Overview on Defocus Incorporated Multiple Segments Lenses: A Novel Perspective in Myopia Progression Management
Abstract
:1. Introduction
2. Approaches for Myopia Management
2.1. Pharmacological Treatment
2.2. Ortho-Keratology
2.3. Contact Lenses
2.4. Spectacle Lenses
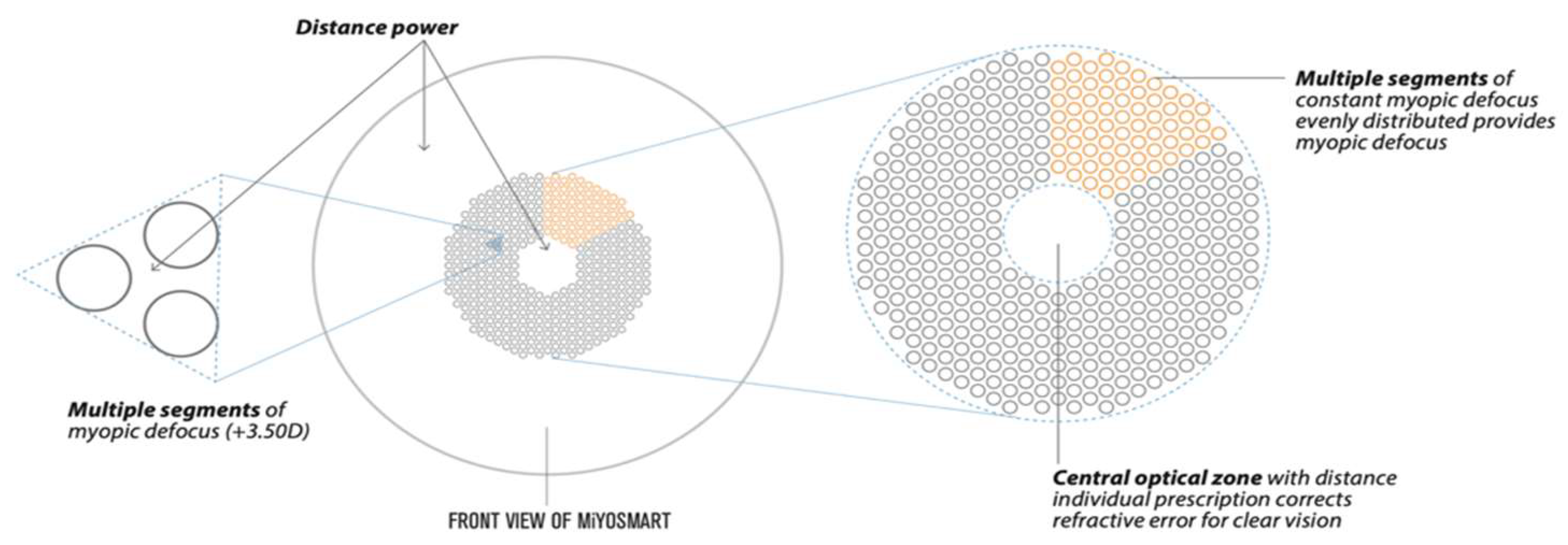
3. DIMS Lens Structure
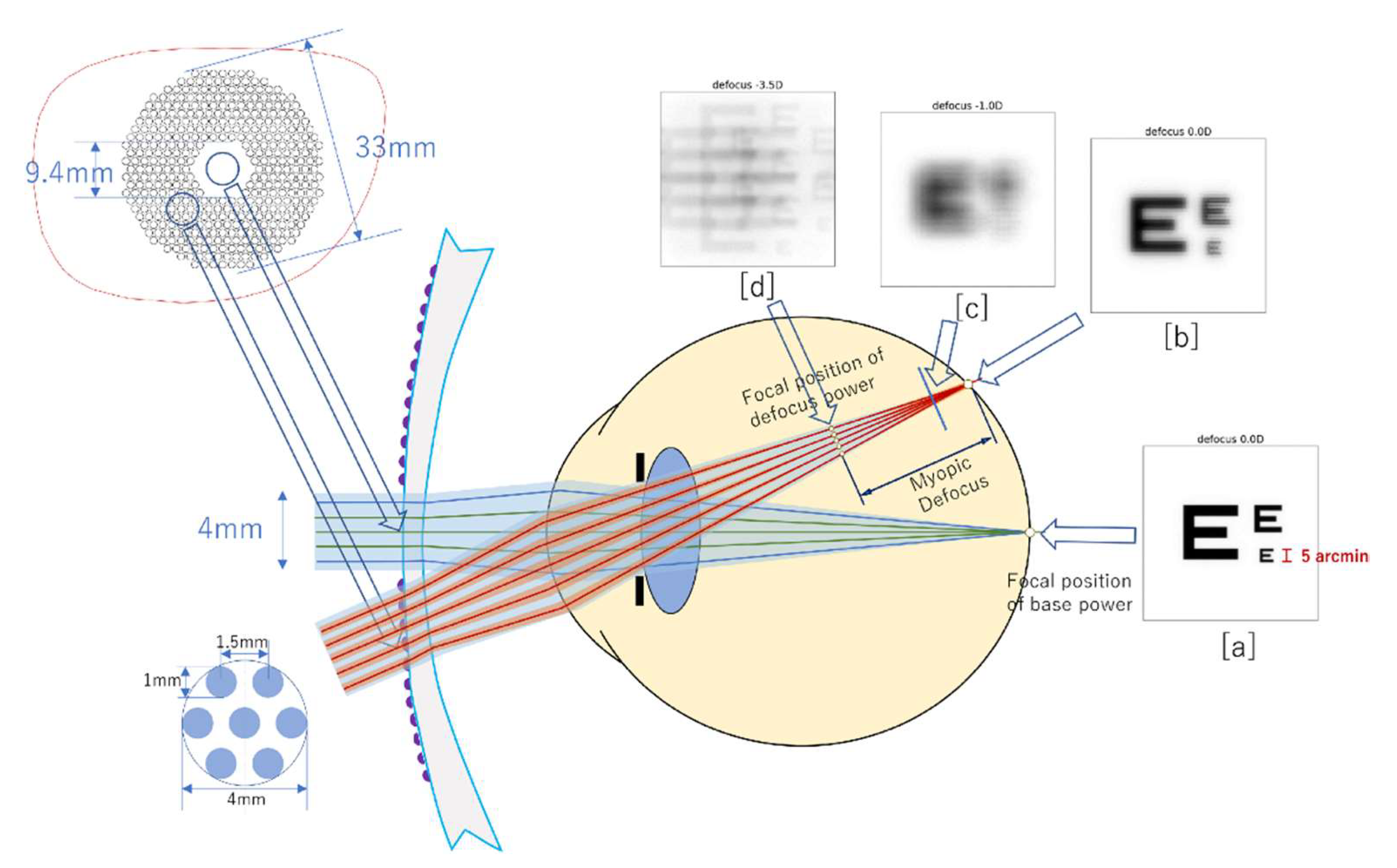
4. Optical Principles
5. Prior Acknowledgements for DIMS Use
6. Outcomes on Myopia Progression
7. Functional and Tolerability Outcomes
8. DIMS Competitors
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI–Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resnikoff, S.; Jonas, J.B.; Friedman, D.; He, M.; Jong, M.; Nichols, J.J.; Ohno-Matsui, K.; Smith, E.L., III; Wildsoet, C.F.; Taylor, H.R. Myopia—A 21st century public health issue. Investig. Ophthalmol. Vis. Sci. 2019, 60, Mi–Mii. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildsoet, C.F.; Chia, A.; Cho, P.; Guggenheim, J.A.; Polling, J.R.; Read, S.; Sankaridurg, P.; Saw, S.-M.; Trier, K.; Walline, J.J. IMI–interventions for controlling myopia onset and progression report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M106–M131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, I.G.; French, A.N.; Ashby, R.S.; Guo, X.; Ding, X.; He, M.; Rose, K.A. The epidemics of myopia: Aetiology and prevention. Prog. Retin. Eye Res. 2018, 62, 134–149. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhou, J.; Zhao, P.; Lian, J.; Zhu, H.; Zhou, Y.; Sun, Y.; Wang, Y.; Zhao, L.; Wei, Y.; et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7504–7509. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.K.; Lee, J.H.; Kakizaki, H.; Jee, D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul, South Korea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5579–5583. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.M.; Bertelsen, G.; Cumberland, P.; Wolfram, C.; Verhoeven, V.J.; Anastasopoulos, E.; Buitendijk, G.H.; Cougnard-Gregoire, A.; Creuzot-Garcher, C.; Erke, M.G.; et al. Increasing Prevalence of Myopia in Europe and the Impact of Education. Ophthalmology 2015, 122, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.M.; Verhoeven, V.J.; Cumberland, P.; Bertelsen, G.; Wolfram, C.; Buitendijk, G.H.; Hofman, A.; van Duijn, C.M.; Vingerling, J.R.; Kuijpers, R.W.; et al. Prevalence of refractive error in Europe: The European Eye Epidemiology (E(3)) Consortium. Eur. J. Epidemiol. 2015, 30, 305–315. [Google Scholar] [CrossRef]
- Ang, M.; Wong, T.Y. Updates on Myopia: A Clinical Perspective; Springer: Berlin, Germany, 2020. [Google Scholar]
- Ruiz-Medrano, J.; Montero, J.A.; Flores-Moreno, I.; Arias, L.; Garcia-Layana, A.; Ruiz-Moreno, J.M. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Prog. Retin. Eye Res. 2019, 69, 80–115. [Google Scholar] [CrossRef]
- Gifford, K.L.; Richdale, K.; Kang, P.; Aller, T.A.; Lam, C.S.; Liu, Y.M.; Michaud, L.; Mulder, J.; Orr, J.B.; Rose, K.A. IMI–clinical management guidelines report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M184–M203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, A.; Chua, W.H.; Cheung, Y.B.; Wong, W.L.; Lingham, A.; Fong, A.; Tan, D. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012, 119, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Chen, C.T.; Lin, K.K.; Sun, C.C.; Kuo, C.N.; Huang, H.M.; Poon, Y.C.; Yang, M.L.; Chen, C.Y.; Huang, J.C.; et al. Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology 2018, 125, 1239–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neitz, J.; Kuchenbecker, J.; Neitz, M. Ophthalmic Lenses for Treating Myopia. 2020. Available online: https://patents.google.com/patent/WO2018026697A1/en (accessed on 30 December 2021).
- Chamberlain, P.; Peixoto-de-Matos, S.C.; Logan, N.S.; Ngo, C.; Jones, D.; Young, G. A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom. Vis. Sci. 2019, 96, 556–567. [Google Scholar] [CrossRef] [Green Version]
- VanderVeen, D.K.; Kraker, R.T.; Pineles, S.L.; Hutchinson, A.K.; Wilson, L.B.; Galvin, J.A.; Lambert, S.R. Use of orthokeratology for the prevention of myopic progression in children: A report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 623–636. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wen, D.; Wang, Q.; McAlinden, C.; Flitcroft, I.; Chen, H.; Saw, S.M.; Chen, H.; Bao, F.; Zhao, Y.; et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology 2016, 123, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Sankaridurg, P.; Naduvilath, T.; Zang, J.; Zou, H.; Zhu, J.; Lv, M.; He, X.; Xu, X. Time spent in outdoor activities in relation to myopia prevention and control: A meta-analysis and systematic review. Acta Ophthalmol. 2017, 95, 551–566. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.S.; Park, S. Recent updates on myopia control: Preventing progression 1 diopter at a time. Curr. Opin. Ophthalmol. 2019, 30, 215–219. [Google Scholar] [CrossRef]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.P.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef]
- Wu, P.-C.; Chuang, M.-N.; Choi, J.; Chen, H.; Wu, G.; Ohno-Matsui, K.; Jonas, J.B.; Cheung, C.M.G. Update in myopia and treatment strategy of atropine use in myopia control. Eye 2019, 33, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.D.M.; Tran, Y.H.; Tran, T.D.; Jong, M.; Coroneo, M.; Sankaridurg, P. A Review of Myopia Control with Atropine. J. Ocul. Pharmacol. Ther. 2018, 34, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.D.; Caroline, P.J.; Harlin, D.D.; Papas, E.B.; Holden, B.A. Morphologic changes in cat epithelium following continuous wear of orthokeratology lenses: A pilot study. Cont. Lens. Anterior Eye 2008, 31, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, T.; Kakita, T.; Okamoto, F.; Takahashi, H.; Oshika, T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: A 5-year follow-up study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3913–3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walline, J.J.; Jones, L.A.; Sinnott, L.T. Corneal reshaping and myopia progression. Br. J. Ophthalmol. 2009, 93, 1181–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.M.; Xie, P. The safety of orthokeratology—a systematic review. Eye Contact Lens. 2016, 42, 35. [Google Scholar] [CrossRef]
- Bullimore, M.A.; Johnson, L.A. Overnight orthokeratology. Cont. Lens Anterior Eye 2020, 43, 322–332. [Google Scholar] [CrossRef]
- Bullimore, M.A.; Sinnott, L.T.; Jones-Jordan, L.A. The risk of microbial keratitis with overnight corneal reshaping lenses. Optom. Vis. Sci. 2013, 90, 937–944. [Google Scholar] [CrossRef]
- Ruiz-Pomeda, A.; Perez-Sanchez, B.; Valls, I.; Prieto-Garrido, F.L.; Gutierrez-Ortega, R.; Villa-Collar, C. MiSight Assessment Study Spain (MASS). A 2-year randomized clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1011–1021. [Google Scholar] [CrossRef]
- Aller, T.A.; Liu, M.; Wildsoet, C.F. Myopia Control with Bifocal Contact Lenses: A Randomized Clinical Trial. Optom. Vis. Sci. 2016, 93, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Anstice, N.S.; Phillips, J.R. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology 2011, 118, 1152–1161. [Google Scholar] [CrossRef]
- Lam, C.S.; Tang, W.C.; Tse, D.Y.; Tang, Y.Y.; To, C.H. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2014, 98, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Sankaridurg, P.; Bakaraju, R.C.; Naduvilath, T.; Chen, X.; Weng, R.; Tilia, D.; Xu, P.; Li, W.; Conrad, F.; Smith, E.L., 3rd; et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol. Opt. 2019, 39, 294–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaridurg, P.; Holden, B.; Smith, E., 3rd; Naduvilath, T.; Chen, X.; de la Jara, P.L.; Martinez, A.; Kwan, J.; Ho, A.; Frick, K.; et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: One-year results. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9362–9367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paune, J.; Morales, H.; Armengol, J.; Quevedo, L.; Faria-Ribeiro, M.; Gonzalez-Meijome, J.M. Myopia Control with a Novel Peripheral Gradient Soft Lens and Orthokeratology: A 2-Year Clinical Trial. BioMed Res. Int. 2015, 2015, 507572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujikado, T.; Ninomiya, S.; Kobayashi, T.; Suzaki, A.; Nakada, M.; Nishida, K. Effect of low-addition soft contact lenses with decentered optical design on myopia progression in children: A pilot study. Clin. Ophthalmol. 2014, 8, 1947–1956. [Google Scholar] [CrossRef] [Green Version]
- Li, S.M.; Kang, M.T.; Wu, S.S.; Meng, B.; Sun, Y.Y.; Wei, S.F.; Liu, L.; Peng, X.; Chen, Z.; Zhang, F. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: A meta-analysis. Ophthalmic Physiol. Opt. 2017, 37, 51–59. [Google Scholar] [CrossRef]
- Walline, J.J.; Walker, M.K.; Mutti, D.O.; Jones-Jordan, L.A.; Sinnott, L.T.; Giannoni, A.G.; Bickle, K.M.; Schulle, K.L.; Nixon, A.; Pierce, G.E.; et al. Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. JAMA 2020, 324, 571–580. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef] [Green Version]
- Tay, S.A.; Farzavandi, S.; Tan, D. Interventions to Reduce Myopia Progression in Children. Strabismus 2017, 25, 23–32. [Google Scholar] [CrossRef]
- Troilo, D.; Smith, E.L.; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.-s. IMI–Report on experimental models of emmetropization and myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef] [Green Version]
- Walline, J.J.; Lindsley, K.B.; Vedula, S.S.; Cotter, S.A.; Mutti, D.O.; Ng, S.M.; Twelker, J.D. Interventions to slow progression of myopia in children. Cochrane Database Syst. Rev. 2020, 1, CD004916. [Google Scholar] [PubMed] [Green Version]
- Kanda, H.; Oshika, T.; Hiraoka, T.; Hasebe, S.; Ohno-Matsui, K.; Ishiko, S.; Hieda, O.; Torii, H.; Varnas, S.R.; Fujikado, T. Effect of spectacle lenses designed to reduce relative peripheral hyperopia on myopia progression in Japanese children: A 2-year multicenter randomized controlled trial. Jpn. J. Ophthalmol. 2018, 62, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, J.E.; Hyman, L.; Norton, T.T.; Hussein, M.E.; Marsh-Tootle, W.; Manny, R.; Wang, Y.; Everett, D. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group. Progressive-addition lenses versus single-vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2749–2757. [Google Scholar]
- Gwiazda, J.; Hyman, L.; Hussein, M.; Everett, D.; Norton, T.T.; Kurtz, D.; Leske, M.C.; Manny, R.; Marsh-Tootle, W.; Scheiman, M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1492–1500. [Google Scholar] [CrossRef]
- Hasebe, S.; Jun, J.; Varnas, S.R. Myopia control with positively aspherized progressive addition lenses: A 2-year, multicenter, randomized, controlled trial. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7177–7188. [Google Scholar] [CrossRef]
- Hasebe, S.; Ohtsuki, H.; Nonaka, T.; Nakatsuka, C.; Miyata, M.; Hamasaki, I.; Kimura, S. Effect of progressive addition lenses on myopia progression in Japanese children: A prospective, randomized, double-masked, crossover trial. Investig. Ophthalmol. Vis. Sci 2008, 49, 2781–2789. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Tang, W.C.; Tse, D.Y.; Lee, R.P.K.; Chun, R.K.M.; Hasegawa, K.; Qi, H.; Hatanaka, T.; To, C.H. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2020, 104, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fu, Y.; Wang, K.; Liu, Z.; Shi, X.; Zhao, M. Evaluating the myopia progression control efficacy of defocus incorporated multiple segments (DIMS) lenses and Apollo progressive addition spectacle lenses (PALs) in 6- to 12-year-old children: Study protocol for a prospective, multicenter, randomized controlled trial. Trials 2020, 21, 279. [Google Scholar]
- Lu, Y.; Lin, Z.; Wen, L.; Gao, W.; Pan, L.; Li, X.; Yang, Z.; Lan, W. The Adaptation and Acceptance of Defocus Incorporated Multiple Segment Lens for Chinese Children. Am. J. Ophthalmol. 2020, 211, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Kaymak, H.; Graff, B.; Neller, K.; Langenbucher, A.; Seitz, B.; Schwahn, H. Myopia treatment and prophylaxis with defocus incorporated multiple segments spectacle lenses. Ophthalmologe 2021, 118, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Mutti, D.O.; Sholtz, R.I.; Friedman, N.E.; Zadnik, K. Peripheral refraction and ocular shape in children. Investig. Ophthalmol Vis. Sci. 2000, 41, 1022–1030. [Google Scholar]
- Sng, C.C.; Lin, X.Y.; Gazzard, G.; Chang, B.; Dirani, M.; Chia, A.; Selvaraj, P.; Ian, K.; Drobe, B.; Wong, T.Y.; et al. Peripheral refraction and refractive error in singapore chinese children. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Sng, C.C.; Lin, X.Y.; Gazzard, G.; Chang, B.; Dirani, M.; Lim, L.; Selvaraj, P.; Ian, K.; Drobe, B.; Wong, T.Y.; et al. Change in peripheral refraction over time in Singapore Chinese children. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7880–7887. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.T.; Cho, P. Relative peripheral refraction in children: Twelve-month changes in eyes with different ametropias. Ophthalmic Physiol. Opt. 2013, 33, 283–293. [Google Scholar] [CrossRef]
- Radhakrishnan, H.; Allen, P.M.; Calver, R.I.; Theagarayan, B.; Price, H.; Rae, S.; Sailoganathan, A.; O’Leary, D.J. Peripheral refractive changes associated with myopia progression. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1573–1581. [Google Scholar] [CrossRef] [Green Version]
- Mutti, D.O.; Sinnott, L.T.; Mitchell, G.L.; Jones-Jordan, L.A.; Moeschberger, M.L.; Cotter, S.A.; Kleinstein, R.N.; Manny, R.E.; Twelker, J.D.; Zadnik, K.; et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Investig. Ophthalmol. Vis. Sci. 2011, 52, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Mutti, D.O.; Hayes, J.R.; Mitchell, G.L.; Jones, L.A.; Moeschberger, M.L.; Cotter, S.A.; Kleinstein, R.N.; Manny, R.E.; Twelker, J.D.; Zadnik, K. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2510–2519. [Google Scholar] [CrossRef]
- Hoogerheide, J.; Rempt, F.; Hoogenboom, W.P. Acquired myopia in young pilots. Ophthalmologica 1971, 163, 209–215. [Google Scholar] [CrossRef]
- Smith, E.L., 3rd; Hung, L.F.; Arumugam, B. Visual regulation of refractive development: Insights from animal studies. Eye 2014, 28, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wildsoet, C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wildsoet, C. The effective add inherent in 2-zone negative lenses inhibits eye growth in myopic young chicks. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5085–5093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, B.; Hung, L.F.; To, C.H.; Holden, B.; Smith, E.L., 3rd. The effects of simultaneous dual focus lenses on refractive development in infant monkeys. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7423–7432. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Hung, L.F.; To, C.H.; Sankaridurg, P.; Smith, E.L., III. The Effects of the Relative Strength of Simultaneous Competing Defocus Signals on Emmetropization in Infant Rhesus Monkeys. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3949–3960. [Google Scholar] [CrossRef] [Green Version]
- Benavente-Perez, A.; Nour, A.; Troilo, D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Investig. Ophthalmol Vis. Sci. 2012, 53, 6479–6487. [Google Scholar] [CrossRef] [Green Version]
- McFadden, S.A.; Tse, D.Y.; Bowrey, H.E.; Leotta, A.J.; Lam, C.S.; Wildsoet, C.F.; To, C.H. Integration of defocus by dual power Fresnel lenses inhibits myopia in the mammalian eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Tarutta, E.; Proskurina, O.V.; Milash, S.; Ibatulin, R.; Tarasova, N.; Kovychev, A.; Smirnova, T.; Markosyan, G.; Khodzhabekyan, N.; Maksimova, M. Peripheral defocus induced by «Perifocal-M» spectacles and myopia progression in children. Russ. Pediatr. Ophthalmol. 2015, 10, 33–37. [Google Scholar]
- Sankaridurg, P.; Donovan, L.; Varnas, S.; Ho, A.; Chen, X.; Martinez, A.; Fisher, S.; Lin, Z.; Smith, E.L., 3rd; Ge, J.; et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom. Vis. Sci. 2010, 87, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.S.Y.; Tang, W.C.; Qi, H.; Radhakrishnan, H.; Hasegawa, K.; To, C.H.; Charman, W.N. Effect of Defocus Incorporated Multiple Segments Spectacle Lens Wear on Visual Function in Myopic Chinese Children. Transl. Vis. Sci. Technol. 2020, 9, 11. [Google Scholar] [CrossRef]
- Stone, R.A.; Flitcroft, D.I. Ocular shape and myopia. Ann. Acad. Med. Singap. 2004, 33, 7–15. [Google Scholar]
- Verkicharla, P.K.; Mathur, A.; Mallen, E.A.; Pope, J.M.; Atchison, D.A. Eye shape and retinal shape, and their relation to peripheral refraction. Ophthalmic Physiol. Opt. 2012, 32, 184–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaskulski, M.; Singh, N.K.; Bradley, A.; Kollbaum, P.S. Optical and imaging properties of a novel multi-segment spectacle lens designed to slow myopia progression. Ophthalmic Physiol. Opt. 2020, 40, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Tideman, J.W.L.; Polling, J.R.; Vingerling, J.R.; Jaddoe, V.W.V.; Williams, C.; Guggenheim, J.A.; Klaver, C.C.W. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018, 96, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Tang, W.C.; Lee, P.H.; Zhang, H.Y.; Qi, H.; Hasegawa, K.; To, C.H. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: Results of a 3-year follow-up study. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Lam, C.S.Y.; Tang, W.C.; Leung, M.; To, C.H. Defocus Incorporated Multiple Segments Spectacle Lenses Changed the Relative Peripheral Refraction: A 2-Year Randomized Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2020, 61, 53. [Google Scholar] [CrossRef]
- Chen, A.H.; O’Leary, D.J.; Howell, E.R. Near visual function in young children. Part I: Near point of convergence. Part II: Amplitude of accommodation. Part III: Near heterophoria. Ophthalmic Physiol. Opt. 2000, 20, 185–198. [Google Scholar] [CrossRef]
- Edwards, M.H.; Law, L.F.; Lee, C.M.; Leung, K.M.; Lui, W.O. Clinical norms for amplitude of accommodation in Chinese. Ophthalmic Physiol. Opt. 1993, 13, 199–204. [Google Scholar] [CrossRef]
- Castagno, V.D.; Vilela, M.A.; Meucci, R.D.; Resende, D.P.; Schneid, F.H.; Getelina, R.; Nasiloski, M.R.; Fassa, A.G. Amplitude of Accommodation in Schoolchildren. Curr. Eye Res. 2017, 42, 604–610. [Google Scholar] [CrossRef]
- Ryu, H.; Ju, U.; Wallraven, C. Myopia-correcting lenses decrease eye fatigue in a visual search task for both adolescents and adults. PLoS ONE 2021, 16, e0258441. [Google Scholar] [CrossRef]
- Bao, J.; Yang, A.; Huang, Y.; Li, X.; Pan, Y.; Ding, C.; Lim, E.W.; Zheng, J.; Spiegel, D.P.; Drobe, B.; et al. One-year myopia control efficacy of spectacle lenses with aspherical lenslets. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
| Treatment Effect in Retarding Myopia Progression over the Study Period | ||||||
|---|---|---|---|---|---|---|
| Study | Period (Months) | Age | Criteria of Rx (D) | Type of Interventions and Sample Size | Mean Difference in D (%) | Mean Difference in mm of AL (%) |
| Gwiazda et al. (2003) [45] | 36 | 6–11 | −1.25 to −4.5 | SVL, n = 233; PAL (2 D Add), n = 229 | −0.20 (14%) | −0.11 (15%) |
| Hasebe et al. (2008) [47] | 18 | 6–12 | −1.25 to −6 | SVL, n = 44; PAL(1.5 D Add), n = 42 | −0.31 (18%) | - |
| COMET2 and PEDIG (2011) [49] | 36 | 8–12 | −0.75 to −2.50 | SV, n = 58 PAL (2 D Add), n = 52 | −0.28 (24%) | - |
| Anstice and Phillips (2011) [32] | 1st: 10 2nd: 20 | 11–14 | −1.25 to −4.5 | SV CL, n = 40 DF (2 D MD), n = 40 | 1st: −0.25 (37%) 2nd: −0.2 (54%) | 1st: −0.11 (49%) 2nd: −0.12 (80%) |
| Sankaridurg et al., (2011) [35] | 12 | 7–14 | −0.75 to −3.5 | SVL, n = 40 novel CL, n = 45 | −0.29 (34%) | −0.13 (33%) |
| Lam et al. (2014) [33] | 24 | 8–13 | −1 to −5 | SV CL, n = 63 DISC, n = 65 | −0.21 (25%) | −0.11 (30%) |
| Chamberlain et al. (2019) [16] | 36 | 8–12 | −0.75 to −4 | SV, n = 74 MiSight CL, n = 70 | −0.73 (59%) | −0.32 (52%) |
| Walline et al. (2020) [39] | 36 | 7–11 | −0.75 to −5 | SV, n = 98 High.add power CL, n = 98 | −0.46 (43%) | −0.23 (36%) |
| Lam et al. (2020) [50] | 24 | 8–13 | −1 to −5 | SV, n = 81 DIMS, n = 79 | −0.55 (52%) | −0.32 (62%) |
| Lam et al. (2021) [76] | 12 (3rd year of previous trial) | 10–15 | −1 to −5 | Control group, n = 76 Control-to-DIMS, n = 55 | −0.30 (86%) | −0.12 (61%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlà, M.M.; Boselli, F.; Giannuzzi, F.; Gambini, G.; Caporossi, T.; De Vico, U.; Savastano, A.; Baldascino, A.; Rizzo, C.; Kilian, R.; et al. Overview on Defocus Incorporated Multiple Segments Lenses: A Novel Perspective in Myopia Progression Management. Vision 2022, 6, 20. https://doi.org/10.3390/vision6020020
Carlà MM, Boselli F, Giannuzzi F, Gambini G, Caporossi T, De Vico U, Savastano A, Baldascino A, Rizzo C, Kilian R, et al. Overview on Defocus Incorporated Multiple Segments Lenses: A Novel Perspective in Myopia Progression Management. Vision. 2022; 6(2):20. https://doi.org/10.3390/vision6020020
Chicago/Turabian StyleCarlà, Matteo Mario, Francesco Boselli, Federico Giannuzzi, Gloria Gambini, Tomaso Caporossi, Umberto De Vico, Alfonso Savastano, Antonio Baldascino, Clara Rizzo, Raphael Kilian, and et al. 2022. "Overview on Defocus Incorporated Multiple Segments Lenses: A Novel Perspective in Myopia Progression Management" Vision 6, no. 2: 20. https://doi.org/10.3390/vision6020020
APA StyleCarlà, M. M., Boselli, F., Giannuzzi, F., Gambini, G., Caporossi, T., De Vico, U., Savastano, A., Baldascino, A., Rizzo, C., Kilian, R., & Rizzo, S. (2022). Overview on Defocus Incorporated Multiple Segments Lenses: A Novel Perspective in Myopia Progression Management. Vision, 6(2), 20. https://doi.org/10.3390/vision6020020






