The Effects of Sex, Oral Contraception, and Menstrual Cycle Phase on Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness: A Descriptive Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sessions
2.3. Procedures
2.3.1. Intraocular Pressure
2.3.2. Central Corneal Thickness
2.3.3. Foveal Thickness
2.4. Data Analysis
3. Results
3.1. Descriptive Results
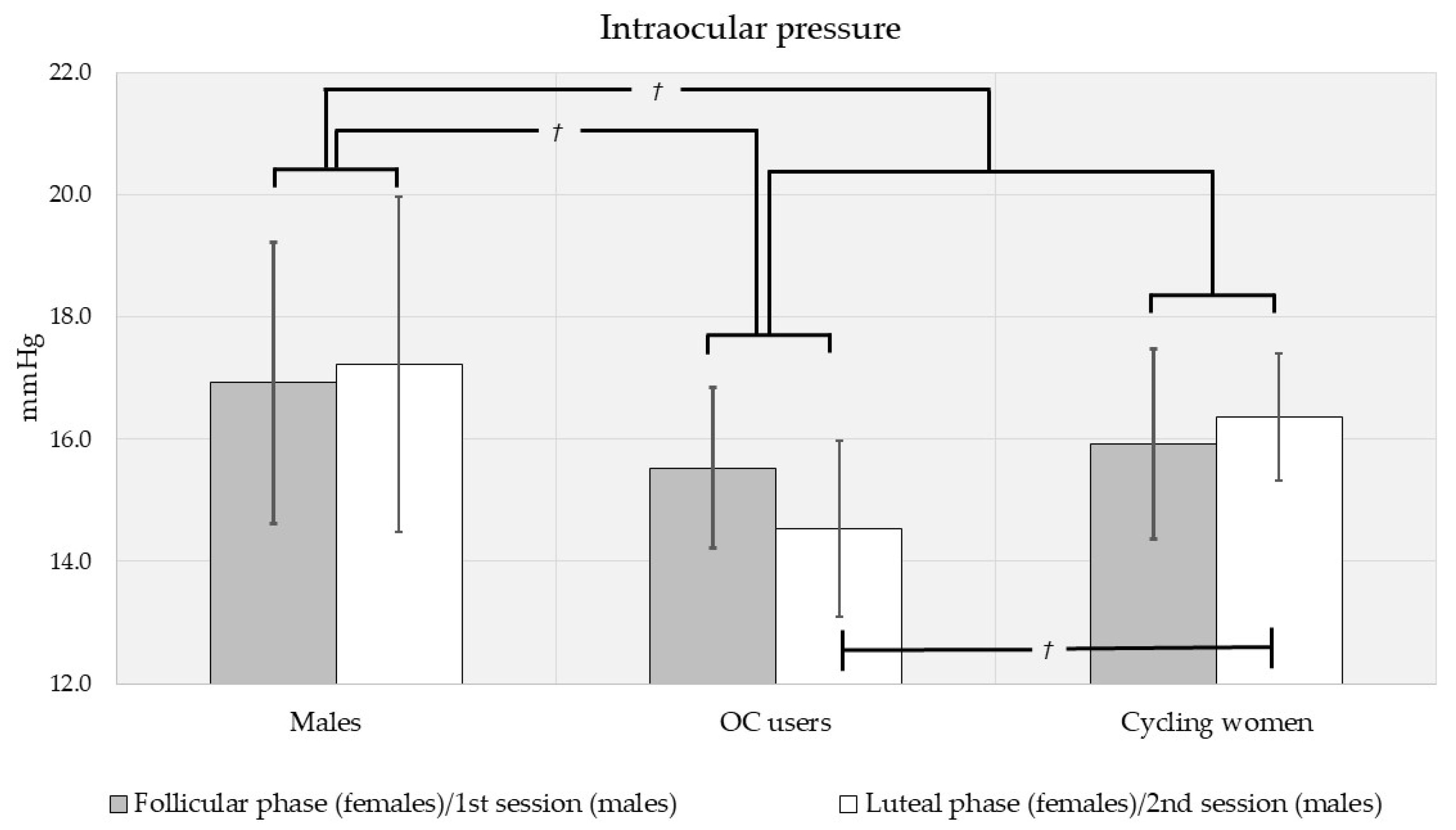
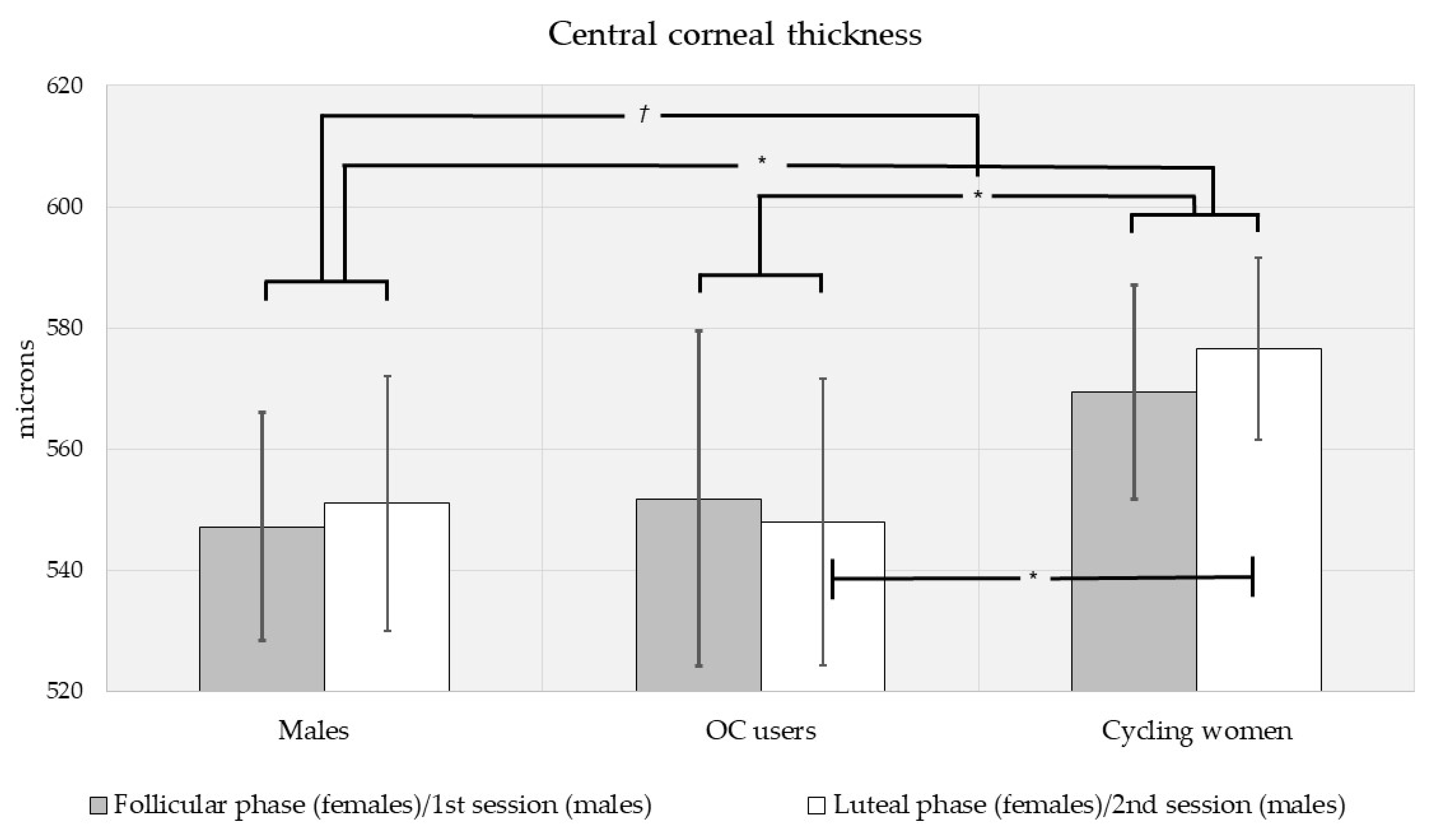
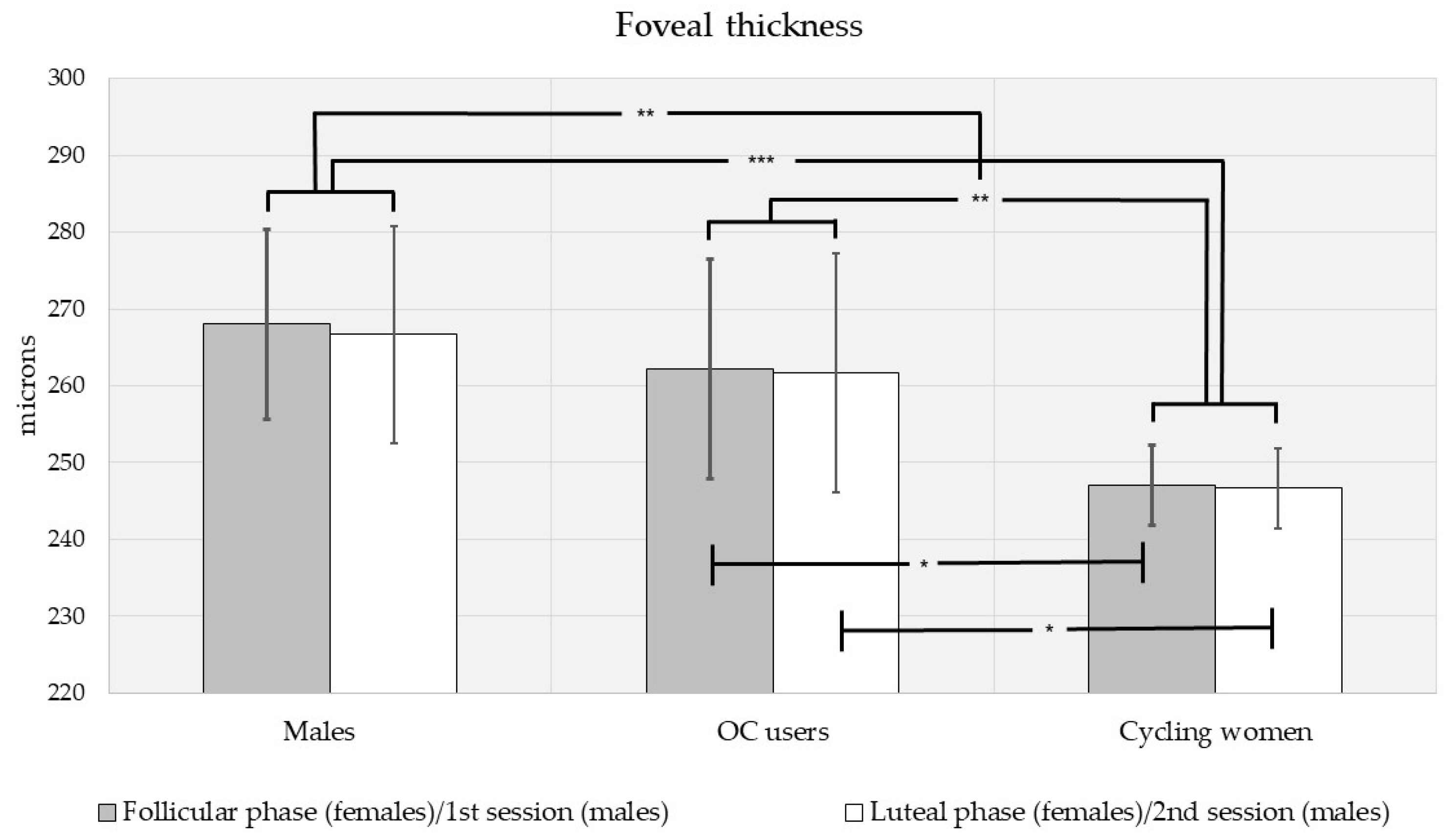
3.2. Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness Analysis by Sex, Contraceptive Use and Menstrual Phase
3.3. Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness Analysis by Sex, Race, and Ethnicity
3.4. Body Mass Index, Blood Glucose Level, and Blood Pressure Analysis by Sex, Contraceptive Use and Menstrual Phase
3.5. Relationships between Ocular Biometrics and Systemic Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mottet, B.; Aptel, F.; Romanet, J.P.; Hubanova, R.; Pépin, J.L.; Chiquet, C. 24-hour intraocular pressure rhythm in young healthy subjects evaluated with continuous monitoring using a contact lens sensor. JAMA Ophthalmol. 2013, 131, 1507–1516. [Google Scholar] [CrossRef]
- Syam, P.P.; Mavrikakis, I.; Liu, C. Importance of early morning intraocular pressure recording for measurement of diurnal variation of intraocular pressure. Br. J. Ophthalmol. 2005, 89, 926–927. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Kang, J.H. Lifestyle, Nutrition and Glaucoma. J. Glaucoma 2009, 18, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.; Chen, B.; Li, Y.; Jiang, B. Prevalence of Primary Angle Closure Glaucoma in the Last 20 Years: A Meta-Analysis and Systematic Review. Front. Med. 2021, 7, 624179. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Bain, J.; Matuk, A.R. Daily assessment of ocular and hormonal variables throughout the menstrual cycle. Arch. Ophthalmol. 1978, 96, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.A.; Xi, X.R.; Wu, X.D.; Pasha, N.; Huang, Y.B. Variations in ocular pressure during menstrual cycle. J. Pak. Med. Assoc. 1998, 48, 37–40. [Google Scholar]
- Wang, Y.E.; Kakigi, C.; Barbosa, D.; Porco, T.; Chen, R.; Wang, S.; Li, Y.; Singh, K.; Pasquale, L.R.; Lin, S.C. Oral Contraceptive Use and Prevalence of Self-Reported Glaucoma or Ocular Hypertension in the United States. Ophthalmology 2016, 123, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Beiser, J.A.; Kass, M.A.; Gordon, M.O. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 2001, 108, 1779–1788. [Google Scholar] [CrossRef]
- Giuffrè, G.; Di Rosa, L.; Fiorino, F.; Bubella, D.M.; Lodato, G. Variations in central corneal thickness during the menstrual cycle in women. Cornea 2007, 26, 144–146. [Google Scholar] [CrossRef]
- Kurtul, B.E.; Inal, B.; Ozer, P.A.; Kabatas, E.U. Impact of oral contraceptive pills on central corneal thickness in young women. Indian J. Pharmacol. 2016, 48, 665–668. [Google Scholar] [CrossRef]
- Milani, P.; Bochicchio, S.; Urbini, L.; Bulone, E.; Callegarin, S.; Pisano, L.; Scotti, L.; Zambon, A.; Bergamini, F. Diurnal Measurements of Macular Thickness and Vessel Density on OCT Angiography in Healthy Eyes and Those with Ocular Hypertension and Glaucoma. J. Glaucoma 2020, 29, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Seong, M.; Sung, K.R.; Choi, E.; Kang, S.Y.; Cho, J.W.; Um, T.W.; Kim, Y.J.; Park, S.B.; Hong, H.E.; Kook, M.S. Macular and Peripapillary Retinal Nerve Fiber Layer Measurements by Spectral Domain Optical Coherence Tomography in Normal-Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1446–1452. [Google Scholar] [CrossRef]
- Adhi, M.; Aziz, S.; Muhammad, K.; Adhi, M.I. Macular Thickness by Age and Gender in Healthy Eyes Using Spectral Domain Optical Coherence Tomography. PLoS ONE 2012, 7, e37638. [Google Scholar] [CrossRef] [PubMed]
- Mashige, K.P.; Oduntan, O.A. Macular thicknesses and their associations with ocular and demographic variables in black South Africans. Afr. Vis. Eye Health 2017, 76, a374. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, Q.; Hu, P.; Jia, L. Macular Thickness Assessed with Optical Coherence Tomography in Young Chinese Myopic Patients. J. Ophthalmol. 2015, 15, 715798. [Google Scholar] [CrossRef] [PubMed]
- Appukuttan, B.; Giridhar, A.; Gopalakrishnan, M.; Sivaprasad, S. Normative spectral domain optical coherence tomography data on macular and retinal nerve fiber layer thickness in Indians. Indian J. Ophthalmol. 2014, 62, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Duker, J.S.; Ko, T.H.; Fujimoto, J.G.; Schuman, J.S. Normal Macular Thickness Measurements in Healthy Eyes Using Stratus Optical Coherence Tomography. Arch. Ophthalmol. 2006, 124, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, Y.M.; Badran, T.A.F. The effect of oral contraceptive pills on the macula, the retinal nerve fiber layer, the ganglion cell layer, and the choroidal thickness. BMC Ophthalmol. 2019, 19, 250. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Duncan, L.; Enyedi, L.B.; Freedman, S.F. Central corneal thickness in children: Racial differences (black vs. white) and correlation with measured intraocular pressure. J. Glaucoma 2006, 15, 520–523. [Google Scholar] [CrossRef]
- Yo, C.; Ariyasu, R.G. Racial differences in central corneal thickness and refraction among refractive surgery candidates. J. Refract. Surg. 2005, 21, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Nannini, D.; Torres, M.; Chen, Y.-D.I.; Taylor, K.D.; Rotter, J.I.; Varma, R.; Gao, X. African Ancestry Is Associated with Higher Intraocular Pressure in Latinos. Ophthalmology 2016, 123, 102–108. [Google Scholar] [CrossRef]
- Asefzadeh, B.; Cavallerano, A.; Fisch, B. Racial Differences in Macular Thickness in Healthy Eyes. Optom. Vis. Sci. 2007, 84, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, F.; Ying-Lai, M.; Azen, S.P.; Varma, R. Los Angeles Latino Eye Study Group. Associations with intraocular pressure in Latinos: The Los Angeles Latino Eye Study. Am. J. Ophthalmol. 2008, 146, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Khabazkhoob, M.; Emamian, M.H.; Shariati, M.; Yekta, A.; Fotouhi, A. Distribution of intraocular pressure and its determinants in an Iranian adult population. Int. J. Ophthalmol. 2016, 9, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Panon, N.; Luangsawang, K.; Rugaber, C.; Tongchit, T.; Thongsepee, N.; Cheaha, D.; Kongjaidee, P.; Changtong, A.; Daradas, A.; Chotimol, P. Correlation between body mass index and ocular parameters. Clin. Ophthalmol. 2019, 13, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Nangia, V.; Jonas, J.B.; Sinha, A.; Matin, A.; Kulkarni, M. Central corneal thickness and its association with ocular and general parameters in Indians: The Central India Eye and Medical Study. Ophthalmology 2010, 117, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Su, D.H.W.; Wong, T.Y.; Foster, P.J.; Tay, W.-T.; Saw, S.-M.; Aung, T. Central Corneal Thickness and its Associations with Ocular and Systemic Factors: The Singapore Malay Eye Study. Am. J. Ophthalmol. 2009, 147, 709–716.e1. [Google Scholar] [CrossRef]
- Jung, S.C.; Choi, Y.R.; Lee, J.S. The Relationship between Intraocular Pressure and Cardiovascular Risk Factors. J. Korean Ophthalmol. Soc. 2005, 46, 1518–1525. [Google Scholar]
- Sanchez-Tocino, H.; Alvarez-Vidal, A.; Maldonado, M.J.; Moreno-Montañés, J.; García-Layana, A. Retinal thickness study with optical coherence tomography in patients with diabetes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1588–1594. [Google Scholar]
- Daniels, K.; Daugherty, J.; Jones, J. Current Contraceptive Status among Women Aged 15-44: United States, 2011–2013. NCHS Data Brief; CDC: Washington, DC, USA, 2014; Volume 173, pp. 1–8. [Google Scholar]
- Bagga, H.; Liu, J.H.; Weinreb, R.N. Intraocular pressure measurements throughout the 24 h. Curr. Opin. Ophthalmol. 2009, 20, 79–83. [Google Scholar] [CrossRef]
- Pointer, J.S. The diurnal variation of intraocular pressure in non-glaucomatous subjects: Relevance in a clinical context. Ophthalmic Physiol. Opt. 1997, 17, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A. Standardizing the measurement of intraocular pressure for clinical research. Guidelines from the Eye Care Technology Forum. Ophthalmology 1996, 103, 183–185. [Google Scholar] [CrossRef]
- Pakrou, N.; Gray, T.; Mills, R.; Landers, J.; Craig, J. Clinical comparison of the Icare tonometer and Goldmann applanation tonometry. J. Glaucoma 2008, 17, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.L.; Cascone, N.C.; Regine, F.; Perdicchi, A.; Cerulli, A.; Recupero, S.M. Validity and limits of the rebound tonometer (ICare®): Clinical study. Eur. J. Ophthalmol. 2011, 21, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 2013, 33, 7–14. [Google Scholar] [CrossRef]
- Rosner, B. Statistical methods in ophthalmology: An adjustment for the intraclass correlation between eyes. Biometrics 1982, 38, 105–114. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Nemesure, B.; Honkanen, R.; Hennis, A.; Wu, Y.; Leske, M.C. Incident Open-angle Glaucoma and Intraocular Pressure. Ophthalmology 2007, 114, 1810–1815. [Google Scholar] [CrossRef]
- Friedman, D.S.; Wilson, M.R.; Liebmann, J.M.; Fechtner, R.D.; Weinreb, R.N. An evidence based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am. J. Ophthalmol. 2004, 138, S19–S31. [Google Scholar] [CrossRef]
- Leske, M.C.; Connell, A.M.; Wu, S.Y.; Hyman, L.; Schachat, A.P. Distribution of intraocular pressure. The Barbados Eye Study. Arch. Ophthalmol. 1997, 115, 1051–1057. [Google Scholar] [CrossRef]
- Ejimadu, C.S.; Chinawa, N.E.; Fiebai, B. Age and Gender Related Changes in Intraocular Pressure among Patients Attending a Peripheral Eye Clinic in Port Harcourt, Nigeria. Austin J. Clin. Ophthalmol. 2018, 5, 1092. [Google Scholar]
- Hohberger, B.; Lucio, M.; Mardin, C.Y.; Lämmer, R. Germany: Longitudinal analysis of intraocular pressure in healthy eyes. Cogent Med. 2020, 7, 1750862. [Google Scholar] [CrossRef]
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernadi, P.; De Franco, I.; Perfetti, S.; Varotto, A.; Tenna, V. Prevalence of glaucoma and intraocular pressure distribution in a defined population: The Egna-Neumarkt Study. Ophthalmology 1998, 105, 209–215. [Google Scholar] [CrossRef]
- Garcia-Medina, M.; García-Medina, J.J.; Garrido-Fernandez, P.; Galvan-Espinosa, J.; Martin-Molina, J.; Garcia-Maturana, C.; Perez-Pardo, S.; Pinazo-Duran, M. Central corneal thickness, intraocular pressure, and degree of myopia in an adult myopic population aged 20 to 40 years in southeast Spain: Determination and relationships. Clin. Ophthalmol. 2011, 5, 249–258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, D.; Kim, M.H.; Pastor-Barriuso, R.; Chang, Y.; Ryu, S.; Zhang, Y.; Rampal, S.; Shin, H.; Kim, J.M.; Friedman, D.S.; et al. A Longitudinal Study of Age-Related Changes in Intraocular Pressure: The Kangbuk Samsung Health Study. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6244–6250. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Piomboni, P.; Morgante, G. Hormonal contraceptives: Pharmacology tailored to women’s health. Hum. Reprod. Update 2016, 22, 634–646. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Kang, J.H. Female reproductive factors and primary open-angle glaucoma in the Nurses’ Health Study. Eye 2011, 25, 633–641. [Google Scholar] [CrossRef]
- Meyer, E.J.; Leibowitz, H.; Christman, E.H.; Niffenegger, J.A. Influence of norethynodrel with mestranol on intraocular pressure in glaucoma. Arch. Ophthalmol. 1966, 75, 157–161. [Google Scholar] [CrossRef]
- Doshi, V.; Mei, Y.-L.; Azen, S.P.; Varma, R.; Los Angeles Latino Eye Study Group. Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension. The Los Angeles Latino Eye Study. Ophthalmology 2008, 115, 639–647.e2. [Google Scholar] [CrossRef]
- Dueker, D.K.; Singh, K.; Lin, S.C.; Fechtner, R.; Minckler, D.S.; Samples, J.R.; Schuman, J.S. Corneal Thickness Measurement in the Management of Primary Open-angle Glaucoma. Ophthalmology 2007, 114, 1779–1787. [Google Scholar] [CrossRef]
- Hashemi, H.; Khabazkhoob, M.; Nabovati, P.; Yazdani, N.; Ostadimoghaddam, H.; Shiralivand, E.; Derakhshan, A.; Yekta, A. Distribution of IOP measured with an air puff tonometer in a young population. J. Curr. Ophthalmol. 2017, 30, 35–41. [Google Scholar] [CrossRef]
- Galgauskas, S.; Garlaite, O.; Juotkaite, G.; Asoklis, R.; Tutkuviene, J. The Correlation Between Central Corneal Thickness, Intraocular Pressure, Age and Gender. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6228. [Google Scholar]
- Hashemi, H.; Mehravaran, S.; Rezvan, F. Changes in corneal thickness, curvature, and anterior chamber depth during the menstrual cycle. Can. J. Ophthalmol. 2010, 45, 67–70. [Google Scholar] [CrossRef]
- Stefan, C.; Dumitrica, D.M.; Tebeanu, E.; Nae, I.; Sapundgieva, A.; Dragomir, L. Prostaglandin analogues and central corneal thickness. Oftalmologia 2007, 51, 95–99. [Google Scholar]
- Grueb, M.; Rohrbach, J.M. Effect of Timolol on Central Corneal Thickness. Eur. J. Ophthalmol. 2013, 23, 784–788. [Google Scholar] [CrossRef]
- Cortés, D.A.; Roca, D.; Navarro, P.I.; Rodríguez, F.J. Macular and choroidal thicknesses in a healthy Hispanic population evaluated by high-definition spectral-domain optical coherence tomography (SD-OCT). Int. J. Retin. Vitr. 2020, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Ulaş, F.; Doğan, U.; Duran, B.; Keleş, A.; Ağca, S.; Celebi, S. Choroidal thickness changes during the menstrual cycle. Curr. Eye Res. 2013, 38, 1172–1181. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, C.; Wang, Z.; Gao, Z.; Zhang, Z.; Pan, Q. Retinal Vascular Changes during the Menstrual Cycle Detected with Optical Coherence Tomography Angiography. J. Ophthalmol. 2021, 2021, 5514575. [Google Scholar] [CrossRef] [PubMed]
- Madendag, Y.; Acmaz, G.; Atas, M.; Sahin, E.; Tayyar, A.T.; Madendag, I.Ç.; Özdemir, F.; Senol, V. The Effect of Oral Contraceptive Pills on the Macula, the Retinal Nerve Fiber Layer, and Choroidal Thickness. Med. Sci. Monit. 2017, 23, 5657–5661. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, B.E.; Klein, R.; Sponsel, W.E.; Franke, T.; Cantor, L.B.; Martone, J.; Menage, M.J. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 1499–1504. [Google Scholar] [CrossRef]
- Qureshi, I.A. Age and intraocular pressure: How are they correlated? J. Pak. Med. Assoc. 1995, 45, 150–152. [Google Scholar] [PubMed]
- Iyamu, E.; Osuobeni, E. Age, gender, corneal diameter, corneal curvature and central corneal thickness in Nigerians with normal intra ocular pressure. J. Optom. 2012, 5, 87–97. [Google Scholar] [CrossRef]
- Siu, A.; Herse, P. The effect of age on human corneal thickness. Statistical implications of power analysis. Acta Ophthalmol. 1993, 71, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Vianna, J.R.; Sharpe, G.P.; Demirel, S.; Girkin, C.A.; Mardin, C.Y.; Scheuerle, A.F.; Burgoyne, C.F. Differential Effects of Aging in the Macular Retinal Layers, Neuroretinal Rim, and Peripapillary Retinal Nerve Fiber Layer. Ophthalmology 2020, 127, 177–185. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Vingrys, A.J.; Armitage, J.A.; Bui, B.V. The role of blood pressure in glaucoma. Clin. Exp. Optom. 2011, 94, 133–149. [Google Scholar] [CrossRef]
- Kong, M.; Kwun, Y.; Sung, J.; Ham, D.I.; Song, Y.M. Association Between Systemic Hypertension and Macular Thickness Measured by Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2144–2150. [Google Scholar] [CrossRef]
- Petitti, D.B. Combination estrogen-progestin oral contraceptives. N. Engl. J. Med. 2003, 349, 1443–1450. [Google Scholar] [CrossRef]
- Fuhrmann, U.; Krattenmacher, R.; Slater, E.P.; Fritzemeier, K.-H. The novel progestin drospirenone and its natural counterpart progesterone: Biochemical profile and antiandrogenic potential. Contraception 1996, 54, 243–251. [Google Scholar] [CrossRef]
- Levin, E.R.; Vitek, W.S.; Hammes, S.R. Estrogens, progestins, and the female reproductivetract. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollman, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2018; pp. 803–833. [Google Scholar]
- DiLiberti, C.E.; O’Leary, C.M.; Hendy, C.H.; Waters, D.H.; Margolis, M.B. Steadystate pharmacokinetics of an extended-regimen oral contraceptive with continuous estrogen. Contraception 2011, 83, 55–61. [Google Scholar] [CrossRef]
- Adhikari, A.; Shet, R.V.; Mandal, R.; Vaghela, Y. Variations in Intraocular Pressure During Different Phases of Menstrual Cycle. J. Ophthalmol. Res. 2021, 4, 183–191. [Google Scholar] [CrossRef]
- Mishra, D.; Bhushan, P.; Sachan, S.; Singh, M.K.; Jayadev, C.; Kusumgar, P. Variations in the central corneal thickness during the menstrual cycle in Indian women. Indian J. Ophthalmol. 2020, 68, 2918–2920. [Google Scholar] [CrossRef] [PubMed]
| Range | Mean ± S.D. | Median | |
|---|---|---|---|
| All subjects (n = 60) | 20–50 yrs | 25.9 ± 5.06 yrs | 24.0 yrs |
| Men (n = 16) | 22–50 yrs | 27.9 ± 6.44 yrs | 26.5 yrs |
| Women (n = 44) | 20–44 yrs | 25.2 ± 4.22 yrs | 24.0 yrs |
| - Cycling (n = 28) | 21–44 yrs | 25.8 ± 5.01 yrs | 24.0 yrs |
| - Oral contraceptive users (n = 16) | 20–28 yrs | 23.9 ± 1.72 yrs | 24.0 yrs |
| IOP | CCT | FT | |
|---|---|---|---|
| (mmHg) | (μm) | (μm) | |
| All subjects, n = 60 | 16.0 ± 3.90 (117) | 560 ± 44.7 (116) | 256 ± 23.3 (116) |
| Men, n = 16 | 17.1 ± 4.97 (31) | 549 ± 38.6 (30) | 267 ± 25.6 (30) |
| - 1st session | 16.9 ± 4.70 (16) | 547 ± 38.5 (16) | 268 ± 25.2 (16) |
| - 2nd session | 17.2 ± 5.40 (15) | 551 ± 40.1 (14) | 267 ± 26.9 (14) |
| Women, n = 44 | 15.7 ± 3.38 (86) | 564 ± 46.1 (86) | 252 ± 21.2 (86) |
| - Follicular | 15.6 ± 3.79 (43) | 564 ± 49.4 (43) | 252 ± 20.5 (43) |
| - Luteal | 15.7 ± 2.97 (43) | 566 ± 43.7 (43) | 252 ± 22.1 (43) |
| Cycling women, n = 28 | 16.1 ± 3.54 (55) | 573 ± 43.6 (55) | 247 ± 13.9 (55) |
| - Follicular | 15.9 ± 4.19 (28) | 569 ± 47.5 (28) | 247 ± 14.1 (28) |
| - Luteal | 16.4 ± 2.76 (27) | 577 ± 39.8 (27) | 247 ± 13.9 (27) |
| OC-users, n = 16 | 14.8 ± 2.95 (31) | 549 ± 47.2 (31) | 262 ± 27.8 (31) |
| - Follicular | 15.1 ± 2.95 (15) | 551 ± 50.8 (15) | 262 ± 26.5 (15) |
| - Luteal | 14.5 ± 3.02 (16) | 547 ± 45.1 (16) | 261 ± 29.8 (16) |
| IOP | CCT | FT | |
|---|---|---|---|
| (mmHg) | (mm) | (mm) | |
| Asian, n = 5 | 14.6 ± 2.45 (10) | 556 ± 49.1 (10) | 265 ± 31.4 (10) |
| - Men, n = 3 | 14.6 ± 2.89 (6) | 541 ± 59.3 (6) | 279 ± 33.7 (6) |
| - Women, n = 2 | 14.5 ± 2.04 (4) | 579 ± 14.6 (4) | 243 ± 6.56 (4) |
| Black, n = 2 (all women) | 17.5 ± 3.76 (4) | 608 ± 52.1 (4) | 239 ± 15.6 (4) |
| South Asian, n = 5 (all women) | 15.1 ± 4.12 (10) | 562 ± 47.3 (10) | 238 ± 16.2 (10) |
| White, n = 48 | 16.2 ± 4.00 (93) | 559 ± 43.2 (92) | 258 ± 22.1 (92) |
| - Men, n = 13 | 17.7 ± 5.22 (25) | 551 ± 33.1 (24) | 265 ± 23.1 (24) |
| Hispanic, n = 2 | 12.0 ± 5.60 (4) | 525 ± 19.0 (4) | 241 ± 12.0 (4) |
| Non-Hispanic, n = 11 | 18.8 ± 4.51 (21) | 556 ± 33.1 (20) | 269 ±21.9 (20) |
| - Women, n = 35 | 15.7 ± 3.33 (68) | 561 ± 46.2 (68) | 256 ± 21.5 (68) |
| Hispanic, n = 5 | 14.9 ± 3.07 (10) | 554 ± 54.8 (10) | 245 ± 11.2 (10) |
| Non-Hispanic, n = 30 | 15.8 ± 3.38 (58) | 562 ± 45.0 (58) | 258 ± 22.3 (58) |
| * BMI | BGL | SBP | DBP | |
|---|---|---|---|---|
| (kg/m2) | (mg/dL) | (mmHg) | (mmHg) | |
| All subjects | 22.5; 6.50 (59) | 85.8 ± 12.2 (89) | 117 ± 12.0 (91) | 72.7 ± 8.09 (90) |
| Men | 25.1; 6.90 (16) | 94.4 ± 12.8 (18) | 126 ± 17.1 (18) | 76.8 ± 9.10 (18) |
| - 1st session | -- | 90.4 ± 11.3 (9) | 125 ± 15.9 (9) | 74.7 ± 9.55 (9) |
| - 2nd session | -- | 98.4 ± 13.6 (9) | 127 ± 19.1 (9) | 78.9 ± 8.64 (9) |
| Women | 22.2; 4.10 (43) | 83.7 ± 11.1 (71) | 114 ± 9.22 (73) | 71.7 ± 7.57 (72) |
| - Follicular | -- | 85.2 ± 11.1 (36) | 114 ± 10.2 (37) | 71.7 ± 7.45 (36) |
| - Luteal | -- | 82.8 ± 10.4 (35) | 115 ± 8.51 (36) | 71.5 ± 7.90 (36) |
| Cycling women | 22.5; 4.40 (28) | 85.7 ± 11.1 (43) | 114 ± 9.50 (45) | 70.4 ± 6.44 (44) |
| - Follicular | -- | 86.9 ± 11.4 (22) | 115 ± 10.6 (23) | 71.7 ± 10.6 (22) |
| - Luteal | -- | 84.4 ± 10.8 (21) | 113 ± 8.41 (22) | 69.1 ± 6.14 (22) |
| OC users | 21.5; 7.30 (15) | 80.7 ± 10.7 (28) | 116 ± 8.85 (28) | 73.7 ± 8.72 (28) |
| - Follicular | -- | 82.6 ± 10.4 (14) | 114 ± 9.95 (14) | 71.7 ± 8.89 (14) |
| - Luteal | -- | 80.4 ± 9.77 (14) | 118 ± 8.08 (14) | 75.4 ± 9.02 (14) |
| All Subjects | Men | Women | Cycling Women | OC Users | |
|---|---|---|---|---|---|
| IOP vs. | -- | -- | -- | -- | -- |
| - CCT | 0.401 (<0.001) | 0.446 (0.014) | 0.457 (<0.001) | 0.621 (<0.001) | 0.116 (0.549) |
| - FT | −0.065 (0.482) | −0.214 (0.255) | −0.061 (0.750) | −0.101 (0.461) | 0.078 (0.688) |
| - BMI † | 0.153 (0.327) | 0.279 (0.314) | 0.059 (0.707) | 0.099 (0.616) | 0.166 (0.554) |
| - BGL | −0.046 (0.665) | 0.069 (0.786) | −0.048 (0.686) | −0.077 (0.625) | −0.161 (0.396) |
| - SBP | 0.057 (0.589) | 0.224 (0.371) | 0.029 (0.803) | 0.066 (0.667) | 0.027 (0.886) |
| - DBP | 0.036 (0.735) | 0.295 (0.234) | −0.023 (0.843) | 0.113 (0.464) | −0.083 (0.663) |
| CCT vs. | -- | -- | -- | -- | -- |
| - FT | −0.224 (0.015) | −0.473 (0.008) | 0.078 (0.481) | −0.105 (0.446) | 0.011 (0.955) |
| - BMI † | −0.113 (0.394) | 0.001 (0.998) | −0.062 (0.693) | 0.017 (0.932) | −0.254 (0.402) |
| - BGL | −0.262 (0.012) | −0.397 (0.103) | −0.162 (0.172) | −0.301 (0.050) | −0.175 (0.355) |
| - SBP | −0.175 (0.094) | 0.034 (0.895) | −0.176 (0.131) | −0.379 (0.010) | 0.098 (0.607) |
| - DBP | −0.046 (0.666) | −0.121 (0.631) | 0.038 (0.746) | −0.184 (0.231) | 0.337 (0.068) |
| FT vs. | -- | -- | -- | -- | -- |
| - BMI † | 0.113 (0.394) | 0.235 (0.381) | −0.020 (0.899) | 0.010 (0.960) | 0.102 (0.718) |
| - BGL | 0.360 (<0.001) | 0.779 (<0.001) | 0.125 (0.292) | 0.019 (0.902) | 0.344 (0.063) |
| - SBP | 0.363 (<0.001) | 0.635 (0.005) | 0.102 (0.384) | 0.250 (0.097) | −0.023 (0.904) |
| - DBP | 0.346 (<0.001) | 0.628 (0.005) | 0.176 (0.133) | 0.276 (0.070) | 0.057 (0.765) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortepiani, L.; Foutch, B.K.; Wilson, M.R. The Effects of Sex, Oral Contraception, and Menstrual Cycle Phase on Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness: A Descriptive Analysis. Vision 2021, 5, 48. https://doi.org/10.3390/vision5040048
Fortepiani L, Foutch BK, Wilson MR. The Effects of Sex, Oral Contraception, and Menstrual Cycle Phase on Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness: A Descriptive Analysis. Vision. 2021; 5(4):48. https://doi.org/10.3390/vision5040048
Chicago/Turabian StyleFortepiani, Lourdes, Brian K. Foutch, and Molly R. Wilson. 2021. "The Effects of Sex, Oral Contraception, and Menstrual Cycle Phase on Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness: A Descriptive Analysis" Vision 5, no. 4: 48. https://doi.org/10.3390/vision5040048
APA StyleFortepiani, L., Foutch, B. K., & Wilson, M. R. (2021). The Effects of Sex, Oral Contraception, and Menstrual Cycle Phase on Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness: A Descriptive Analysis. Vision, 5(4), 48. https://doi.org/10.3390/vision5040048







