Fibroblast Growth Factor-2 (FGF-2) Expression in Pterygia Using Cell Spot Arrays
Abstract
1. Introduction
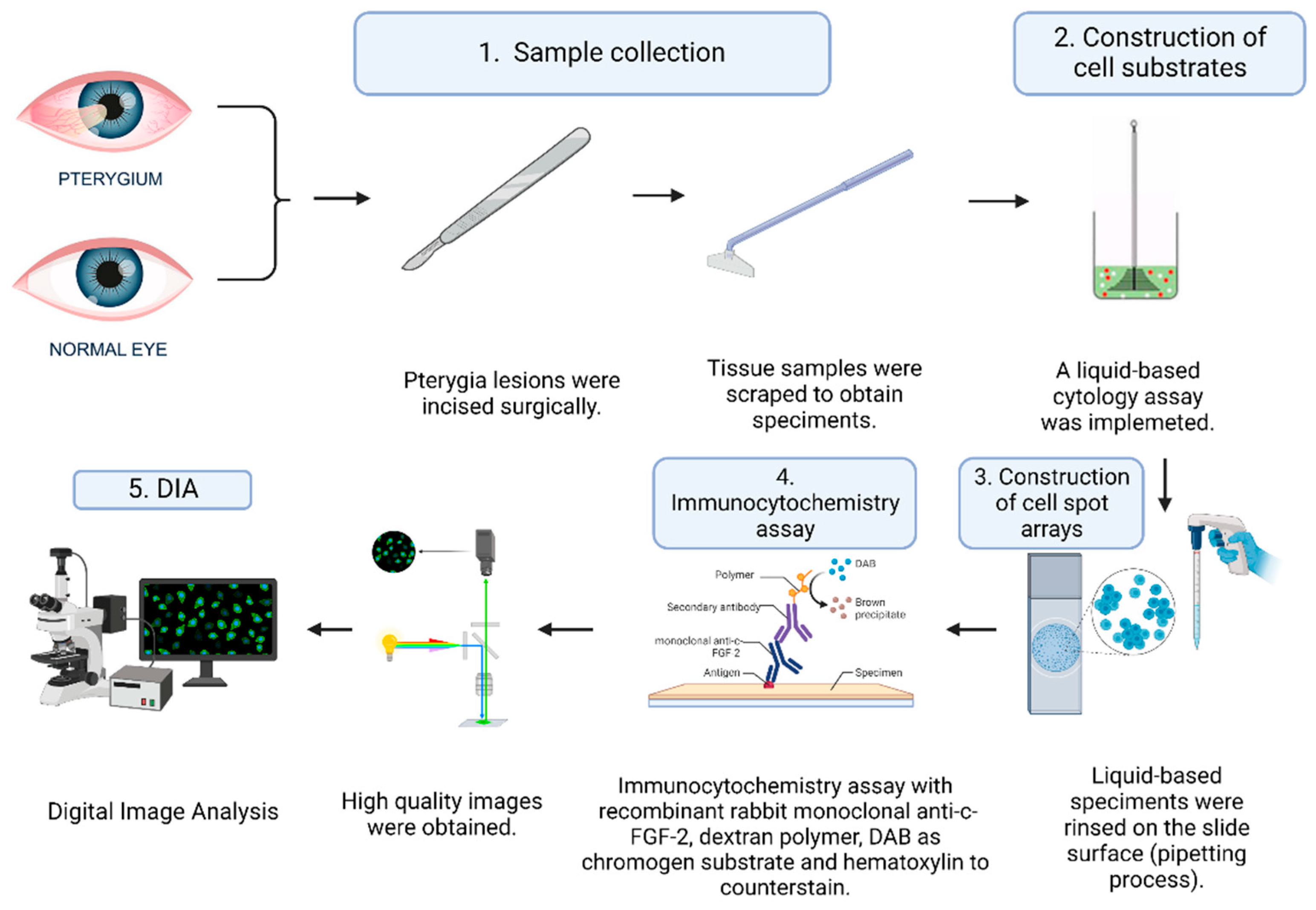
2. Materials and Methods
2.1. Study Design and Patients
2.2. Cell Substrates
2.3. Construction of the Slide for the Cell Spot Array
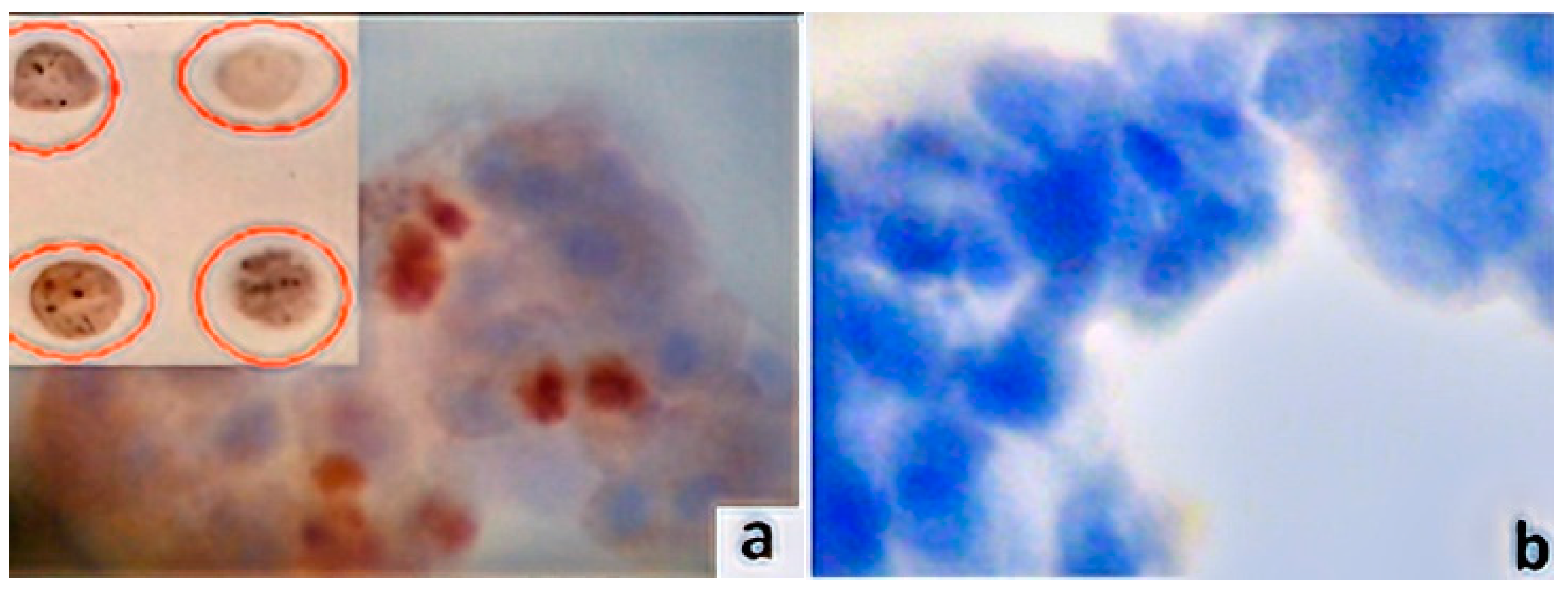
2.4. Immunocytochemistry Assays (ICC) and Antibodies
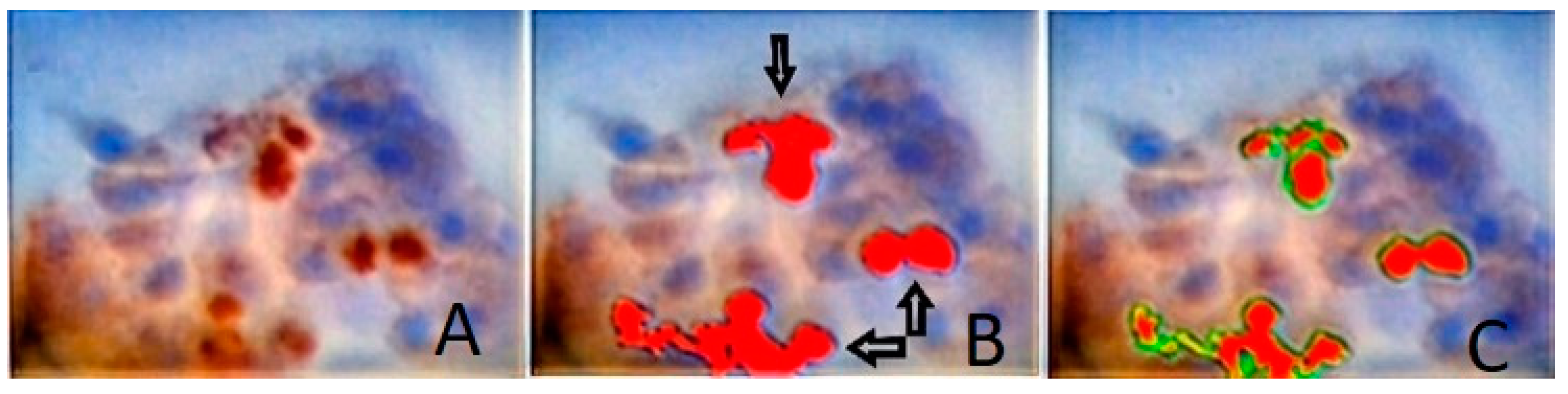
2.5. Digital Image Analysis (DIA)
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, J.C.; Yang, W.; Bradley, R.H.; Reid, T.W.; Schwab, I.R. The science of pterygia. Br. J. Ophthalmol. 2010, 94, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Detorakis, E.T.; Spandidos, D.A. Pathogenetic mechanisms and treatment options for ophthalmic pterygium: Trends and perspectives. Int. J. Mol. Med. 2009, 23, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Xie, L. Progress in the pathogenesis of pterygium. Curr. Eye Res. 2013, 38, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.J.; Sikder, S. Surgical management of pterygium. Ocul. Surf. 2014, 12, 112–119. [Google Scholar] [CrossRef]
- Singh, H.; Laad, S.; Pattebahadur, R.S.; Saluja, P.; Ramnani, P. A comparative study of postoperative outcome after pterygium excision using autologous blood and sutures. J. Evid. Based Med. 2017, 4, 6022–6027. [Google Scholar]
- Rezvan, F.; Khabazkhoob, M.; Hooshmand, E.; Yekta, A.; Saatchi, M.; Hashemi, H. Prevalence and risk factors of pterygium: A systematic review and meta-analysis. Surv. Ophthalmol. 2018, 63, 719–735. [Google Scholar] [CrossRef]
- Cárdenas-Cantú, E.; Zavala, J.; Valenzuela, J. Molecular Basis of Pterygium Development. Semin. Ophthalmol. 2016, 31, 567–583. [Google Scholar] [CrossRef]
- Kim, K.W.; Ha, H.S.; Kim, J.C. Ischemic tissue injury and progenitor cell tropism: Significant contributors to the pathogenesis of pterygium. Histol. Histopathol. 2015, 30, 311–320. [Google Scholar]
- Dolivo, D.M. Anti-fibrotic effects of pharmacologic FGF-2: A review of recent literature. J. Mol. Med. 2022, 100, 847–860. [Google Scholar] [CrossRef]
- Tamburello, M.; Altieri, B.; Sbiera, I.; Sigala, S.; Berruti, A.; Fassnacht, M.; Sbiera, S. FGF/FGFR signaling in adrenocortical development and tumorigenesis: Novel potential therapeutic targets in adrenocortical carcinoma. Endocrine 2022, 77, 411–418. [Google Scholar] [CrossRef]
- Chen, K.; Rao, Z.; Dong, S.; Chen, Y.; Wang, X.; Luo, Y.; Gong, F.; Li, X. Roles of the fibroblast growth factor signal transduction system in tissue injury repair. Burns Trauma 2022, 10, tkac005. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.H.; Chee, S.-P.; Dear, K.B.G.; Lim, A.S.M. Effect of Pterygium Morphology on Pterygium Recurrence in a Controlled Trial Comparing Conjunctival Autografting with Bare Sclera Excision. Arch. Ophthalmol. 1997, 115, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.; Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013, 14, 166–180. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, R.T.; Pollet, N.; Delius, H.; Niehrs, C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat. Cell Biol. 2004, 6, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, M.; Trueb, B. Characterization of a novel protein (FGFRL1) from human cartilage related to FGF receptors. Genomics 2000, 69, 275–279. [Google Scholar] [CrossRef]
- Sanchez-Heras, E.; Howell, F.V.; Williams, G.; Doherty, P. The fibroblast growth factor receptor acid box is essential for interactions with N-cadherin and all of the major isoforms of neural cell adhesion molecule. J. Biol. Chem. 2006, 281, 35208–35216. [Google Scholar] [CrossRef]
- Qian, X. N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene 2014, 33, 3411–3421. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181–189. [Google Scholar] [CrossRef]
- Gupta, M.; Orozco, G.; Rao, M.; Gedaly, R.; Malluche, H.H.; Neyra, J.A. The Role of Alterations in Alpha-Klotho and FGF-23 in Kidney Transplantation and Kidney Donation. Front. Med. 2022, 9, 803016. [Google Scholar] [CrossRef]
- Helsten, T.; Schwaederle, M.; Kurzrock, R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: Biologic and clinical implications. Cancer Metastasis Rev. 2015, 34, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Loda, A.; Turati, M.; Semeraro, F.; Rezzola, S.; Ronca, R. Exploring the FGF/FGFR System in Ocular Tumors: New Insights and Perspectives. Int. J. Mol. Sci. 2022, 23, 3835. [Google Scholar] [CrossRef] [PubMed]
- Chua, V.; Orloff, M.; Teh, J.L.; Sugase, T.; Liao, C.; Purwin, T.J.; Lam, B.Q.; Terai, M.; Ambrosini, G.; Carvajal, R.D. Stromal fibroblast growth factor 2 reduces the efficacy of bromodomain inhibitors in uveal melanoma. EMBO Mol. Med. 2019, 11, e9081. [Google Scholar] [CrossRef] [PubMed]
- Schweigerer, L.; Neufeld, G.; Gospodarowicz, D. Basic fibroblast growth factor is present in cultured human retinoblastoma cells. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1838–1843. [Google Scholar]
- Ronca, R.; Giacomini, A.; Rusnati, M.; Presta, M. The potential of fibroblast growth factor/fibroblast growth factor receptor signaling as a therapeutic target in tumor angiogenesis. Expert Opin. Ther. Targets 2015, 19, 1361–1377. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Fu, Q.; Zhang, X.; Chen, Y. Efficacy of bevacizumab in the treatment of pterygium: An updated meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2021, 98, 107921. [Google Scholar] [CrossRef]
- Martín-López, J.; Pérez-Rico, C.; García-Honduvilla, N.; Buján, J.; Pascual, G. Elevated blood/lymphatic vessel ratio in pterygium and its relationship with vascular endothelial growth factor (VEGF) distribution. Histol. Histopathol. 2019, 34, 917–929. [Google Scholar]
- Liu, D.; Peng, C.; Jiang, Z.; Tao, L. Relationship between expression of cyclooxygenase 2 and neovascularization in human pterygia. Oncotarget 2017, 8, 105630–105636. [Google Scholar] [CrossRef][Green Version]
- Feng, Q.-Y.; Hu, Z.-X.; Song, X.-L.; Pan, H.-W. Aberrant Expression of Genes and Proteins in Pterygium and Their Implications in the Pathogenesis. Int. J. Ophthalmol. 2017, 10, 973–981. [Google Scholar] [CrossRef]
- Kria, L.; Ohira, A.; Amemiya, T. Growth factors in cultured pterygium fibroblasts: Immunohistochemical and ELISA analysis. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 702–708. [Google Scholar] [CrossRef]
- Solomon, A.; Li, D.Q.; Lee, S.B.; Tseng, S.C. Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2154–2163. [Google Scholar]
- Reid, T.W.; Dushku, N. What a study of pterygia teaches us about the cornea? Molecular mechanisms of formation. Eye Contact Lens. 2010, 36, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Kria, L.; Ohira, A.; Amemiya, T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996, 98, 195–201. [Google Scholar] [CrossRef]
- Lee, S.B.; Li, D.Q.; Tan, D.T.; Meller, D.C.; Tseng, S.C. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr. Eye Res. 2000, 20, 325–334. [Google Scholar] [CrossRef]
- Bianchi, E.; Scarinci, F.; Grande, C.; Plateroti, R.; Plateroti, P.; Plateroti, A.M.; Fumagalli, L.; Capozzi, P.; Feher, J.; Artico, M. Immunohistochemical profile of VEGF, TGF-β and PGE₂ in human pterygium and normal conjunctiva: Experimental study and review of the literature. Int. J. Immunopathol. Pharmacol. 2012, 25, 607–615. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Soliman, W.; Fathalla, A.M.; El Refaie, A. Effect of single subconjunctival injection of bevacizumab on primary pterygium: Clinical, histopathological and immunohistochemical study. Int. J. Ophthalmol. 2018, 11, 797–801. [Google Scholar]
- Mastronikolis, S.; Adamopoulou, M.; Tsiambas, E.; Makri, O.Ε.; Pagkalou, M.; Thomopoulou, V.K.; Georgakopoulos, C.D. Vascular Endothelial Growth Factor Expression Patterns in non-Human Papillomavirus-Related Pterygia: An Experimental Study on Cell Spot Arrays Digital Analysis. Curr. Eye Res. 2022, 47, 1003–1008. [Google Scholar] [CrossRef]
- Meng, Q.; Qin, Y.; Deshpande, M.; Kashiwabuchi, F.; Rodrigues, M.; Lu, Q.; Ren, H.; Elisseeff, J.H.; Semenza, G.L.; Montaner, S.V.; et al. Hypoxia-Inducible Factor-Dependent Expression of Angiopoietin-Like 4 by Conjunctival Epithelial Cells Promotes the Angiogenic Phenotype of Pterygia. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4514–4523. [Google Scholar]
| Clinicopathological Parameters | FGF-2 Expression | p-Value | ||
|---|---|---|---|---|
| n (%) | H and M | L | ||
| Pterygia Controls | n = 60 n = 20 | 60/60 (100%) 3/20 (15%) | 0/60 (0%) 17/20 (85%) | 0.001 |
| Gender | 0.469 | |||
| Male | 20/60 (33%) | 20/20 (100%) | 0/20 (0%) | |
| Female | 40/60 (67%) | 40/40 (100%) | 0/40 (0%) | |
| Anatomic location | 0.341 | |||
| Central | 32/60 (53%) | 32/32 (100%) | 0/32 (0%) | |
| Peripheral | 28/60 (47%) | 28/28 (100%) | 0/28 (0%) | |
| Morphology type | 0.007 | |||
| Flat | 36/60 (60%) | 36/36 (100%) | 0/36 (0%) | |
| Fleshy | 24/60 (40%) | 24/24 (100%) | 0/24 (0%) | |
| Recurrence status | 0.999 | |||
| Relapse | 8/60 (16%) | 8/8 (100%) | 0/8 (0%) | |
| Non-relapse | 52/60 (84%) | 52/52 (100%) | 0/52 (0%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastronikolis, S.; Tsiambas, E.; Kagkelaris, K.; Pagkalou, M.; Plotas, P.; Mastronikoli, S.; Roukas, D.; Georgakopoulos, C.D. Fibroblast Growth Factor-2 (FGF-2) Expression in Pterygia Using Cell Spot Arrays. Vision 2022, 6, 58. https://doi.org/10.3390/vision6040058
Mastronikolis S, Tsiambas E, Kagkelaris K, Pagkalou M, Plotas P, Mastronikoli S, Roukas D, Georgakopoulos CD. Fibroblast Growth Factor-2 (FGF-2) Expression in Pterygia Using Cell Spot Arrays. Vision. 2022; 6(4):58. https://doi.org/10.3390/vision6040058
Chicago/Turabian StyleMastronikolis, Stylianos, Evangelos Tsiambas, Konstantinos Kagkelaris, Marina Pagkalou, Panagiotis Plotas, Sofianiki Mastronikoli, Dimitrios Roukas, and Constantinos D. Georgakopoulos. 2022. "Fibroblast Growth Factor-2 (FGF-2) Expression in Pterygia Using Cell Spot Arrays" Vision 6, no. 4: 58. https://doi.org/10.3390/vision6040058
APA StyleMastronikolis, S., Tsiambas, E., Kagkelaris, K., Pagkalou, M., Plotas, P., Mastronikoli, S., Roukas, D., & Georgakopoulos, C. D. (2022). Fibroblast Growth Factor-2 (FGF-2) Expression in Pterygia Using Cell Spot Arrays. Vision, 6(4), 58. https://doi.org/10.3390/vision6040058






