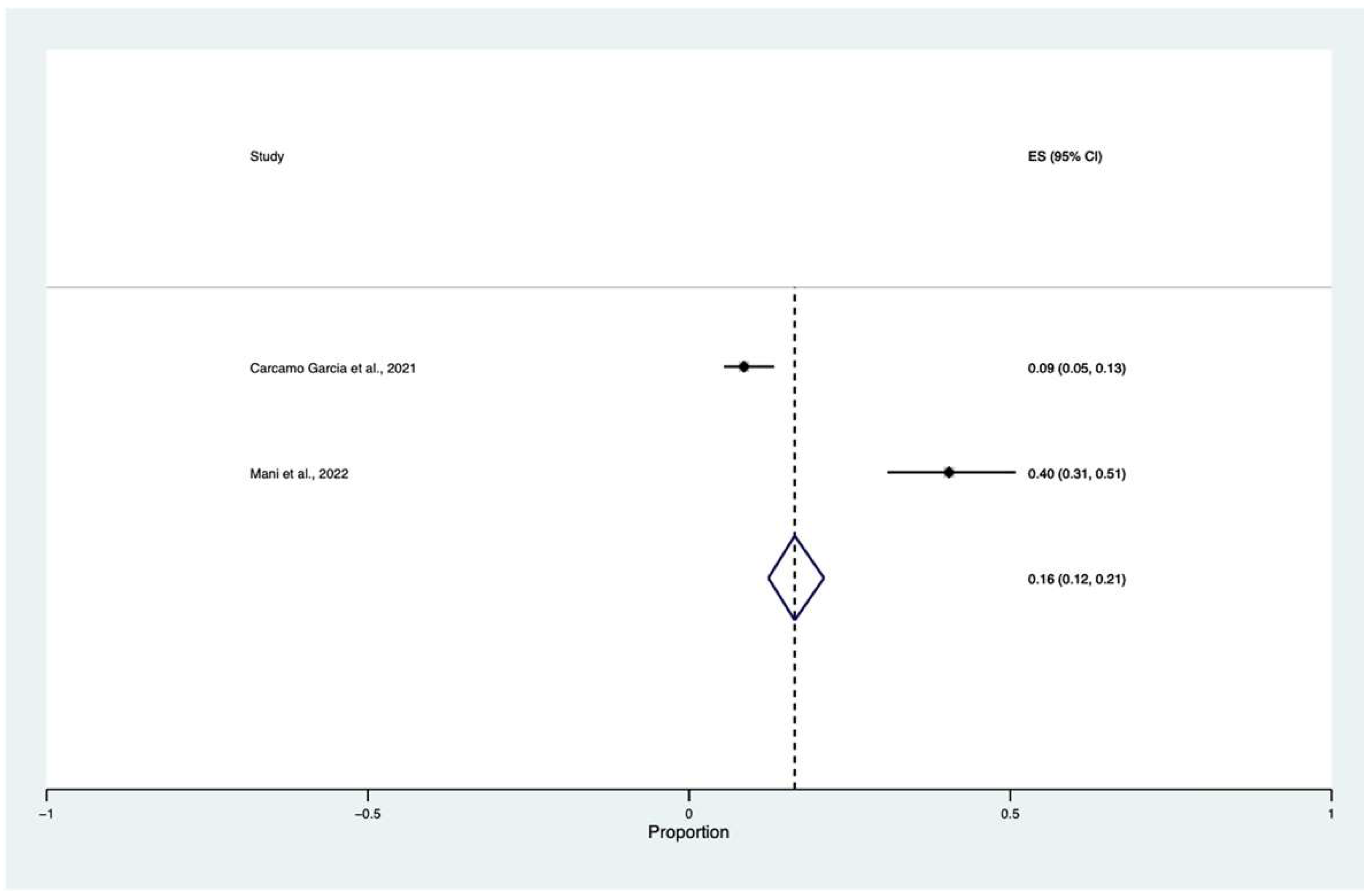
3.4. Meta-Analysis
3.5. Risk of Bias and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nabil, A.; Uto, K.; Elshemy, M.M.; Soliman, R.; Hassan, A.A.; Ebara, M.; Shiha, G. Current Coronavirus (SARS-CoV-2) Epidemiological, Diagnostic and Therapeutic Approaches: An Updated Review until June 2020. EXCLI J. 2020, 19, 992–1016. [Google Scholar] [CrossRef] [PubMed]
- Savastano, A.; Ripa, M.; Savastano, M.C.; Kilian, R.; Marchini, G.; Rizzo, S. Impact of the COVID-19 Pandemic on Ophthalmologic Outpatient Care: Experience from an Italian Tertiary Medical Center. Ann. Med. 2021, 53, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Eastin, C.; Eastin, T. Clinical Characteristics of Coronavirus Disease 2019 in China. J. Emerg. Med. 2020, 58, 711. [Google Scholar] [CrossRef]
- Gandhi, M.; Yokoe, D.S.; Havlir, D.V. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control Covid-19. N. Engl. J. Med. 2020, 382, 2158–2160. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef]
- Inomata, T.; Kitazawa, K.; Kuno, T.; Sung, J.; Nakamura, M.; Iwagami, M.; Takagi, H.; Midorikawa-Inomata, A.; Zhu, J.; Fujimoto, K.; et al. Clinical and Prodromal Ocular Symptoms in Coronavirus Disease: A Systematic Review and Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sacchetti, D.; Finn, A.; Dapaah-Afriyie, K. Acute Vision Loss in a Patient with COVID-19. Rhode Isl. Med. J. 2020, 103, 37–38. [Google Scholar]
- Mani, S.; Thirunavukkarasu, A. A Clinico-Pathological Study of COVID-19 Associated Rhino-Orbital-Cerebral Mucormycosis. Indian J. Ophthalmol. 2022, 70, 1013–1018. [Google Scholar] [CrossRef]
- Katti, V.; Ramamurthy, L.; Kanakpur, S.; Shet, S.; Dhoot, M. Neuro-Ophthalmic Presentation of COVID-19 Disease: A Case Report. Indian J. Ophthalmol. 2021, 69, 992–994. [Google Scholar] [CrossRef]
- Kaya, Y.; Kara, S.; Akinci, C.; Kocaman, A.S. Transient Cortical Blindness in COVID-19 Pneumonia; a PRES-like Syndrome: Case Report. J. Neurol. Sci. 2020, 413, 116858. [Google Scholar] [CrossRef] [PubMed]
- Atum, M.; Demiryüre, B.E. Sudden Bilateral Vision Loss in a COVID-19 Patient: A Case Report. Indian J. Ophthalmol. 2021, 69, 2227–2228. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, M.S.; Romero-Castro, R.M.; Alvarado-de la Barrera, C.; González-Cannata, M.G.; García-Morales, A.K.; Ávila-Ríos, S. Optic Neuritis Following SARS-CoV-2 Infection. J. NeurozVirology 2021, 27, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Veisi, A.; Bagheri, A.; Eshaghi, M.; Rikhtehgar, M.H.; Rezaei Kanavi, M.; Farjad, R. Rhino-Orbital Mucormycosis during Steroid Therapy in COVID-19 Patients: A Case Report. Eur. J. Ophthalmol. 2021, 32, NP11–NP16. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, M.; Saidahmed, O.; Adebayo, A.; Archibald, N. Persistent Cortical Blindness Following Posterior Reversible Encephalopathy Syndrome (PRES) as a Complication of COVID-19 Pneumonia. Cureus 2021, 13, e12794. [Google Scholar] [CrossRef]
- Gonzalez, M.P.; Rios, R.; Pappaterra, M.; Hernandez, M.; Toledo, A.; Santos, C.; Emanuelli, A.; Kurup, S.K.; Oliver, A.L. Reactivation of Acute Retinal Necrosis Following SARS-CoV-2 Infection. Case Rep. Ophthalmol. Med. 2021, 2021, 7336488. [Google Scholar] [CrossRef]
- Gascon, P.; Briantais, A.; Bertrand, E.; Ramtohul, P.; Comet, A.; Beylerian, M.; Sauvan, L.; Swiader, L.; Durand, J.M.; Denis, D. COVID-19-Associated Retinopathy: A Case Report. Ocul. Immunol. Inflamm. 2020, 28, 1293–1297. [Google Scholar] [CrossRef]
- Eslamiyeh, H.; Jafari, M. Binocular Optic Neuritis in an Eight-Year-Old Boy Due to COVID-19 Infection. Iran. J. Pediatrics 2021, 31, e111798. [Google Scholar] [CrossRef]
- Khan, A.W.; Ullah, I.; Khan, K.S. Ischemic Stroke Leading to Bilateral Vision Loss in COVID-19 Patient—A Rare Case Report. J. Med. Virol. 2021, 93, 683–685. [Google Scholar] [CrossRef]
- Liu, L.; Cai, D.; Huang, X.; Shen, Y. COVID-2019 Associated with Acquired Monocular Blindness. Curr. Eye Res. 2021, 46, 1247–1250. [Google Scholar] [CrossRef]
- Benito-Pascual, B.; Gegúndez, J.A.; Díaz-Valle, D.; Arriola-Villalobos, P.; Carreño, E.; Culebras, E.; Rodríguez-Avial, I.; Benitez-Del-Castillo, J.M. Panuveitis and Optic Neuritis as a Possible Initial Presentation of the Novel Coronavirus Disease 2019 (COVID-19). Ocul. Immunol. Inflamm. 2020, 28, 922–925. [Google Scholar] [CrossRef]
- da Silva Catharino, A.M.; Neves, M.A.O.; dos Santos Moraes Nunes, N.; do Nascimento, J.S.F.; do Nascimento, J.K.F.; Castro, R.R.T.; Azizi, M.A.A.; Airão, A.R.; Alvarenga, R.M.P. COVID-19 Related Optic Neuritis: Case Report. J. Clin. Neurol. Neurosci. 2020. [Google Scholar] [CrossRef]
- Carcamo Garcia, M.H.; Garcia Choza, D.D.; Salazar Linares, B.J.; Diaz, M.M. Neurological Manifestations of Patients with Mild-to-Moderate COVID-19 Attending a Public Hospital in Lima, Peru. eNeurologicalSci 2021, 23, 100338. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.M.; Riga, V.; Shirodkar, A.L.; Meyer, J. Proning Related Bilateral Anterior Ischaemic Optic Neuropathy in a Patient with COVID-19 Related Acute Respiratory Distress Syndrome. BMC Ophthalmol. 2021, 21, 276. [Google Scholar] [CrossRef]
- Crane, A.B.; Abreu Diaz, M.C.; Jiang, Y.; Pergament, K.M. Rare Case of Endogenous Klebsiella Endophthalmitis Associated with Emphysematous Prostatitis in a Patient with Diabetes, Cirrhosis and COVID-19. BMJ Case Rep. 2021, 14, e240425. [Google Scholar] [CrossRef] [PubMed]
- Cyr, D.G.; Vicidomini, C.M.; Siu, N.Y.; Elmann, S.E. Severe Bilateral Vision Loss in 2 Patients with Coronavirus Disease 2019. J. Neuroophthalmol. 2020, 40, 403–405. [Google Scholar] [CrossRef]
- Eswaran, S.; Balan, S.K.; Saravanam, P.K. Acute Fulminant Mucormycosis Triggered by COVID 19 Infection in a Young Patient. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–5. [Google Scholar] [CrossRef]
- de Souza, E.C.; de Campos, V.E.; Duker, J.S. Atypical Unilateral Multifocal Choroiditis in a COVID-19 Positive Patient. Am. J. Ophthalmol. Case Rep. 2021, 22, 101034. [Google Scholar] [CrossRef]
- Zhou, S.; Jones-lopez, E.C.; Soneji, D.J.; Azevedo, C.J.; Patel, V.R. Myelin Oligodendrocyte Glycoprotein Antibody–Associated Optic Neuritis and Myelitis in COVID-19. Wolters Kluwer Public Health Emerg. Collect. 2020, 40, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Pellegrini, M.; Messenio, D.; Cereda, M.; Olivieri, P.; Brambilla, A.M.; Staurenghi, G. Impending Central Retinal Vein Occlusion in a Patient with Coronavirus Disease 2019 (COVID-19). Ocul. Immunol. Inflamm. 2020, 28, 1290–1292. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.; Sarfraz, A.; Sarfraz, Z.; Valentine, D.; Idowu, A.R.; Sanchez, V. Unilateral Optic Neuritis Associated with SARS-CoV-2 Infection: A Rare Complication. Am. J. Case Rep. 2021, 22, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Malek, I.; Sayadi, J.; Lahiani, R.; Boumediene, M.; ben Salah, M.; Jrad, M.; Khairallah, M.; Nacef, L. Acute Bilateral Blindness in a Young COVID-19 Patient with Rhino-Orbito-Cerebral Mucormycosis. J. Ophthalmic Inflamm. Infect. 2021, 11, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Micieli, J.A.; Yu, C.W. Optic Neuritis Associated with SARS-CoV-2 B.1.1.7 Variant of Concern. Can. J. Neurol. Sci. 2021, 49, 591–592. [Google Scholar] [CrossRef]
- Murchison, A.P.; Sweid, A.; Dharia, R.; Theofanis, T.N.; Tjoumakaris, I.; Jabbour, P.M.; Bilyk, J.R. Monocular Visual Loss as the Presenting Symptom of COVID-19 Infection. Clin. Neurol. Neurosurg. 2020, 201, 106440. [Google Scholar] [CrossRef]
- Reich, M.; Pauleikhoff, L.; Schröter, N.; Spang, S.; Lübke, J.; Lange, C.; Lagrèze, W.A. Sehverlust Nach Assistierter Mechanischer Beatmung Aufgrund von SARS-CoV-2-Eine Fallserie. Dtsch. Arztebl. Int. 2020, 117, 561–562. [Google Scholar] [CrossRef]
- François, J.; Collery, A.S.; Hayek, G.; Sot, M.; Zaidi, M.; Lhuillier, L.; Perone, J.M. Coronavirus Disease 2019–Associated Ocular Neuropathy with Panuveitis: A Case Report. JAMA Ophthalmol. 2021, 139, 247–249. [Google Scholar] [CrossRef]
- Cleo, G.; Scott, A.M.; Islam, F.; Julien, B.; Beller, E. Usability and Acceptability of Four Systematic Review Automation Software Packages: A Mixed Method Design. Syst. Rev. 2019, 8, 145. [Google Scholar] [CrossRef]
- Lo, C.K.L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Borges Migliavaca, C.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting Proportional Meta-Analysis in Different Types of Systematic Reviews: A Guide for Synthesisers of Evidence. BMC Med Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- Sen, M.; Honavar, S.G.; Sharma, N.; Sachdev, M.S. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J. Ophthalmol. 2021, 69, 488–509. [Google Scholar] [CrossRef] [PubMed]
- Al-Moujahed, A.; Boucher, N.; Fernando, R.; Saroj, N.; Vail, D.; Rosenblatt, T.R.; Moshfeghi, D.M. Incidence of Retinal Artery and Vein Occlusions During the COVID-19 Pandemic. Ophthalmic. Surg. Lasers Imaging Retin. 2022, 53, 22–30. [Google Scholar] [CrossRef]
- Franceschi, A.M.; Arora, R.; Wilson, R.; Giliberto, L.; Libman, R.B.; Castillo, M. Neurovascular Complications in COVID-19 Infection: Case Series. Am. J. Neuroradiol. 2020, 41, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Zhang, B.; Wu, C.; Zhou, J.; Zhang, Y.; Yang, W.; Li, Z.; Shi, S. Coagulopathy Is Associated with Multiple Organ Damage and Prognosis of COVID-19. EXCLI J. 2021, 20, 174–191. [Google Scholar] [CrossRef]
- Heimfarth, L.; dos Santos, M.A.; Barreto-Filho, J.A.; Barreto, A.S.; Macedo, F.N.; de Souza Araújo, A.A.; Martins-Filho, P.; Scotti, M.T.; Scotti, L.; Quintans-Júnior, L.J. Insights into the Actions of Angiotensin-1 Receptor (AT1R) Inverse Agonists: Perspectives and Implications in COVID-19 Treatment. EXCLI J. 2021, 20, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]

| Study Name | Country | Publication Date | Sample Size Study Design Age (Media ± SD) Gender | Visual Impairment Description | “Laterality” | Visual Acuity | Duration between the Onset of COVID-19 Symptoms and Ocular Symptoms | Comorbidities | Patients with COVID-19 (N and %) | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaya et al. [11] | Turkey | 28 April 2020 | N = 1 Case report 38 Male | Vision loss in both eyes | Bilateral | OU: perception light | 5 days | NA | 1 | PRES |
| Selvaraj et al. [8] | USA | 10 June 2020 | N = 1 Case Report 50 Female | Acute, painless RE monocular visual disturbance, described as a white cloud and blurriness involving most of her RE, sparing the superior nasal aspect. | Monocular | RE: 20/70 | 7 days | NA | 1 | PION |
| Reich et al. [35] | Germany | 17 August 2020 | N = 1 each case Case sries Case 1: 64 Case 2: 43 Case 3: 60 Case 1: Female Case 2 and 3: Male | Immediately after regaining full consciousness, the patients reported visual impairment. | Case 1 and 3: Bilateral Case 2: Monolateral | Case 1: OU: 0.5 LogMar Case 2: LE: 1 LogMar Case 3: RE: light perception LE: 1.0 LogMar | Soon after extubation (after 7–16 days of mechanical ventilation) | Case 1: obesity Case 2: arterial hypertension and medical history of carbon monoxide poisoning Case 3: arterial hypertension, insulin-dependent diabetes mellitus, grade 1 obesity, and nicotine abuse | 1 each case | Case 1: Optic Atrophy Case 2: High Intraocular Pressure (IOP > 50) Case 3: Optic Atrophy |
| Cyr et al. [26] | USA | September 2020 | N = 1 each case Case series Patient 1: 61 Patient 2: 34 patient 1: male patient 2: Female | Patient 1: sudden, painless loss of vision for 2 days Patient 2: sudden, painless loss of vision of two-day duration. | Patient 1: bilateral Patient 2: bilateral | Patient 1: light perception Patient 2: light perception | Patient 1: 7 days Patient 2: 10 days | Patient 1: non–insulin-dependent diabetes mellitus Patient 2: four systemic lupus erythematosus, hypertension, end-stage renal disease on hemodialysis, chronic obstructive pulmonary disease | 1 each case | Patient 1: acute bilateral occipital territorial ischemic infarct Patient 2: acute infarct in the right frontal lobe, acute left posterior temporal-occipital territorial infarction and bilateral medial occipital |
| Zhou et al. [29] | USA | September 2020 | N = 1 Case report 26 Male | Bilateral, subacute, sequential vision loss first affecting the LE, then the RE 3 days later | Bilateral | RE: HM LE: 20/250 | “Few days” | None | 1 | Severe optic neuritis and myelitis |
| Benito Pascual et al. [21] | Spain | 1 September 2020 | N = 1 Case Report 60 Female | Ocular pain, blurred vision, and redness in her LE | Monolateral | LE: 20/200 | 14 days | NA | 1 | Panuveitis and Optic Neuritis |
| Khan et al. [19] | Pakistan | 3 September 2020 | N = 1 Case report 60 Male | Bilateral visual loss | Bilateral | NA | 24 h | NA | 1 | Cortical blindness secondary to occipital lobe stroke |
| Invernizzi et al. [30] | Italy | 25 September 2020 | N = 1 Case report 54 Female | Scotomas and decreased vision in her RE | Monolateral | RE: 20/40 | 10 days | NA | 1 | Impending Central Retinal Vein Occlusion |
| Gascon et al. [17] | France | 06 Oct 2020 | N = 1 Case Report 50 Male | LE: negative scotoma and acute onset of dyschromatopsia and decreased visual acuity | Monolateral | LE: 20/63 | 8 days | Splenectomy and RE: Glaucoma | 1 | Acute macular neuroretinopathy and paracentral acute middle maculopathy |
| Catharino et al. [22] | Brazil | 18 November 2020 | N = 1 Case Report 64 Male | NA | Monolateral | NA | The same day | hypertension | 1 | Optic Neuritis |
| Murchison et al. [34] | USA | 15 December 2020 | N = 1 Case Report “Fifth decade” Male | RE: Acute onset of painless visual loss | Monolateral | RE: HM | 3-weeks | Hypertension, tobacco use, and occasional marijuana use | 1 | CRAO |
| Francois et al. [36] | France | 17 December 2020 | N = 1 Case Report “Late 50 s” Female | Blurred vision and redness in her RE and temporary (eight-day history) pain when mobilizing the globe | Monolateral | RE: HM | 2 days | NA | 1 | Ocular neuropathy and panuveitis |
| Elhassan 2021 [15] | UK | 19 January 2021 | N = 1 Case Report 52 Female | Complete cortical blindness with poor insight into the extent of her visual impairment, often claiming to be able to see (Anton’s syndrome) and hallucinations | Bilateral | No perception light | 31 | None | 1 | PRES |
| Liu et al. [20] | China | 03 February 2021 | N = 1 Case Report 66 Female | “Shadows similar to cotton wool” with her LE followed by monocular blindness | Monolateral | No light perception | First symptoms 10 days after COVID-19, blindness after 3 weeks from initial symptoms | None | 1 | Acute viral retinitis, optic neuritis, uveitis and secondary glaucoma |
| De Souza et al. [28] | Brazil | 19 February 2021 | N = 1 Case Report 23 Male | Acute painless loss of central vision in his RE | Monolateral | RE: 20/800 | NA | NA | 1 | Multifocal choroiditis |
| Katti et al. [10] | India | 16 March 2021 | N = 1 Case Report 66 Male | Sudden bilateral loss of vision | Bilateral | RE/LE: no light perception | 10 days | None | 1 | Pituitary macroadenoma with apoplexy and stroke |
| Rodríguez-Rodríguez et al. [13] | Mexico | 23 March 2021 | N = 1 Case Report 55 Female | Unilateral, gradual visual loss, decreasing visual acuity, and chromatic impairment | Bilateral | RE: 20/40 LE: 20/200 | NA | None | 1 | Optic neuritis |
| Veisi et al. [14] | Iran | 10 April 2021 | N = 1 each case Case Series Case 1: 40 Case 2: 54 Case 1: Female Case 2: Male | Case 1: bilateral visual loss and complete ophthalmoplegia of the RE Case 2: vision loss, proptosis, orbital inflammation, and complete ophthalmoplegia on the left side | Case 1: bilateral Case 2: monolateral | Case 1: no light perception Case 2: LE light percepetion | Case 1: 15 days Case 2: 7 days | Case 1 None Case 2 Non-insulin-dependent diabetes mellitus | 1 per case | Case 1: Mucormycosis Case 2: Rhino-orbital mucormycosis |
| Carcamo Garcia et al. [23] | Perù | 14 April 2021 | N = 199 Cross-sectional study 42.8 ±15.1 85 males and 114 females | Visual changes 24 (12%): Visual symptoms: 23 (11.6%) Unilateral 0 (0%) Bilateral 15 (65.2%) Deficient color vision 4 (17.4%) Vision loss 17 (73.9%) Double vision 3 (13%) | NA | NA | 8 ± 6 days | Hypercholesterolemia (12%), followed by hypertension (10%), prior history of tuberculosis or other pulmonary disease (9%) and diabetes (7%), cancer (4%); chronic kidney disease (2%) cerebrovascular disease or stroke (1%). Nearly 10% of the cohort had a history of smoking or were current smokers. | 199 | Bilateral visual changes and decreased visual acuity were the most common symptoms in patients with mild-moderate COVID-19 infection. |
| Crane et al. [25] | USA | 21 April 2021 | N = 1 Case Report 35 Male | Vision loss with no associated pain or redness started in the LE but very quickly involving the RE | Bilateral | RE/LE: Light perception | NA | diabetes, cirrhosis | 1 | Endogenous Klebsiella endophthalmitis. |
| Deane et al. [31] | USA | 13 June 2021 | N = 1 Case Report 21 Female | Blurry vision in her LE associated with one-week history of severe headaches with pain with movements in all directions in her LE | Monolateral | LE: Hand Motion | NA | NA | 1 | Optic Neuritis |
| Eswaran et al. [27] | India | 13 June 2021 | N = 1 Case Report 31 Male | Bilateral proptosis, loss of vision and ophthalmoplegia | Bilateral | NA | NA | Diabetes | 1 | Mucormycosis |
| Clarke et al. [24] | UK | 13 July 2021 | N = 1 Case Report 55 Male | Profound bilateral vision loss after cessation of sedation | Bilateral | LE: 3/30 unaided RE: counting fingers | NA | Hypercholesterolemia and hypertension | 1 | NAION due to Prone Position |
| Gonzalez et al. [16] | USA | 19 July 2021 | N = 1 Case Report 32 Female | Sudden vision loss in her LE, associated with a one-week history of pain, redness, and photophobia | Monocular | LE: perception light | 24 h | Left retinal detachment secondary to necrotizing herpetic retinitis | 1 | Acute retinal necrosis |
| Atum et al. [12] | Turkey | 23 July 2021 | N = 1 Case Report 84 Male | Sudden vision loss | Bilateral | HM | 5 days | NA | 1 | Bilateral occipital ischemic stroke |
| Micieli et al. [33] | Canada | 29 July 2021 | N = 1 Case Report 31 Male | RE vision loss after 10-day history of pain that worsened with eye movements and blurred vision | Monolateral | RE: CF at 4 feet | 12 | None | 1 | Optic Neuritis |
| Eslamiyeh et al. [18] | Iran | 8 August 2021 | N = 1 Case Report 8 Male | Sudden bilateral and progressive blurring of vision in the RE | Bilateral | RE: 2/10 LE: 4/10 | NA | NA | 1 | Optic Neuritis |
| Malek et al. [32] | Tunisie | 18 October 2021 | N = 1 Case Report 20 Male | Rapid bilateral visual loss with left periorbital pain, proptosis, palpebral edema, and swelling | Bilateral | No LP | 7 days | NA | 1 | Rhino-orbito-cerebral mucormycosis, left cavernous sinus and internal carotid thrombosis together with a right CRAO |
| Mani et al. [9] | India | 25 February 2022 | N = 89 Cross-sectional study 54.71 ± 11.03 70 males and 19 females | ROCM (stage 3c): 1 patient: Bilateral orbital involvement with loss of vision ROCM (stage 3d) 35 patients: central retinal artery occlusion or involvement of orbital apex, superior orbital fissure, inferior orbital fissure with loss of vision | One patient bilateral, 35 patients monolateral | NA | NA | Diabetes | 89 | Rhino-orbital-cerebral mucormycosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripa, M.; Motta, L.; Schipa, C.; Rizzo, S.; Sollazzi, L.; Aceto, P. “Vision Loss” and COVID-19 Infection: A Systematic Review and Meta-Analysis. Vision 2022, 6, 60. https://doi.org/10.3390/vision6040060
Ripa M, Motta L, Schipa C, Rizzo S, Sollazzi L, Aceto P. “Vision Loss” and COVID-19 Infection: A Systematic Review and Meta-Analysis. Vision. 2022; 6(4):60. https://doi.org/10.3390/vision6040060
Chicago/Turabian StyleRipa, Matteo, Lorenzo Motta, Chiara Schipa, Stanislao Rizzo, Liliana Sollazzi, and Paola Aceto. 2022. "“Vision Loss” and COVID-19 Infection: A Systematic Review and Meta-Analysis" Vision 6, no. 4: 60. https://doi.org/10.3390/vision6040060
APA StyleRipa, M., Motta, L., Schipa, C., Rizzo, S., Sollazzi, L., & Aceto, P. (2022). “Vision Loss” and COVID-19 Infection: A Systematic Review and Meta-Analysis. Vision, 6(4), 60. https://doi.org/10.3390/vision6040060






