Validation of Digital Applications for Evaluation of Visual Parameters: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
- Articles showing the use of tests in healthy patients to evaluate any aspect of the visual function using tablets or smartphones;
- Articles written in English.
(“visual function” OR “visual acuity”) AND (“iPad” OR “app”) NOT (“Mice model” OR “Animal model”) NOT (“Amblyopia treatment” OR “ocular diseases”)
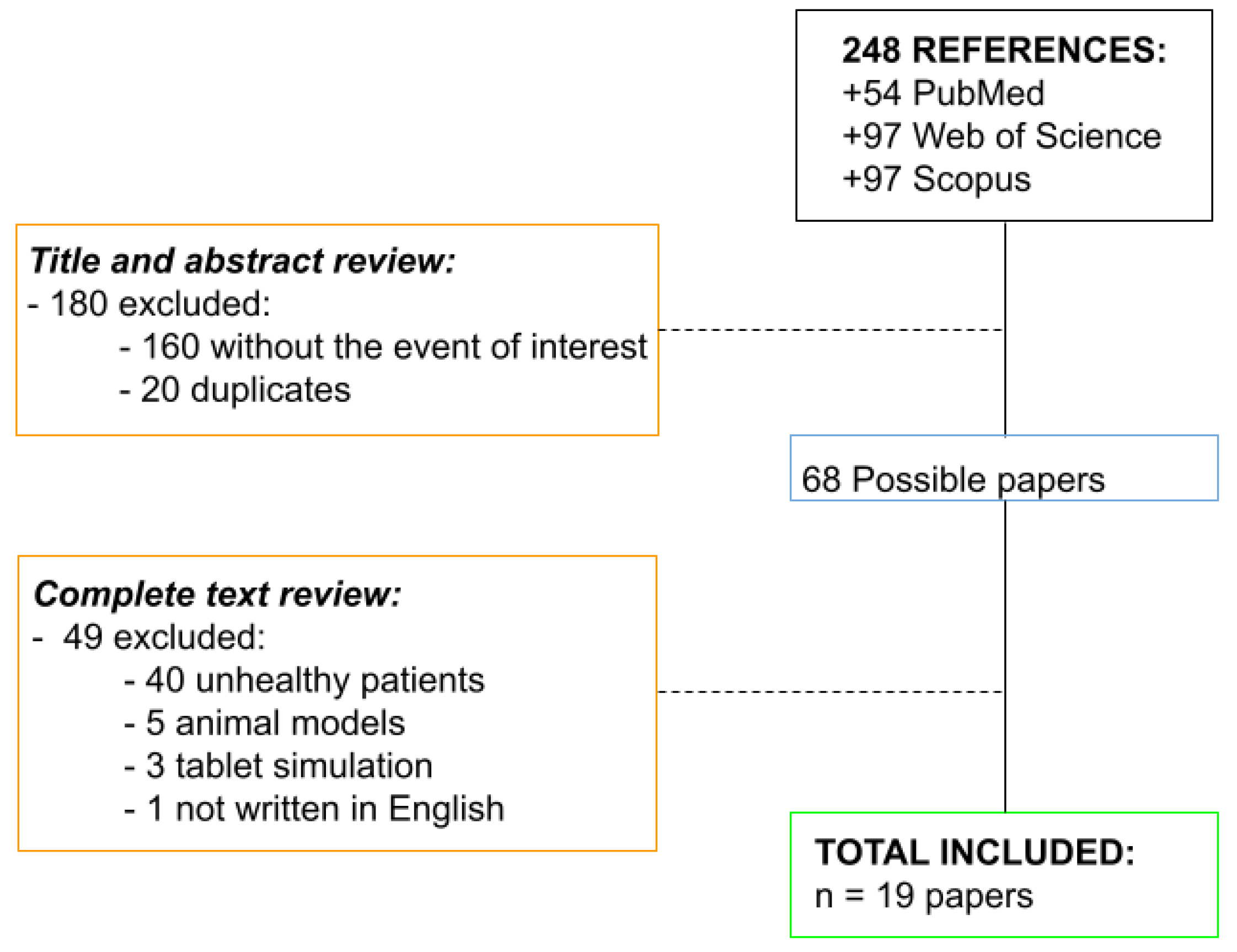
3. Results
3.1. Search Results
3.2. Analysis of the Articles Included and Excluded
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, S.; McAndrew, D.J. The validity of visual acuity assessment using mobile technology devices in the primary care setting. Aust. Fam. Physician 2016, 45, 212–215. [Google Scholar]
- de Fez, D.; Luque, M.J.; Matea, L.; Piñero, D.P.; Camps, V.J. New iPAD-based test for the detection of color vision deficiencies. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2349–2360. [Google Scholar] [CrossRef] [Green Version]
- de Fez, D.; Luque, M.J.; García-Domene, M.C.; Camps, V.; Piñero, D. Colorimetric characterization of mobile devices for vision applications. Optom. Vis. Sci. 2015, 93, 85–93. [Google Scholar] [CrossRef]
- Montanera, R.; Julia, V. Study of Social Networks. 2018. Available online: https://iabspain.es/wp-content/uploads/estudio-redes-sociales-2018_vreducida.pdf (accessed on 2 June 2020).
- Vázquez Martínez, R.; Martínez López, M. Citizens before e-Health. Opinions and Expectations of Citizens on the Use and Application of ICT in the Health Field. 2016. Available online: https://www.ontsi.red.es/ontsi/sites/ontsi/files/los_ciudadanos_ante_la_e-sanidad.pdf (accessed on 2 June 2020).
- Hogarty, D.T.; Hogarty, J.P.; Hewitt, A.W. Smartphone use in ophthalmology: What is their place in clinical practice? Surv. Ophthalmol. 2020, 65, 250–262. [Google Scholar] [CrossRef]
- de Fez, D.; Luque, M.J.; García-Domene, M.C.; Caballero, M.T.; Camps, V.J. Can applications designed to evaluate visual function be used in different iPads? Optom. Vis. Sci. 2018, 95, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Molina-Martín, A.; Piñero, D.P.; Coco-Martín, M.B.; Leal-Vega, L.; de Fez, D. Reproduction between Electronic Devices for Visual Assessment: Clinical Implications. Technologies 2021, 9, 68. [Google Scholar] [CrossRef]
- Wroblewski, D.; Francis, B.A.; Sadun, A.; Vakili, G.; Chopra, V. Testing of visual field with virtual reality goggles in manual and visual grasp modes. BioMed Res. Int. 2014, 2014, 206082. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, I.A.; Lok, A.S.; Tarbert, C. New mobile technologies and visual acuity. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 2189–2192. [Google Scholar] [PubMed]
- Tahir, H.J.; Murray, I.J.; Parry, N.R.; Aslam, T.M. Optimisation and assessment of three modern touch screen tablet computers for clinical vision testing. PLoS ONE 2014, 9, e95074. [Google Scholar] [CrossRef] [PubMed]
- Benjumea, J.; Ropero, J.; Rivera-Romero, O.; Dorronzoro-Zubiete, E.; Carrasco, A. Privacy Assessment in Mobile Health Apps: Scoping Review. JMIR mHealth uHealth 2020, 8, e18868. [Google Scholar] [CrossRef]
- Health App & Privacy. Available online: https://www.apple.com/legal/privacy/data/en/health-app/ (accessed on 4 November 2021).
- Black, J.M.; Jacobs, R.J.; Phillips, G.; Chen, L.; Tan, E.; Tran, A.; Thompson, B. An assessment of the iPad as a testing platform for distance visual acuity in adults. BMJ Open 2013, 3, e002730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.T.; Zhang, S.C.; Huang, X.G.; Liang, L.Y. A pilot trial of the iPad tablet computer as a portable device for visual acuity testing. J. Telemed. Telecare 2013, 19, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kingsnorth, A.; Wolffsohn, J.S. Mobile app reading speed test. Br. J. Ophthalmol. 2015, 99, 536–539. [Google Scholar] [CrossRef]
- Norgett, Y.; Siderov, J. Foveal crowding differs in children and adults. J. Vis. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, A.A.; Lagamayo, M.A.N. A comparison of distance visual acuity testing using a standard ETDRS chart and a tablet device. Philipp. J. Ophthalmol. 2015, 40, 88–92. [Google Scholar]
- Perera, C.; Chakrabarti, R.; Islam, F.M.A.; Crowston, J. The eye phone study: Reliability and accuracy of assessing Snellen visual acuity using smartphone technology. Eye 2015, 29, 888–894. [Google Scholar] [CrossRef]
- Tofigh, S.; Shortridge, E.; Elkeeb, A.; Godley, B.F. Effectiveness of a smartphone application for testing near visual acuity. Eye 2015, 29, 1464–1468. [Google Scholar] [CrossRef]
- Kingsnorth, A.; Drew, T.; Grewal, B.; Wolffsohn, J.S. Mobile app Aston contrast sensitivity test. Clin. Exp. Optom. 2016, 99, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Pathipati, A.S.; Wood, E.H.; Lam, C.K.; Sales, C.S.; Moshfeghi, D.M. Visual acuity measured with a smartphone app is more accurate than Snellen testing by emergency department providers. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1175–1180. [Google Scholar] [CrossRef]
- Phung, L.; Gregori, N.Z.; Ortiz, A.; Schiffman, J.C. Reproducibility and comparison of visual acuity obtained with sightbook mobile application to near card and Snellen chart. Retina 2016, 36, 1009–1020. [Google Scholar] [CrossRef]
- Rhiu, S.; Lee, H.J.; Goo, Y.S.; Cho, K.; Kim, J.H. Visual acuity testing using a random method visual acuity application. Telemed. J. e-Health 2016, 22, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vallejo, M.; Llorens-Quintana, C.; Furlan, W.D.; Monsoriu, J.A. Visual acuity and contrast sensitivity screening with a new iPad application. Displays 2016, 44, 15–20. [Google Scholar] [CrossRef]
- Rhiu, S.; Kim, M.; Kim, J.H.; Lee, H.J.; Lim, T.H. Korean version self-testing application for reading speed. Korean J. Ophthalmol. 2017, 31, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodduluri, L.; Boon, M.Y.; Ryan, M.; Dain, S.J. Normative values for a tablet computer-based application to assess chromatic contrast sensitivity. Behav. Res. Methods 2018, 50, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Azis, N.N.N.; Chew, F.L.M.; Rosland, S.F.; Ramlee, A.; Che-Hamzah, J. Parents’ performance using the AAPOS vision screening app to test visual acuity in Malaysian preschoolers. JAAPOS J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2019, 23, 268.e1–268.e6. [Google Scholar] [CrossRef]
- Brucker, J.; Bhatia, V.; Sahel, J.A.; Girmens, J.F.; Mohand-Saïd, S. Odysight: A mobile medical application designed for remote monitoring—A prospective study comparison with standard clinical eye tests. Ophthalmol. Ther. 2019, 8, 461–476. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Rodríguez-Vallejo, M.; Tauste, A.; Albarrán, C.; Basterra, I.; Piñero, D. Fast measure of visual acuity and contrast sensitivity defocus curves with an iPad application. Open Ophthalmol. J. 2019, 13, 15–22. [Google Scholar] [CrossRef]
- Rodríguez-Vallejo, M.; Remón, L.; Monsoriu, J.A.; Furlan, W.D. Designing a new test for contrast sensitivity function measurement with iPad. J. Optom. 2015, 8, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nes, F.L.; Bouman, M.A. Spatial modulation transfer in the human eye. J. Opt. Soc. Am. 1967, 57, 401–406. [Google Scholar] [CrossRef]
- Kollbaum, P.S.; Jansen, M.E.; Kollbaum, E.J.; Bullimore, M.A. Validation of an iPad test of letter contrast sensitivity. Optom. Vis. Sci. 2014, 91, 291–296. [Google Scholar] [CrossRef]
- Habtamu, E.; Bastawrous, A.; Bolster, N.M.; Tadesse, Z.; Callahan, E.K.; Gashaw, B.; Macleod, D.; Burton, M.J. Development and validation of a smartphone-based contrast sensitivity test. Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.A.; Thapa, S.; Kong, Y.X.G.; Robin, A.L. Performance of an iPad application to detect moderate and advanced visual field loss in Nepal. Am. J. Ophthalmol. 2017, 182, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, H.; Law, C.; Chriqui, E.; Leclerc, B.S.; Kergoat, M.J. Tool for screening visual acuity in older individuals with dementia. Am. J. Alzheimers Dis. Other Dement. 2017, 32, 96–100. [Google Scholar] [CrossRef]
- Vingrys, A.J.; Healey, J.K.; Liew, S.; Saharinen, V.; Tran, M.; Wu, W.; Kong, G.Y.X. Validation of a tablet as a tangent perimeter. Transl. Vis. Sci. Technol. 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Turpin, A.; Lawson, D.J.; McKendrick, A.M. PsyPad: A platform for visual psychophysics on the iPad. J. Vis. 2014, 14, 16. [Google Scholar] [CrossRef] [Green Version]
| Authors | Sample | Device | Apps or Applications | Measures | Main Results and Conclusions |
|---|---|---|---|---|---|
| Black et al. [14] 2013 | n = 85 participants | iPad | Visual Acuity XL app | VA (Bailey Lovie and HOTV chart) |
|
| Zhang et al. [15] 2013 | n = 120 participants | iPad 2 | Eye Chart Pro app | VA (E Snellen optotype) |
|
| Kingsnorth et al. [16] 2014 | n = 21 participants | iPad 3 | 2 custom-made mobile app reading speed charts | Reading speed (Radner reading chart) |
|
| Norgett et al. [17] 2014 | n = 89 participants | iPad 2 | Custom-designed visual acuity test | VA (Sloan and ETDRS optotypes) |
|
| Manzanaro et al. [18] 2015 | n = 46 participants | iPad | 2020 Duo FLEX Visual Acuity Chart | VA (ETDRS optotype) |
|
| Perera et al. [19] 2015 | n = 88 participants | iPhone 4 | “Snellen” DrBloggs Ltd. app | VA (6 S VA chart) |
|
| Tofigh et al. [20] 2015 | n = 100 participants | iPhone 5 | EyeHand Book app | VA (app vs. Rosenbaum near optotype) |
|
| Kingsnorth et al. [21] 2016 | n = 20 participants | iPad | Aston near app and Aston distance app | CS (CSV-100 and Pelli–Robson tests) |
|
| Pathipati et al. [22] 2016 | n = 64 participants | iPhone | Paxos Checkup app | VA (Snellen optotype vs. app) |
|
| Phung et al. [23] 2016 | n = 30 participants | iPad | SightBook mobile app | VA near and far (ETDRS optotype vs. app) |
|
| Rhiu et al. [24] 2016 | n = 43 participants | iPad | iPad-based app: Snellen chart, Tumbling Echart, Landolt C chart, and Arabic figures chart | VA (Snellen, Landolt C optotypes) |
|
| Rodriguez-Vallejo et al. [25] 2016 | n = 45 participants | iPad | Self-developed app for IOS | VA and CS (ETDRS optotypes) |
|
| Rhiu et al. [26] 2017 | n = 65 participants | iPad | Korean version reading speed chart | Reading speed |
|
| de Fez et al. [2] 2018 | n = 407 participants | iPad | Optopad | Color vision |
|
| Bodduluri et al. [27] 2018 | n = 100 participants | iPad | Three self-developed games | Chromatic contrast sensitivity |
|
| Azis et al. [28] 2019 | n = 195 participants | iPad | AAPOS Vision screening app | VA (ETDRS and Sloan optotypes vs. app) |
|
| Brucker et al. [29] 2019 | n = 120 participants | iPad | Odysight app | VA and CS (app vs. ETDRS test) |
|
| Fernández et al. [30] 2019 | n = 127 participants | iPad | Defocus curve app (version 1.0.8) | VA (E Snellen optotype) and CS (CSF test) |
|
| Hogarti et al. [6] 2020 | - | iPhone | 45 apps (Google play store) and 23 apps (Apple store) | Visual function (mainly VA) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-Guevara, K.J.; Piñero, D.P.; de Fez, D. Validation of Digital Applications for Evaluation of Visual Parameters: A Narrative Review. Vision 2021, 5, 58. https://doi.org/10.3390/vision5040058
Mena-Guevara KJ, Piñero DP, de Fez D. Validation of Digital Applications for Evaluation of Visual Parameters: A Narrative Review. Vision. 2021; 5(4):58. https://doi.org/10.3390/vision5040058
Chicago/Turabian StyleMena-Guevara, Kevin J., David P. Piñero, and Dolores de Fez. 2021. "Validation of Digital Applications for Evaluation of Visual Parameters: A Narrative Review" Vision 5, no. 4: 58. https://doi.org/10.3390/vision5040058
APA StyleMena-Guevara, K. J., Piñero, D. P., & de Fez, D. (2021). Validation of Digital Applications for Evaluation of Visual Parameters: A Narrative Review. Vision, 5(4), 58. https://doi.org/10.3390/vision5040058






