The Impact of Shape-Based Cue Discriminability on Attentional Performance
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Apparatus and Stimuli
2.2.1. Apparatus
2.2.2. Shape-Based Spatial Cue Generating
2.2.3. Stimuli
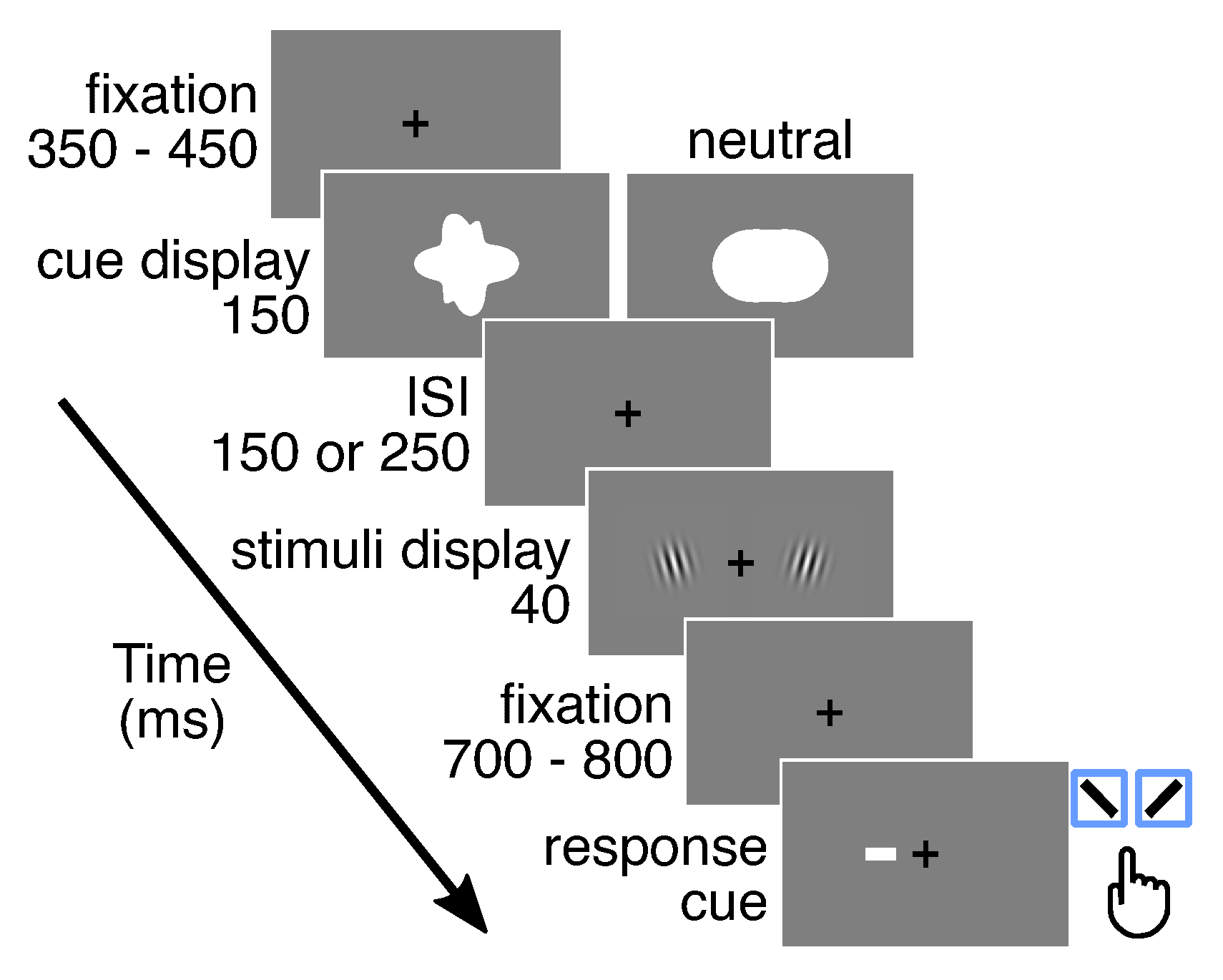
2.3. Experimental Procedure
2.3.1. General Procedure
2.3.2. Cue Sensitivity Test
2.3.3. Practice Session
2.3.4. Main Experiment
2.4. Analysis
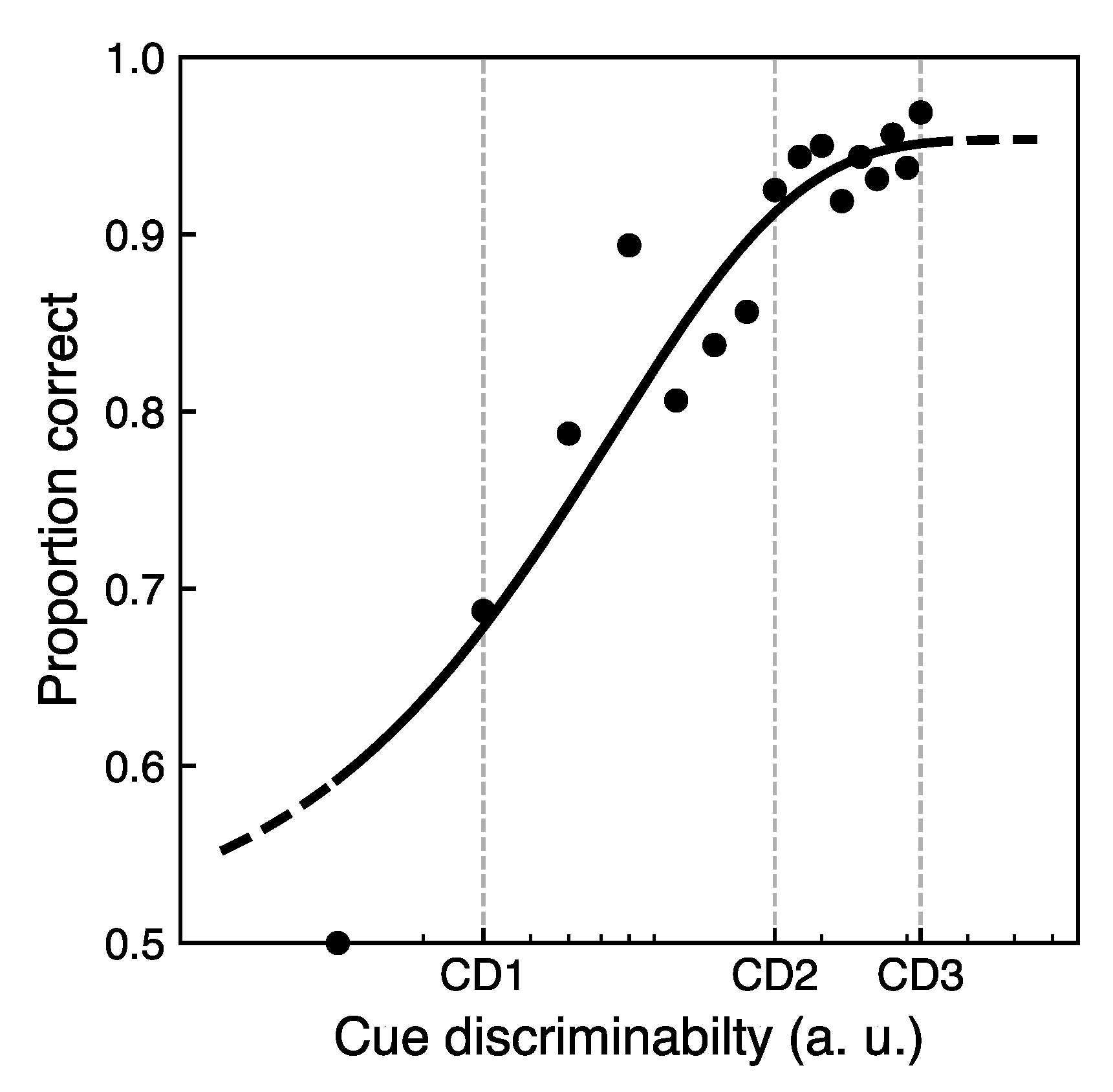
2.4.1. Cue Sensitivity Test
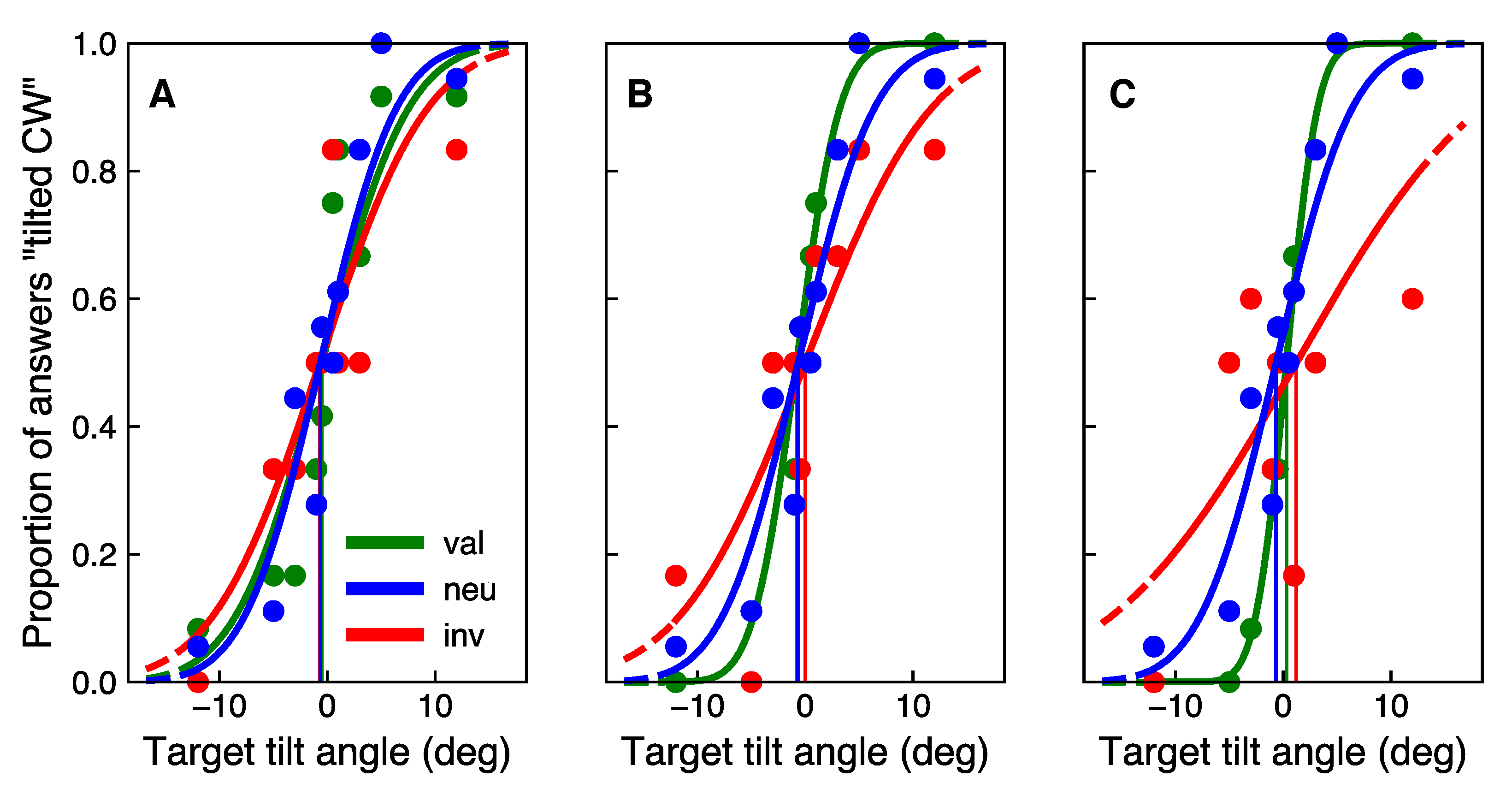
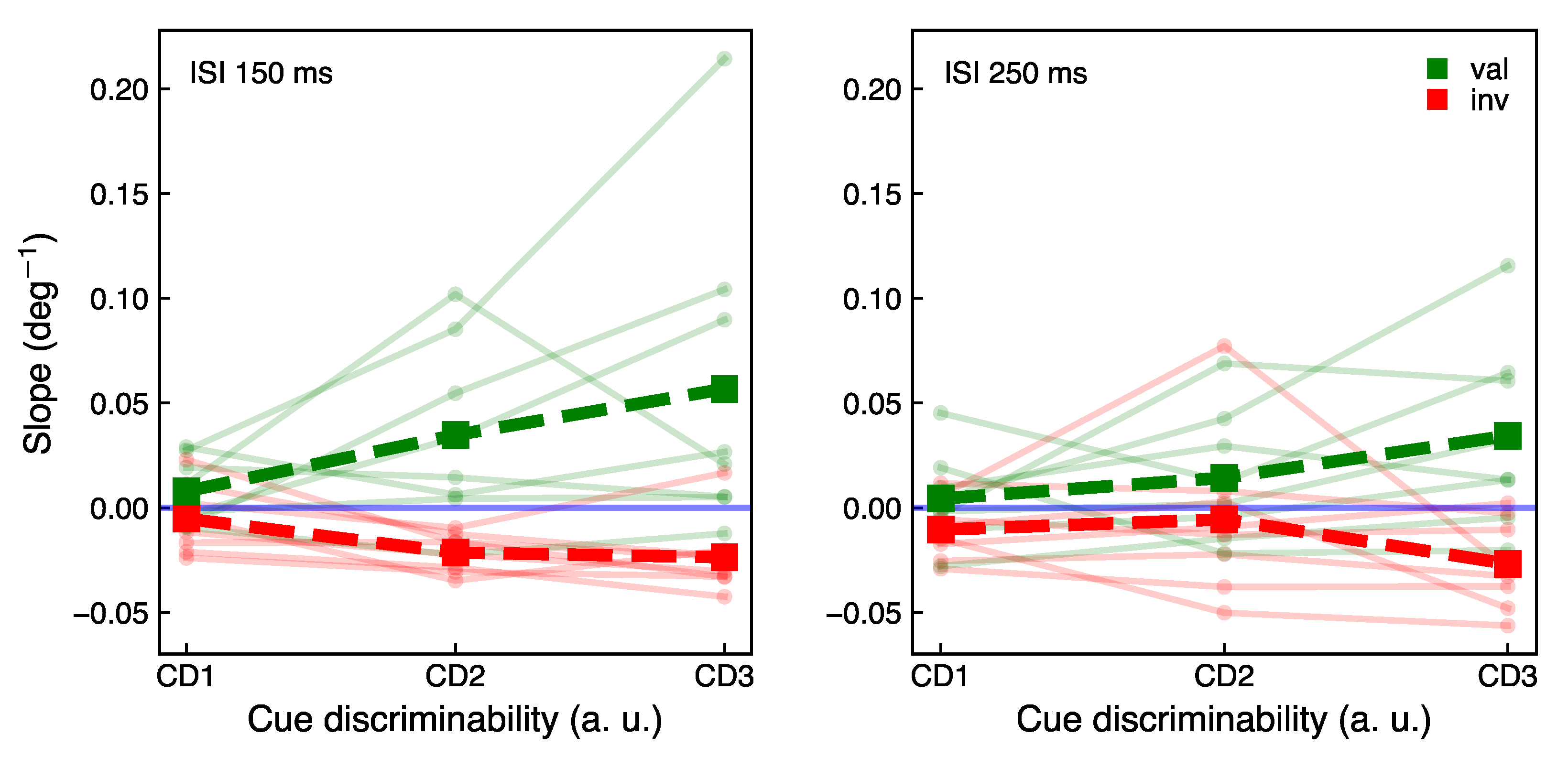
2.4.2. Main Experiment
2.4.3. Cue Processing Temporal Cost
3. Results
3.1. Cue Sensitivity
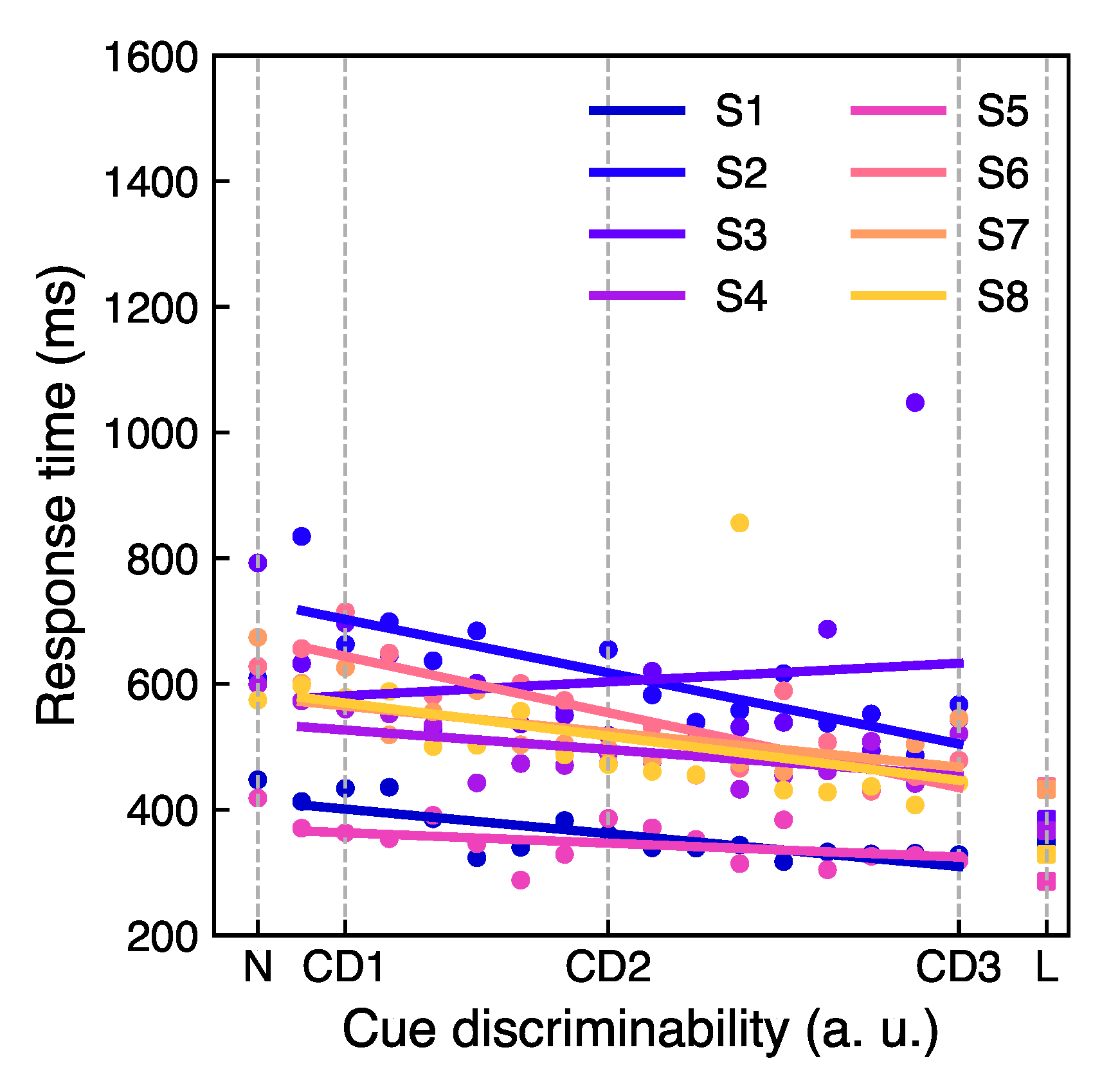
3.2. Main Experiment
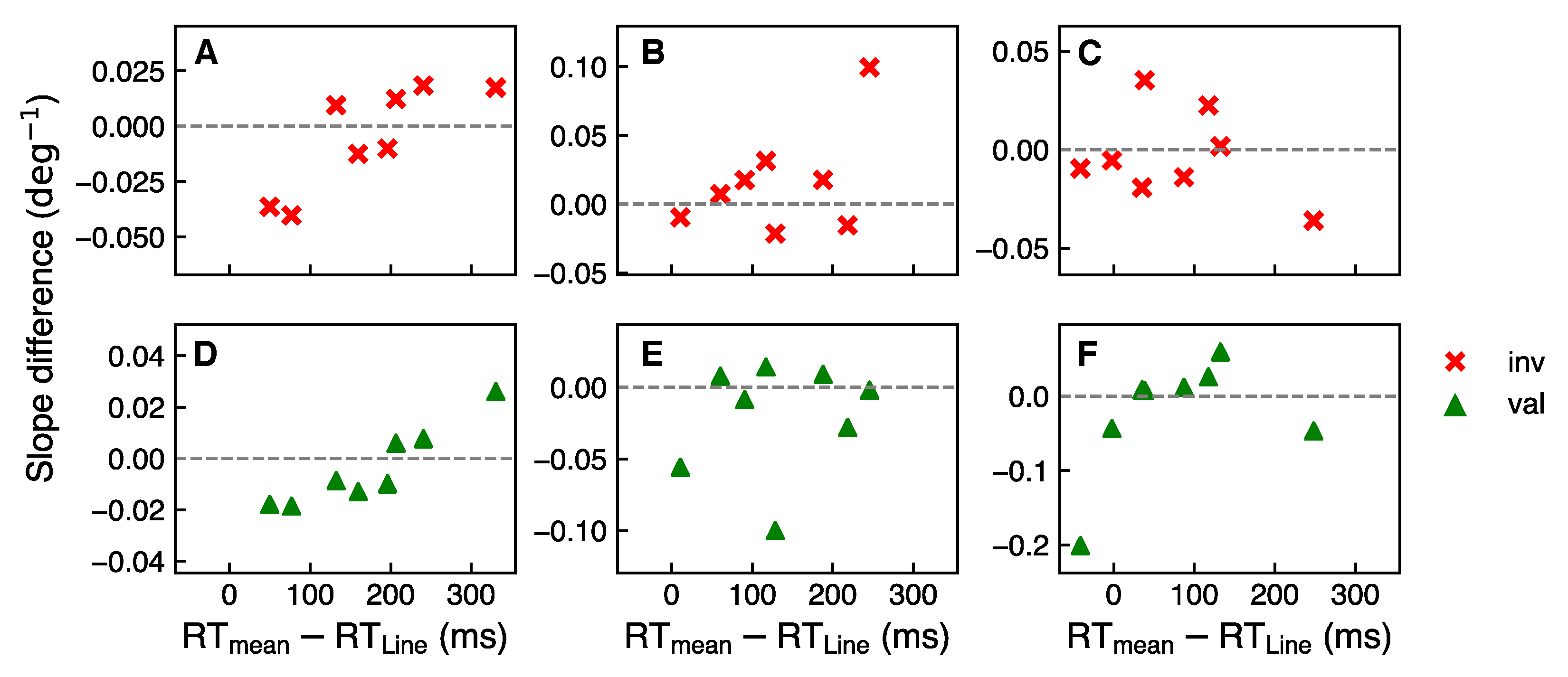
3.3. Cue Processing Temporal Costs
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2AFC | two-alternative-forced-choice |
| SOA | stimulus-onset asynchrony |
| ISI | inter-stimulus interval |
References
- Madsen, A.; Rouinfar, A.; Larson, A.M.; Loschky, L.C.; Rebello, N.S. Can short duration visual cues influence students’ reasoning and eye movements in physics problems? Phys. Rev. Spec. Top. Phys. Educ. Res. 2013, 9, 020104. [Google Scholar] [CrossRef]
- Awan, O.A. Virtual Radiology Readouts After the COVID-19 Pandemic. AJR Am. J. Roentgenol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M. Visual attention: The past 25 years. Vis. Res. 2011, 51, 1484–1525. [Google Scholar] [CrossRef]
- Eckstein, M.P. Visual search: A retrospective. J. Vis. 2011, 11, 14. [Google Scholar] [CrossRef]
- Carrasco, M.; Williams, P.E.; Yeshurun, Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. J. Vis. 2002, 2, 4. [Google Scholar] [CrossRef]
- Carrasco, M.; Loula, F.; Ho, Y.X. How attention enhances spatial resolution: Evidence from selective adaptation to spatial frequency. Percept. Psychophys. 2006, 68, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Pestilli, F.; Carrasco, M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vis. Res. 2005, 45, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Montagna, B.; Pestilli, F.; Carrasco, M. Attention trades off spatial acuity. Vis. Res. 2009, 49, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.; Barbot, A.; Carrasco, M. Voluntary attention increases perceived spatial frequency. Atten. Percept. Psychophys. 2010, 72, 1510–1521. [Google Scholar] [CrossRef]
- Liu, T.; Abrams, J.; Carrasco, M. Voluntary attention enhances contrast appearance. Psychol. Sci. 2009, 20, 354–362. [Google Scholar] [CrossRef]
- Lu, W.; Duh, B.L.H.; Feiner, S. Subtle cueing for visual search in augmented reality. In Proceedings of the 2012 IEEE International Symposium on Mixed and Augmented Reality (ISMAR), Atlanta, GA, USA, 5–8 November 2012; pp. 161–166. [Google Scholar] [CrossRef]
- Orlosky, J.; Liu, C.; Kalkofen, D.; Kiyokawa, K. Visualization-guided attention direction in dynamic control tasks. In Proceedings of the Adjunct Proceedings of the 2019 IEEE International Symposium on Mixed and Augmented Reality Adjunct (ISMAR-Adjunct), Beijing, China, 10–18 October 2019; pp. 372–373. [Google Scholar] [CrossRef]
- Raja, V.; Calvo, P. Augmented reality: An ecological blend. Cogn. Syst. Res. 2017, 42, 58–72. [Google Scholar] [CrossRef]
- Müller, N.G.; Bartelt, O.A.; Donner, T.H.; Villringer, A.; Brandt, S.A. A physiological correlate of the “zoom lens” of visual attention. J. Neurosci. 2003, 23, 3561–3565. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Park, Y.; Carrasco, M. Cue contrast modulates the effects of exogenous attention on appearance. Vis. Res. 2009, 49, 1825–1837. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Carrasco, M. The effects of transient attention on spatial resolution and the size of the attentional cue. Percept. Psychophys. 2008, 70, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.M.; Horowitz, T.S. What attributes guide the deployment of visual attention and how do they do it? Nat. Rev. Neurosci. 2004, 5, 495–501. [Google Scholar] [CrossRef]
- Carrasco, M. How visual spatial attention alters perception. Cogn. Process. 2018, 19, 77–88. [Google Scholar] [CrossRef]
- Folk, C.L.; Remington, R.W.; Johnston, J.C. Involuntary Covert Orienting Is Contingent on Attentional Control Settings. J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 1030–1044. [Google Scholar] [CrossRef]
- Bertleff, S.; Fink, G.R.; Weidner, R. Attentional capture: Role of top-down focused spatial attention and the need to search among multiple locations. Vis. Cogn. 2017, 25, 326–342. [Google Scholar] [CrossRef]
- Goldsmith, M.; Yeari, M. Central-Cue Discriminability Modulates Object-Based Attention by Influencing Spatial Attention. Exp. Psychol. 2012, 59, 132–137. [Google Scholar] [CrossRef]
- Brignani, D.; Guzzon, D.; Marzi, C.; Miniussi, C. Attentional orienting induced by arrows and eye-gaze compared with an endogenous cue. Neuropsychologia 2009, 47, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Watanabe, K. Gaze cueing by pareidolia faces. i-Perception 2013, 4, 490–492. [Google Scholar] [CrossRef]
- Trachel, R.E.; Clerc, M.; Brochier, T.G. Decoding covert shifts of attention induced by ambiguous visuospatial cues. Front. Hum. Neurosci. 2015, 9, 358. [Google Scholar] [CrossRef]
- Jonides, J. Voluntary vs. automatic control over the mind’s eye’s movement. In Attention and Performance; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1980. [Google Scholar]
- Müller, H.J.; Rabbitt, P.M. Reflexive and Voluntary Orienting of Visual Attention: Time Course of Activation and Resistance to Interruption. J. Exp. Psychol. Hum. Percept. Perform. 1989, 15, 315–330. [Google Scholar] [CrossRef]
- Cheal, M.; Lyon, D.R. Central and Peripheral Precuing of Forced-Choice Discrimination. Q. J. Exp. Psychol. Sect. A 1991, 43, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Pierce, L. Moving attention: Evidence for time-invariant shifts of visual selective attention. Percept. Psychophys. 1984, 35, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Stevens, S.T.; Carrasco, M. Comparing the time course and efficacy of spatial and feature-based attention. Vis. Res. 2007, 47, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Dalmaijer, E.S.; Mathôt, S.; Van der Stigchel, S. PyGaze: An open-source, cross-platform toolbox for minimal-effort programming of eyetracking experiments. Behav. Res. Methods 2014, 46, 913–921. [Google Scholar] [CrossRef]
- Guzzon, D.; Brignani, D.; Miniussi, C.; Marzi, C.A. Orienting of attention with eye and arrow cues and the effect of overtraining. Acta Psychol. 2010, 134, 353–362. [Google Scholar] [CrossRef]
- Wilkinson, F.; Wilson, H.R.; Habak, C. Detection and recognition of radial frequency patterns. Vis. Res. 1998, 38, 3555–3568. [Google Scholar] [CrossRef]
- Giordano, A.M.; McElree, B.; Carrasco, M. On the automaticity and flexibility of covert attention: A speed-accuracy trade-off analysis. J. Vis. 2009, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Block, R.A.; Aguinis, H. Cautionary Note on Reporting Eta-Squared Values from Multifactor ANOVA Designs. Educ. Psychol. Meas. 2004, 64, 916–924. [Google Scholar] [CrossRef]
- Klein, R.M. Inhibition of return. Trends Cogn. Sci. 2000, 4, 138–147. [Google Scholar] [CrossRef]
- Pratt, J.; Fischer, M.H. Examining the role of the fixation cue in inhibition of return. Can. J. Exp. Psychol. 2002, 56, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.S.; Bryant, T.A. Variation in cue duration reveals top-down modulation of involuntary orienting to uninformative symbolic cues. Percept. Psychophys. 2005. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukashova-Sanz, O.; Wahl, S.; Wallis, T.S.A.; Rifai, K. The Impact of Shape-Based Cue Discriminability on Attentional Performance. Vision 2021, 5, 18. https://doi.org/10.3390/vision5020018
Lukashova-Sanz O, Wahl S, Wallis TSA, Rifai K. The Impact of Shape-Based Cue Discriminability on Attentional Performance. Vision. 2021; 5(2):18. https://doi.org/10.3390/vision5020018
Chicago/Turabian StyleLukashova-Sanz, Olga, Siegfried Wahl, Thomas S. A. Wallis, and Katharina Rifai. 2021. "The Impact of Shape-Based Cue Discriminability on Attentional Performance" Vision 5, no. 2: 18. https://doi.org/10.3390/vision5020018
APA StyleLukashova-Sanz, O., Wahl, S., Wallis, T. S. A., & Rifai, K. (2021). The Impact of Shape-Based Cue Discriminability on Attentional Performance. Vision, 5(2), 18. https://doi.org/10.3390/vision5020018





