Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outcome Measures
2.2. Procedures and Instruments
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Financial Disclosure
References
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.-M.; Bahreini, E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- WHO. Weekly Epidemiological Update on COVID-19, 84th ed.; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Zhu, J.; Zhong, Z.; Ji, P.; Li, H.; Li, B.; Pang, J.; Zhang, J.; Zhao, C. Clinicopathological characteristics of 8697 patients with COVID-19 in China: A meta-analysis. Fam. Med. Community Health 2020, 8, e000406. [Google Scholar] [CrossRef] [PubMed]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Navarin, N.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020, 584, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Anyfanti, P.; Gavriilaki, M.; Lazaridis, A.; Douma, S.; Gkaliagkousi, E. Endothelial dysfunction in COVID-19: Lessons learned from coronaviruses. Curr. Hypertens. Rep. 2020, 22, 63. [Google Scholar] [CrossRef]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC council of basic cardiovascular science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Deanfield, J.; Donald, A.; Ferri, C.; Giannattasio, C.; Halcox, J.; Halligan, S.; Lerman, A.; Mancia, G.; Oliver, J.J.; Pessina, A.C.; et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: A statement by the working group on endothelin and endothelial factors of the European society of hypertension. J. Hypertens. 2005, 23, 7–17. [Google Scholar] [CrossRef]
- Charakida, M.; Masi, S.; Lüscher, T.F.; Kastelein, J.J.P.; Deanfield, J.E. Assessment of atherosclerosis: The role of flow-mediated dilatation. Eur. Heart J. 2010, 31, 2854–2861. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Santoro, L.; Falsetti, L.; Zaccone, V.; Nesci, A.; Tosato, M.; Giupponi, B.; Savastano, M.C.; Moroncini, G.; Gasbarrini, A.; Landi, F.; et al. Impaired Endothelial Function in Convalescent Phase of COVID-19: A 3 Month Follow up Observational Prospective Study. J. Clin. Med. 2022, 11, 1774. [Google Scholar] [CrossRef]
- Savastano, A.; Crincoli, E.; Savastano, M.C.; Younis, S.; Gambini, G.; De Vico, U.; Cozzupoli, G.M.; Culiersi, C.; Rizzo, S.; Gemelli against COVID-19 Post-Acute Care Study Group. Peripapillary retinal vascular involvement in early post-COVID-19 patients. J. Clin. Med. 2020, 9, 2895. [Google Scholar] [CrossRef]
- Invernizzi, A.; Torre, A.; Parrulli, S.; Zicarelli, F.; Schiuma, M.; Colombo, V.; Giacomelli, A.; Cigada, M.; Milazzo, L.; Ridolfo, A.; et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine 2020, 27, 100550. [Google Scholar] [CrossRef]
- Gemelli against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: The need for an interdisciplinary approach. Aging Clin. Exp. Res. 2020, 32, 1613–1620. [Google Scholar] [CrossRef]
- Daniels, A.B.; Froehler, M.T.; Nunnally, A.H.; Pierce, J.M.; Bozic, I.; Stone, C.A.; Santapuram, P.R.; Tao, Y.K.; Boyd, K.L.; Himmel, L.E.; et al. Rabbit model of intra-arterial chemotherapy toxicity demonstrates retinopathy and vasculopathy related to drug and dose, not procedure or approach. Invest. Ophthalmol. Vis. Sci. 2019, 60, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Maruhashi, T.; Kajikawa, M.; Kishimoto, S.; Hashimoto, H.; Takaeko, Y.; Yamaji, T.; Harada, T.; Han, Y.; Aibara, Y.; Yusoff, F.M.; et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J. Am. Heart Assoc. 2020, 9, e013915. [Google Scholar] [CrossRef]
- Minnella, A.M.; Barbano, L.; Verrecchia, E. Macular impairment in Fabry disease: A morpho-functional assessment by swept-source OCT angiography and focal electroretinography. Invest. Ophthalmol. Vis. Sci. 2019, 60, 2667–2675. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wang, J.C.; Zeng, R.; Katz, R.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Quantitative comparison of microvascular metrics on three optical coherence tomography angiography devices in chorioretinal disease. Clin. Ophthalmol. 2019, 13, 2063–2069. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.-L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 66008. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.J.; Chang, R.; LeTran, V.; Vu, B.; Burkemper, B.; Chu, Z.; Fard, A.; Kashani, A.; Xu, B.; Wang, R.; et al. Ocular determinants of peripapillary vessel density in healthy african americans: The African American eye disease study. Invest. Ophthalmol. Vis. Sci. 2019, 60, 3368–3373. [Google Scholar] [CrossRef]
- Walters, J.F.; Hampton, S.M.; Deanfield, J.E.; Donald, A.E.; Skene, D.J.; Ferns, G.A.A. Circadian variation in endothelial function is attenuated in postmenopausal women. Maturitas 2006, 54, 294–303. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; Matsumoto, T.; Oda, N.; et al. Endothelial dysfunction, increased arterial stiffness, and cardiovascular risk prediction in patients with coronary artery disease: FMD-J (Flow-Mediated Dilation Japan) study A. J. Am. Heart Assoc. 2018, 7, e008588. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Carmeliet, P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 2019, 30, 414–433. [Google Scholar] [CrossRef]
- Wang, M.; Hao, H.; Leeper, N.J.; Zhu, L.; Early career committee. Thrombotic regulation from the endothelial cell perspectives. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e90–e95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vabret, N.; Samstein, R.; Fernandez, N.; Merad, M.; Sinai Immunology Review Project. Advancing scientific knowledge in times of pandemics. Nat. Rev. Immunol. 2020, 20, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, T.; Xiao, R.; Lin, G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: A double-edged sword? FASEB J. 2020, 34, 6017–6026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, S. Defining vascular aging and cardiovascular risk. J. Hypertens. 2012, 30, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, Y.-Y.; Lv, T.-T.; Guan, S.-Y.; Li, X.-M.; Li, X.-P.; Pan, H.-F. Subclinical atherosclerosis in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. Angiology 2019, 70, 141–159. [Google Scholar] [CrossRef]
- Pereira, L.A.; Soares, L.C.M.; Nascimento, P.A.; Cirillo, L.R.N.; Sakuma, H.T.; Veiga, G.L.D.; Fonseca, F.L.A.; Lima, V.L.; Abucham-Neto, J.Z. Retinal findings in hospitalised patients with severe COVID-19. Br. J. Ophthalmol. 2020, 106, 102–105. [Google Scholar] [CrossRef]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef]
- Landecho, M.F.; Yuste, J.R.; Gándara, E.; Sunsundegui, P.; Quiroga, J.; Alcaide, A.B.; García-Layana, A. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J. Intern. Med. 2020, 289, 116–120. [Google Scholar] [CrossRef]
- Cennamo, G.; Reibaldi, M.; Montorio, D.; D’Andrea, L.; Fallico, M.; Triassi, M. Optical Coherence Tomography Angiography Features in Post-COVID-19 Pneumonia Patients: A Pilot Study. Am. J. Ophthalmol. 2021, 227, 182–190. [Google Scholar] [CrossRef]
- Flammer, J.; Konieczka, K.; Bruno, R.M.; Virdis, A.; Flammer, A.J.; Taddei, S. The eye and the heart. Eur. Heart J. 2013, 34, 1270–1278. [Google Scholar] [CrossRef]



| Variable | Total Population (82 Pts) | Impaired FMD (31/82 Pts) | Normal FMD (51 Pts) | p Univariate | p Regression |
|---|---|---|---|---|---|
| ANAMNESTIC DATA | |||||
| Age (years) | 52.9 ± 13.5 | 60.12 ± 11.9 (55.9–64.3) | 48.2 ± 12.6 (45.0–51.9) | <0.001 | 0.048 |
| Sex | M = 48/82 (58.5%) | 21/31 (67.7%) | 27/51(52.9%) | 0.18 | 0.411 |
| Systemic arterial hypertension | 19/82 (23.2%) | 13/31 (41.9%) | 6/51 (11.76%) | 0.002 | 0.417 |
| Diabetes | 36/82 (43.9%) | 18/31 (58.1%) | 18/51 (35.3%) | 0.04 | 0.046 |
| Autoimmune diseases | 21/82 (25.6%) | 7/31 (22.58%) | 14/51 (27.45%) | 0.62 | 0.211 |
| BMI score | 25.7 ± 4.3 | 25.5 ± 4.0 (24.0–26.9) | 25.7 ± 4.6 (24.5–27.0) | 0.78 | |
| BMI > 30 | 8/82 (9.7%) | 1/31 (3.2%) | 7/51 (13.7%) | 0.12 | 0.514 |
| Chronic kidney disease | 8/82 (9.8%) | 5/31 (16.1%) | 3/51 (5.9%) | 0.08 | 0.324 |
| Cognitive impairment | 7/82 (8.5%) | 4/31 (12.9%) | 3/51 (5.9%) | 0.12 | 0.731 |
| ADMISSION DATA | |||||
| Hydroxychloroquine | 57/82 (69.5%) | 25/31 (80.6%) | 32/51 (62.7%) | 0.09 | |
| Lopinavir + Ritonavir | 27/82 (32.9%) | 13/31 (41.9%) | 14/51 (27.5%) | 0.17 | 0.24 |
| Darunavir + Ritonavir | 35/82 (42.7%) | 14/31 (45.2%) | 21/51 (41.2%) | 0.72 | 0.36 |
| Heparin | 28/82 (34.1%) | 11/31 (35.5%) | 17/51(33.3%) | 0.84 | |
| Azithromycin | 33/82 (40.2%) | 14/31 (45.2%) | 14/51 (27.4%) | 0.10 | |
| Antiplatelet therapy | 6/82 (7.31%) | 4/31 (12.9%) | 2/51 (3.9%) | 0.12 | |
| Corticosteroids | 4/82 (4.87%) | 1/31 (3.2%) | 3/51 (5.8%) | 0.59 | |
| ICU admission | 9/82 (10.9%) | 5/31 (16.1%) | 4/51 (7.8%) | 0.62 | 0.581 |
| Oxygen therapy | 33/82 (40.2%) | 14/31 (45.2%) | 19/51 (37.3%) | 0.49 | 0.612 |
| Non-invasive ventilation | 11/82 (13.4%) | 4/31 (12.9%) | 7/51 (13.7%) | 0.93 | 0.866 |
| Invasive ventilation | 4/82 (4.9%) | 2/31 (6.5%) | 2/51 (3.9%) | 0.76 | 0.643 |
| Pulmonary embolism | 2/82 (2.4%) | 1/31 (3.2%) | 1/51 (2%) | 0.88 | |
| Venous thrombosis | 2/82 (2.4%) | 2/31 (6.5%) | 0/51 (0%) | 0.19 | |
| Variable | Linear Correlation To FMD | p | ||
|---|---|---|---|---|
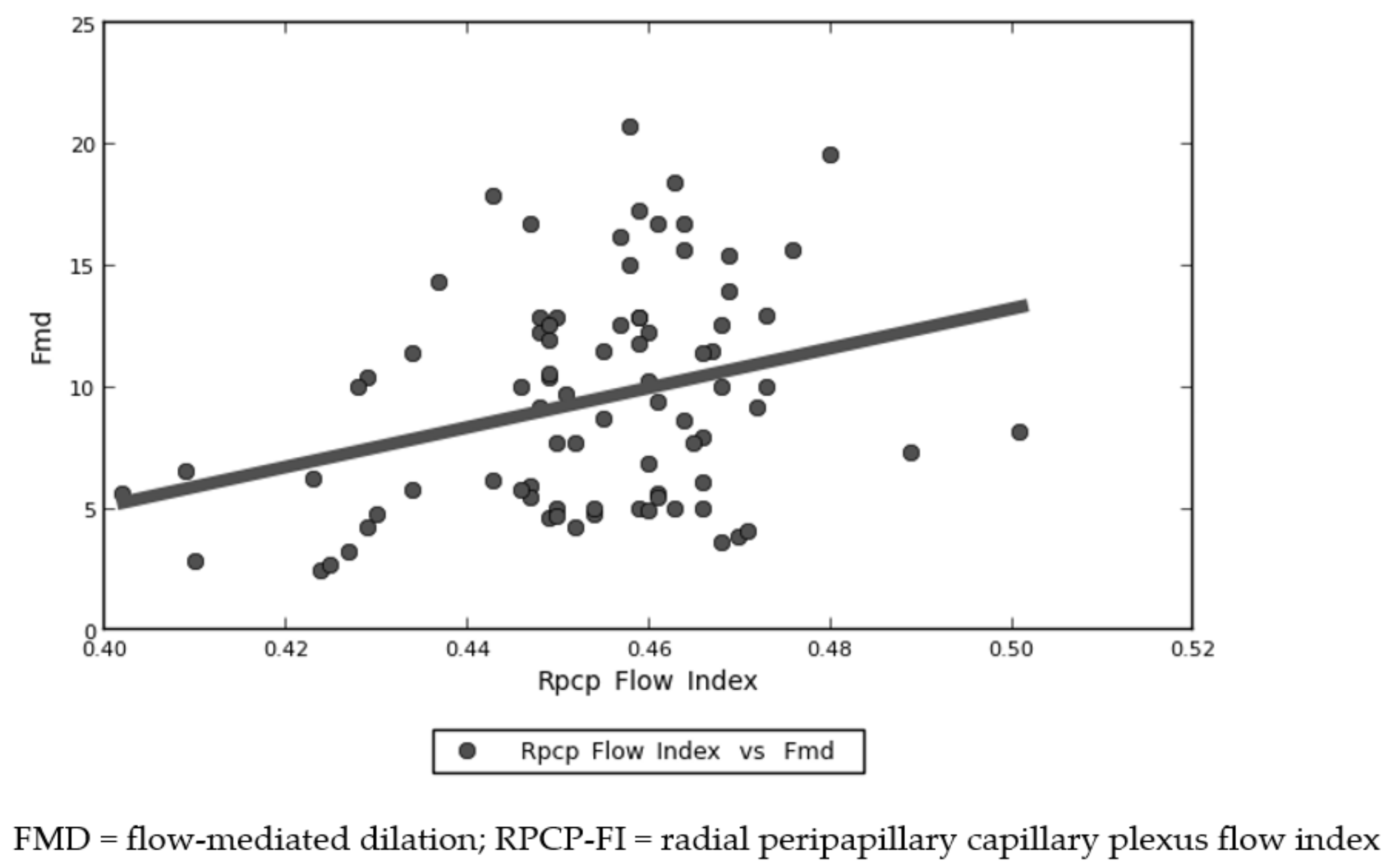
| RPCP-FI | R = 0.244 | Slope = 81.455 | Intercept = −27.467 | 0.027 |
| RPCP-D | R = 0.212 | Slope = 26.916 | Intercept = −2.289 | 0.055 |
| SCP-D | R = 0.116 | Slope = 0.428 | Intercept = 0.488 | 0.31 |
| DCP-D | R = 0.110 | Slope = 0.523 | Intercept = 0.451 | 0.46 |
| SCT | R = 0.2 | Slope = 0.014 | Intercept = 5.02 | 0.072 |
| FAZ area | R = −0.031 | Slope = −3.14 | Intercept = 10.329 | 0.78 |
| FAZ perimeter | R = −0.117 | Slope = −1.251 | Intercept = 12.16 | 0.31 |
| Variable | Total Population (82 Patients) | Impaired FMD (31/82 Patients) | Normal FMD (51/82 Patients) | p Univariate | p Regression |
|---|---|---|---|---|---|
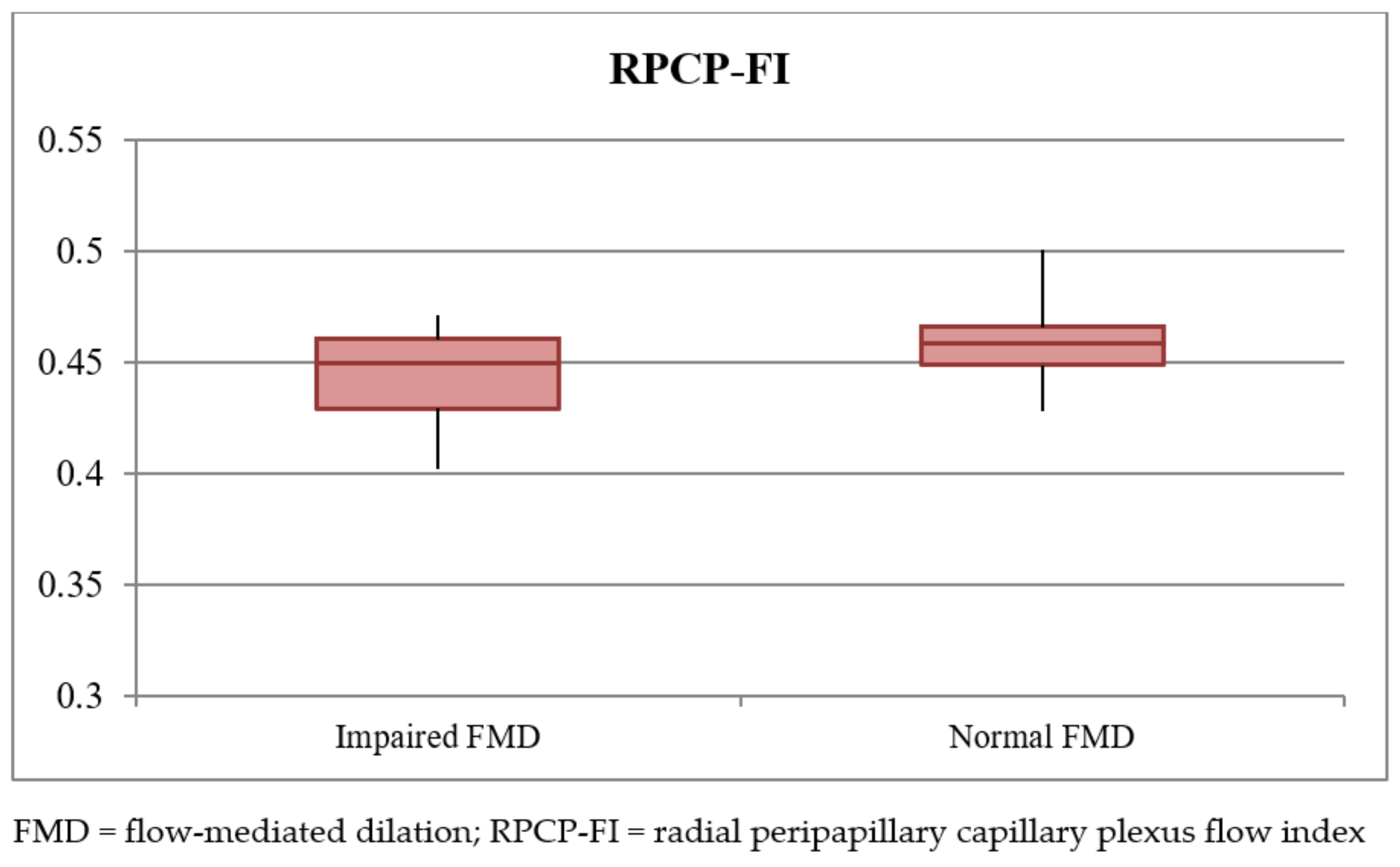
| RPCP-FI | 0.452 ± 0.017 | 0.445 ± 0.019 (0.439–0.452) | 0.458 ± 0.014 (0.455–0.462) | <0.001 | 0.047 |
| RPCP-D | 0.437 ± 0.031 | 0.432 ± 0.037 (0.419–0.445) | 0.441 ± 0.027 (0.433–0.448) | 0.21 | 0.055 |
| SCP-D | 21.27 ± 1.32 | 21.05 ± 1.50 (20.50–21.59) | 21.40 ± 1.22 (21.07–21.74) | 0.25 | 0.31 |
| SCP-P | 0.385 ± 0.021 | 0.371 ± 0.08 (0.380–0.369) | 0.390 ± 0.12 (0.396–0.377) | 0.45 | 0.63 |
| DCP-D | 21.82 ± 2.51 | 21.96 ± 2.38 (21.44–22.23) | 21.56 ± 2.52 (21.24–21.73) | 0.37 | 0.43 |
| DCP-P | 0.456 ± 0.03 | 0.453 ± 0.05 (CI 0.448–0.460) | 0.459 ± 0.01 (CI 0.453–0.465) | 0.86 | 0.77 |
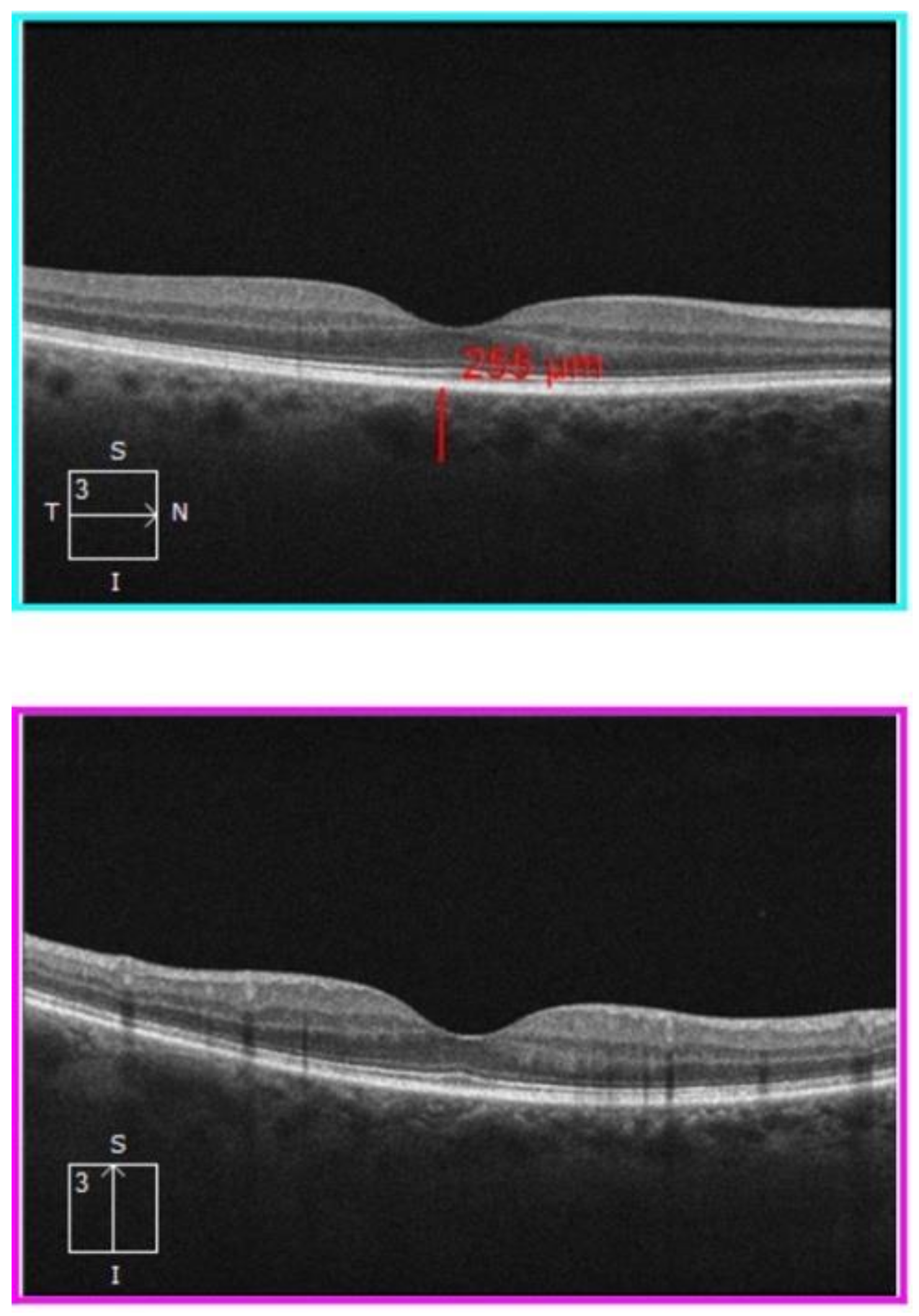
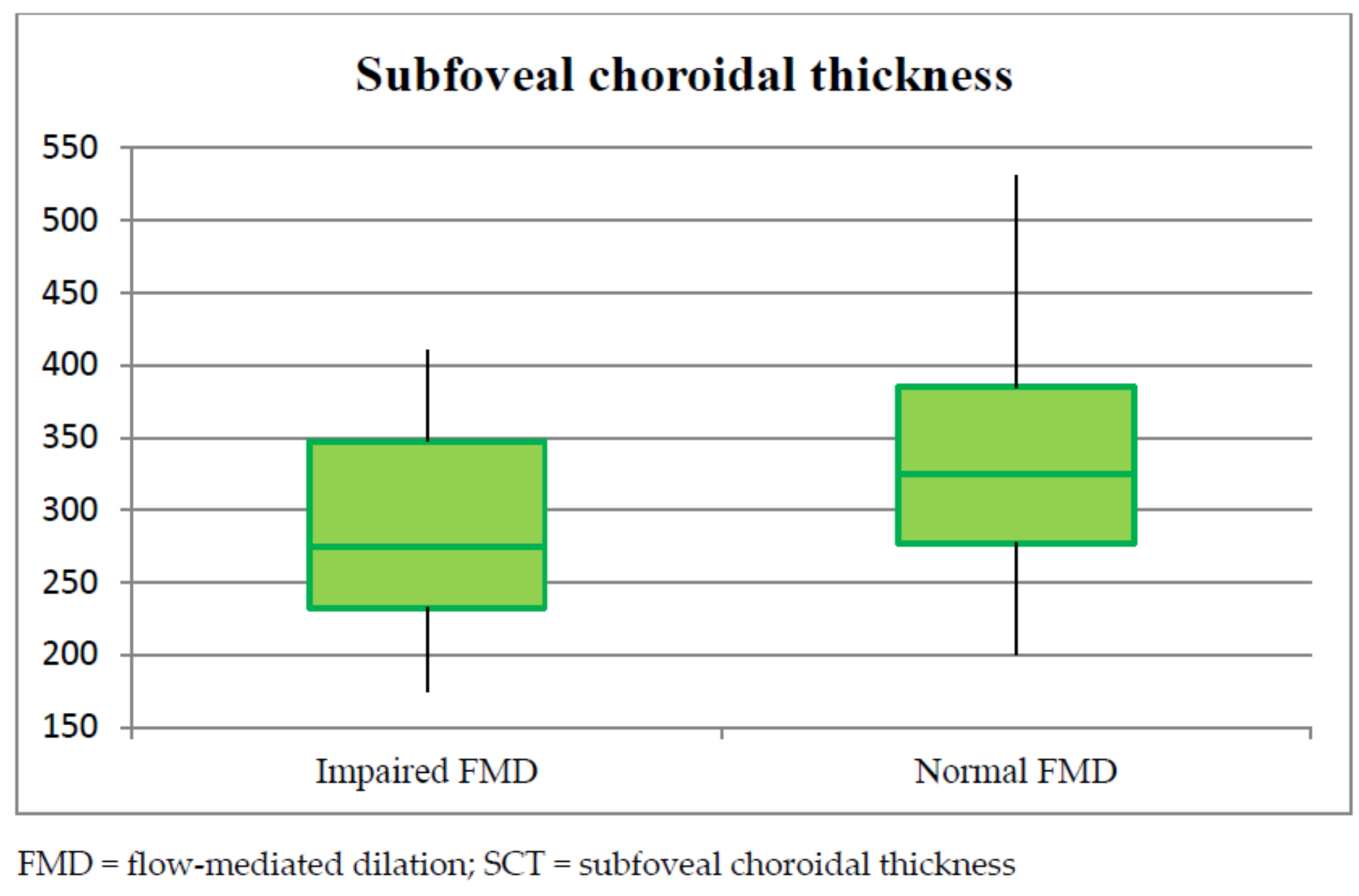
| SCT | 310.46 ± 43.13 | 278.45 ± 79.36 (250.51–306.39) | 329.92 ± 47.38 (308.69–351.16) | 0.004 | 0.07 |
| FAZ area | 0.237 ± 0.106 | 0.236 ± 0.099 (0.200–0.271) | 0.238 ± 0.112 (0.207–0.269) | 0.91 | 0.85 |
| FAZ perimeter | 2.058 ± 0.506 | 2.082 ± 0.427 (1.926–2.237) | 2.045 ± 0.553 (1.892–2.199) | 0.76 | 0.76 |
| Cotton wool spots | 10/82 (12.2%) | 4/31 (12.9%) | 6/51 (11.8%) | 0.32 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savastano, M.C.; Santoro, L.; Crincoli, E.; Fossataro, C.; Gambini, G.; Savastano, A.; De Vico, U.; Santoliquido, A.; Nesci, A.; Landi, F.; et al. Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection. Vision 2022, 6, 26. https://doi.org/10.3390/vision6020026
Savastano MC, Santoro L, Crincoli E, Fossataro C, Gambini G, Savastano A, De Vico U, Santoliquido A, Nesci A, Landi F, et al. Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection. Vision. 2022; 6(2):26. https://doi.org/10.3390/vision6020026
Chicago/Turabian StyleSavastano, Maria Cristina, Luca Santoro, Emanuele Crincoli, Claudia Fossataro, Gloria Gambini, Alfonso Savastano, Umberto De Vico, Angelo Santoliquido, Antonio Nesci, Francesco Landi, and et al. 2022. "Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection" Vision 6, no. 2: 26. https://doi.org/10.3390/vision6020026
APA StyleSavastano, M. C., Santoro, L., Crincoli, E., Fossataro, C., Gambini, G., Savastano, A., De Vico, U., Santoliquido, A., Nesci, A., Landi, F., Rizzo, S., & on behalf of Gemelli against COVID-19 Post-Acute Care Study Group. (2022). Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection. Vision, 6(2), 26. https://doi.org/10.3390/vision6020026







