Reliability and Generalizability of Similarity-Based Fusion of MEG and fMRI Data in Human Ventral and Dorsal Visual Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
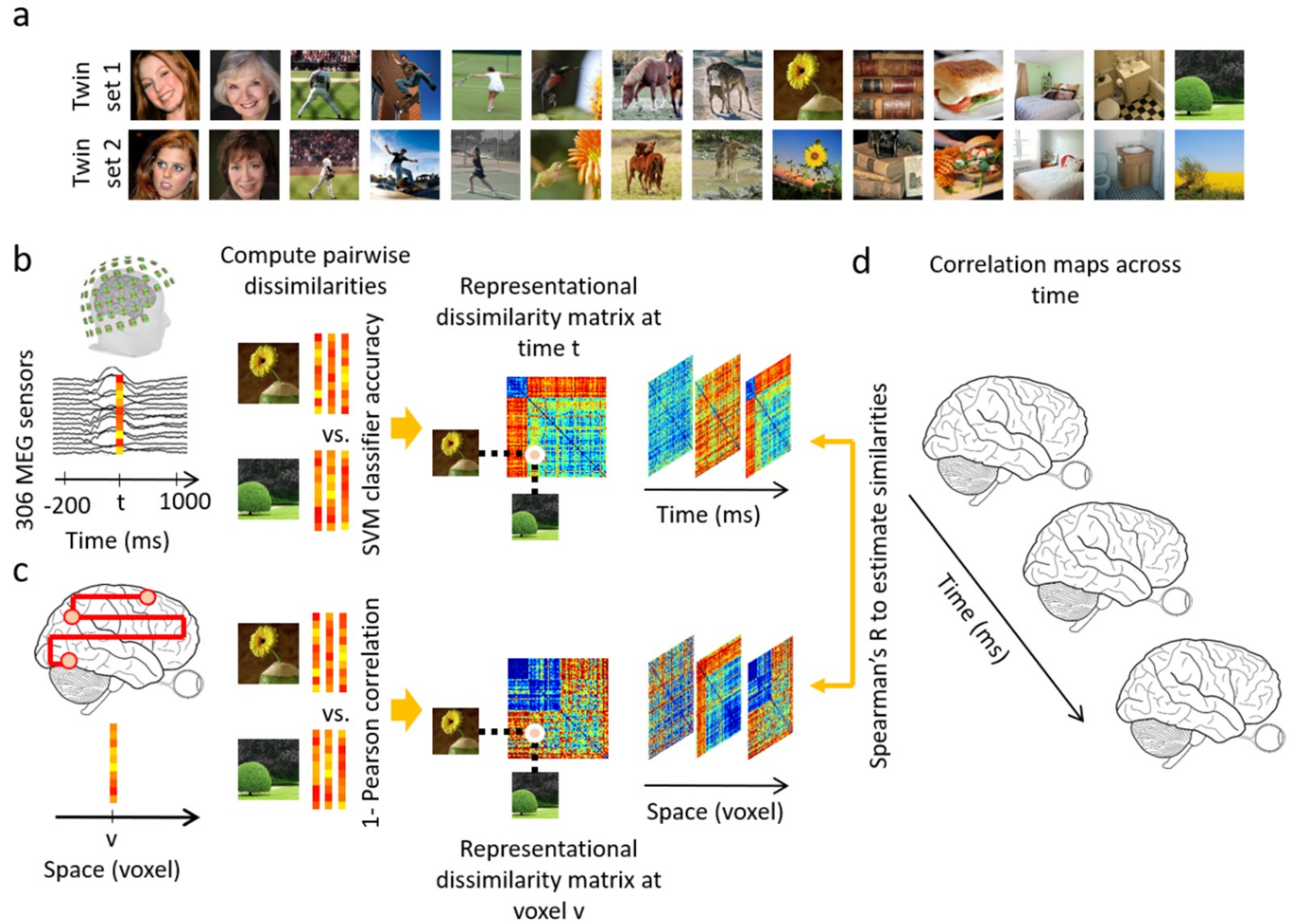
2.2. Stimulus Set
2.3. fMRI and MEG Acquisition
2.4. Data Analyses
3. Results
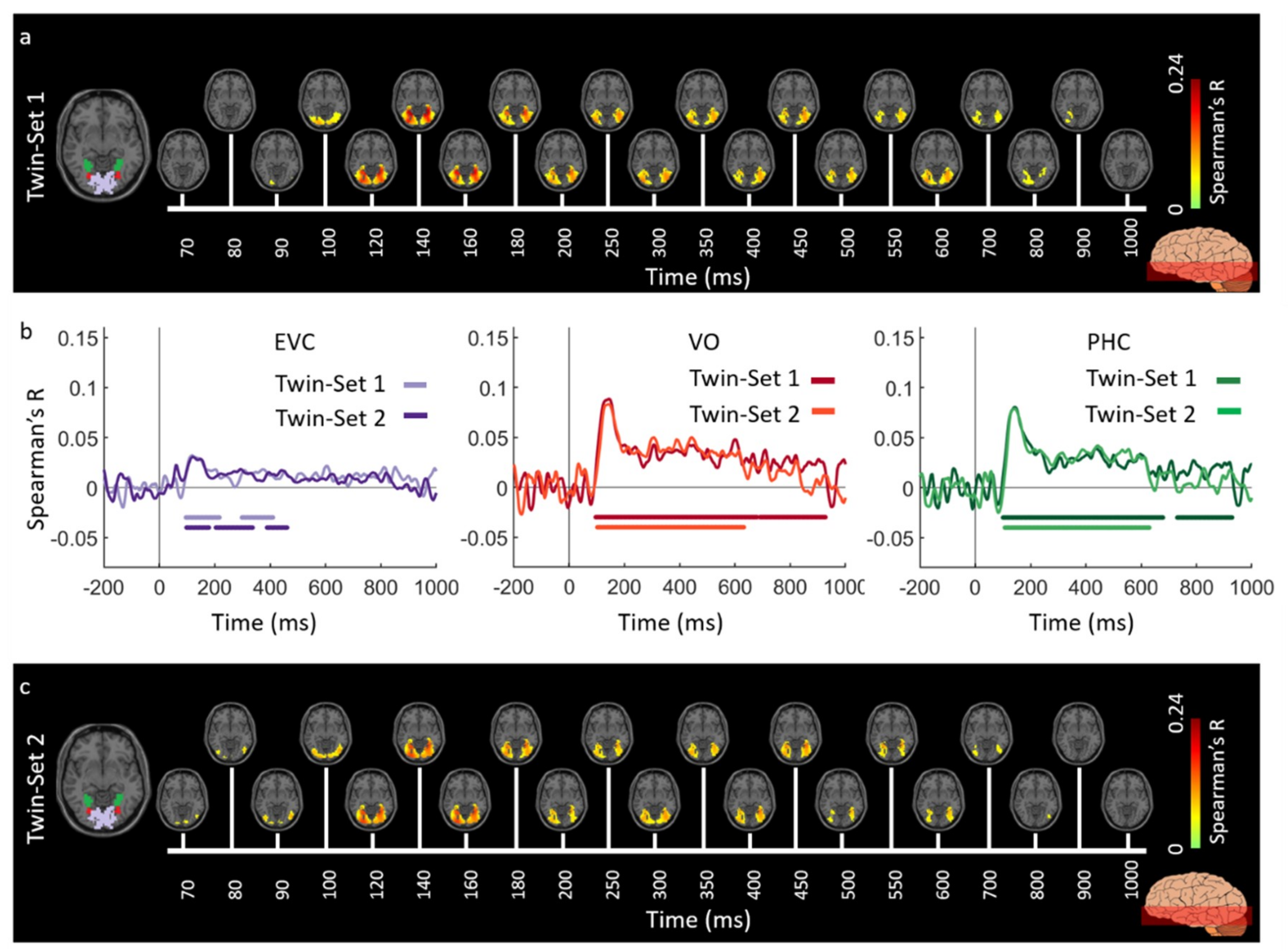
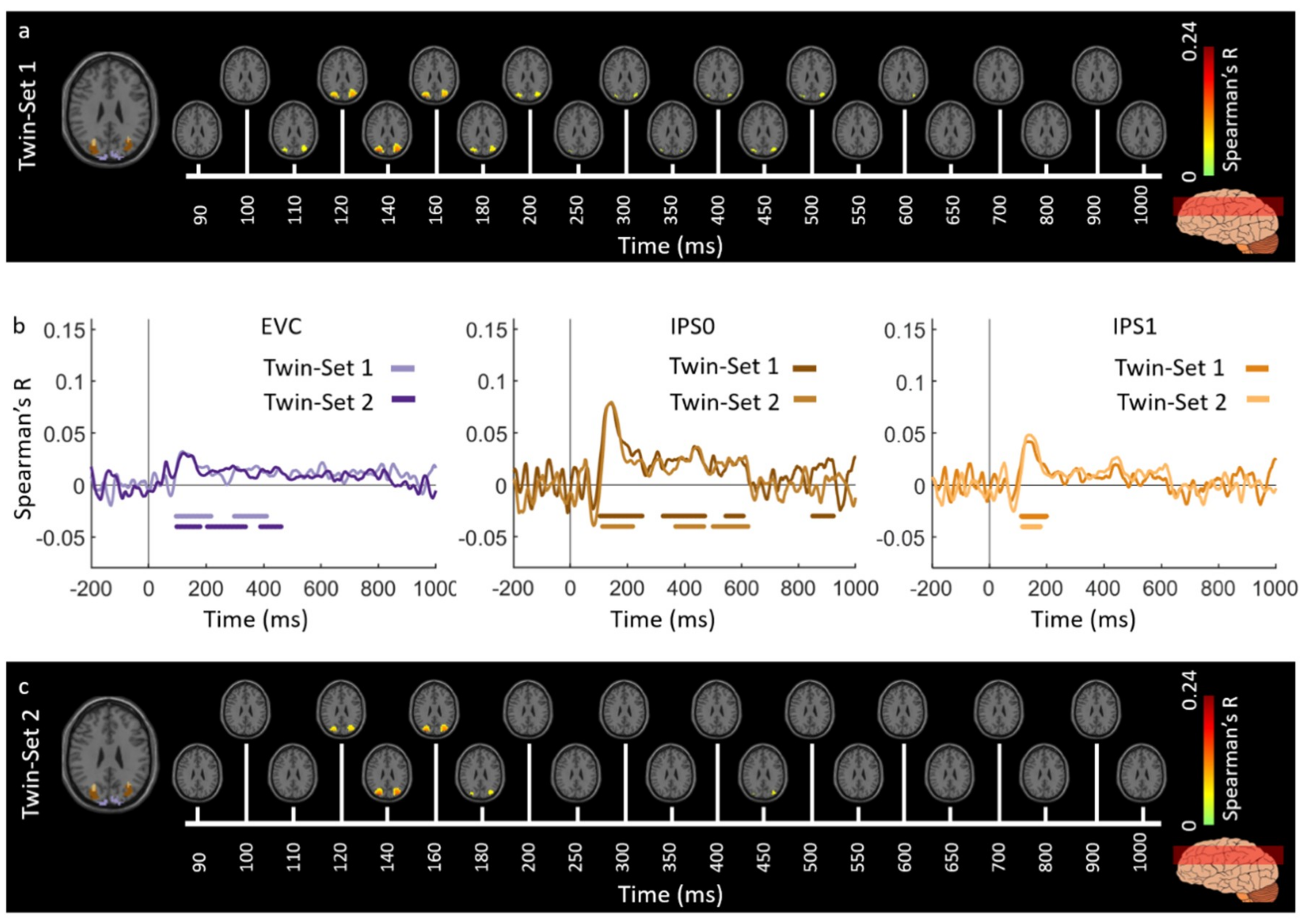
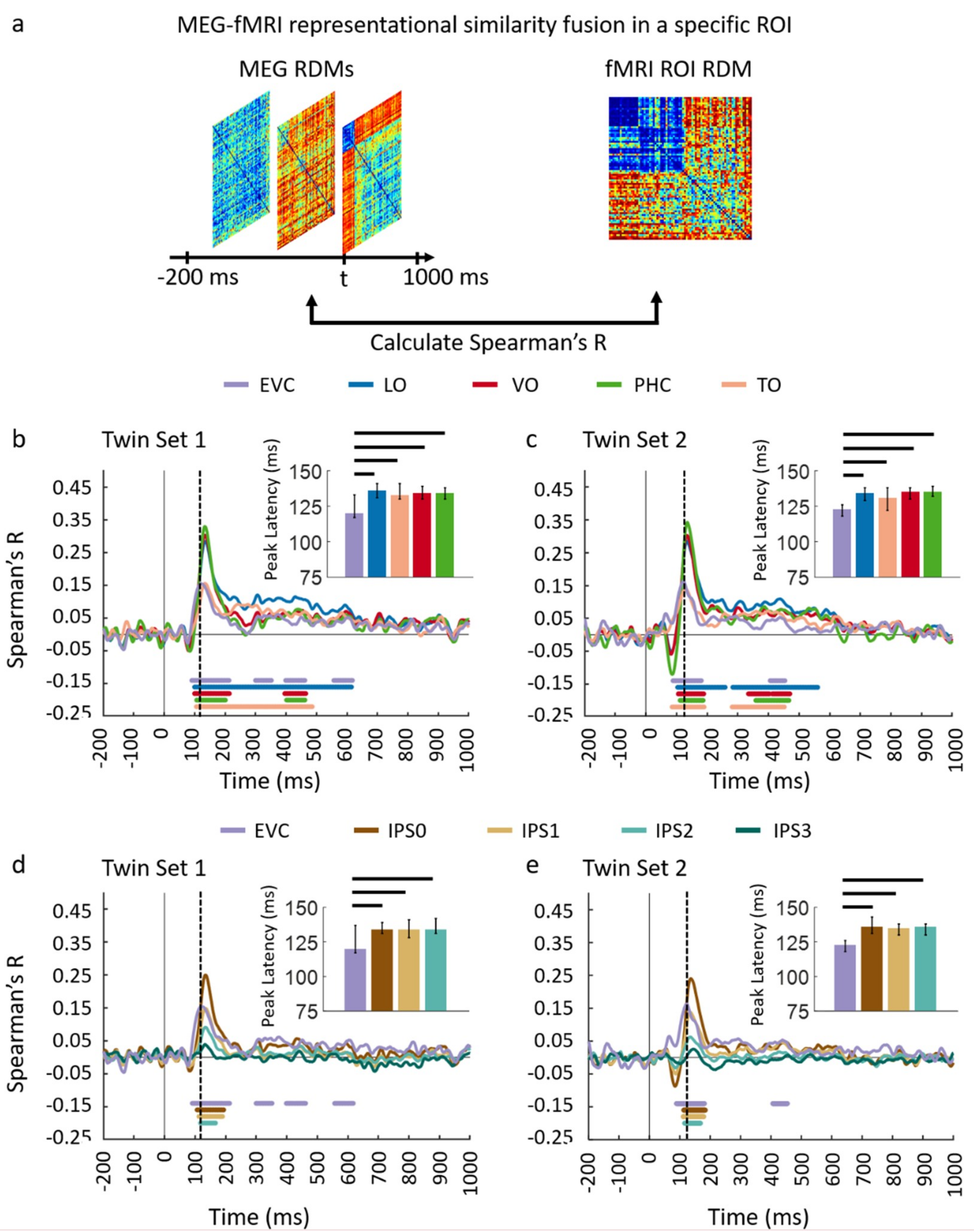
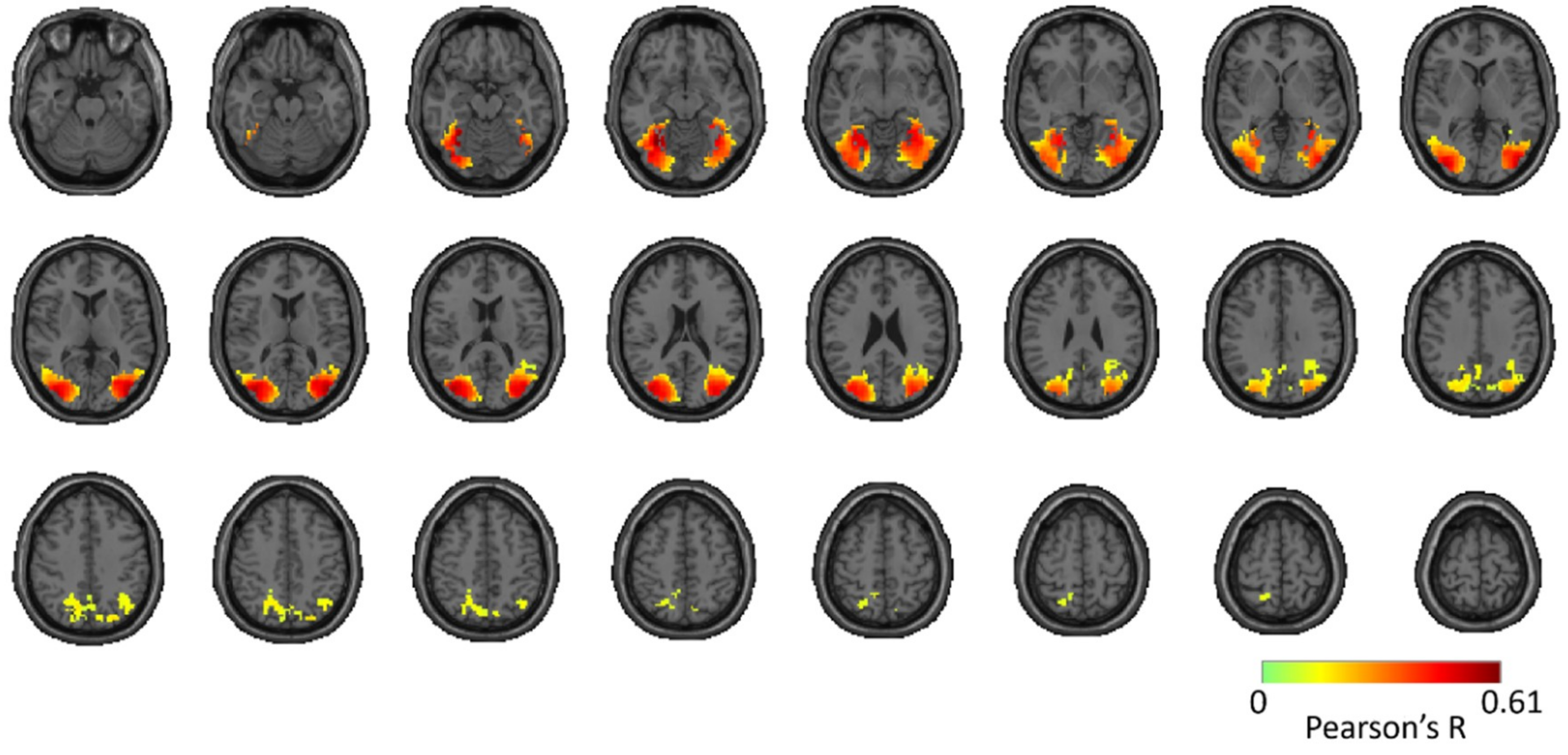
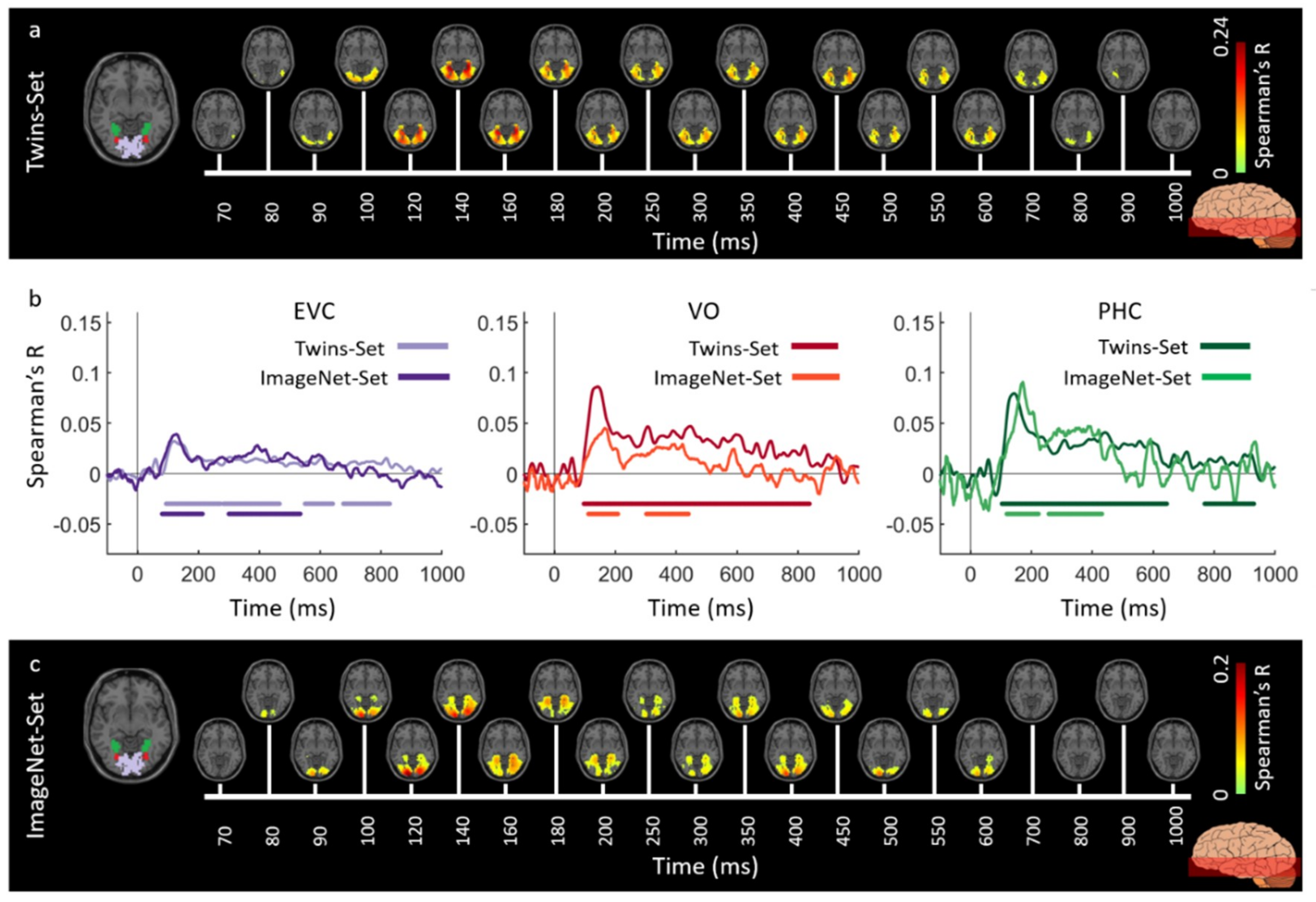
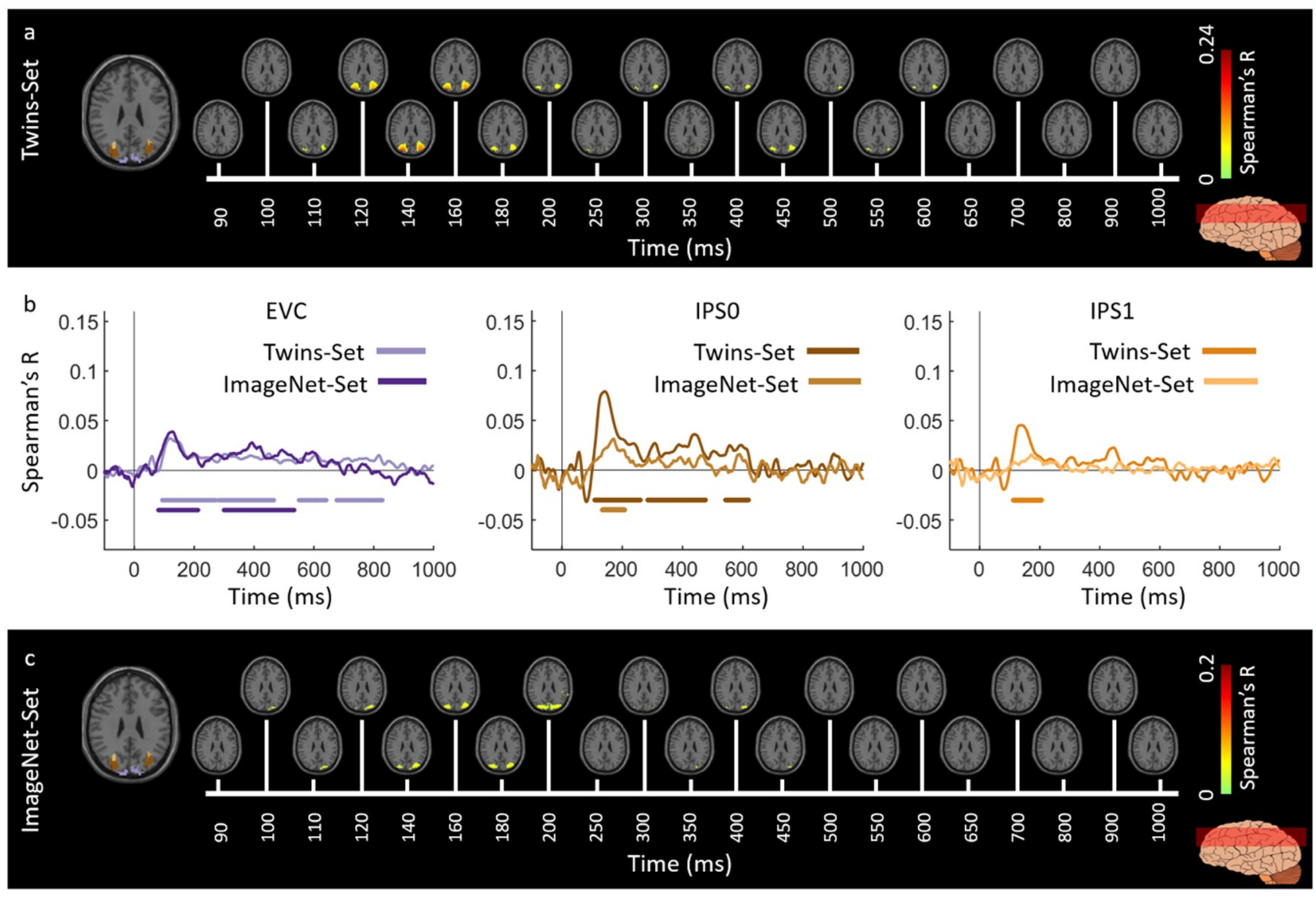
3.1. Reliability of Similarity-Based fMRI-MEG Fusion Method
3.2. Generalizability of Similarity-Based fMRI-MEG Fusion Method
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Spectral Energy Level (Spatial Frequency) | ||||
|---|---|---|---|---|
| 10% | 30% | 70% | 90% | |
| t value | −0.43 | 0.87 | 0.37 | −0.37 |
| p value | 0.67 | 0.37 | 0.7 | 0.72 |
| Color Distribution | ||||||
|---|---|---|---|---|---|---|
| RGB Space | Lab Space (Brightness and Contrast) | |||||
| R | G | B | L | a | b | |
| t value | 0.65 | 0.99 | 0.71 | 0.87 | −0.80 | 0.05 |
| p value | 0.51 | 0.32 | 0.48 | 0.38 | 0.43 | 0.96 |
Appendix B
MEG Acquisition and Analysis
fMRI Acquisition and Analysis
Appendix C
Appendix D
Region-of-Interest Analysis
Appendix E
Reliability Maps
Appendix F
Statistical Testing
References
- Malach, R.; Reppas, J.B.; Benson, R.R.; Kwong, K.K.; Jiang, H.; Kennedy, W.A.; Ledden, P.J.; Brady, T.J.; Rosen, B.R.; Tootell, R.B. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl. Acad. Sci. USA 1995, 92, 8135–8139. [Google Scholar] [CrossRef] [PubMed]
- Grill-Spector, K.; Kourtzi, Z.; Kanwisher, N. The lateral occipital complex and its role in object recognition. Vis. Res. 2001, 41, 1409–1422. [Google Scholar] [CrossRef]
- Johnson, J.S.; Olshausen, B.A. Timecourse of neural signatures of object recognition. J. Vis. 2003, 3, 4. [Google Scholar] [CrossRef]
- Op de Beeck, H.P.; Baker, C.I. The neural basis of visual object learning. Trends Cogn. Sci. 2010, 14, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.R.; Steeves, J.K.E. Consecutive TMS-fMRI Reveals an Inverse Relationship in BOLD Signal between Object and Scene Processing. J. Neurosci. 2013, 33, 19243–19249. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, J.J.; Zoccolan, D.; Rust, N.C. How Does the Brain Solve Visual Object Recognition? Neuron 2012, 73, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Konkle, T.; Oliva, A. A Real-World Size Organization of Object Responses in Occipitotemporal Cortex. Neuron 2012, 74, 1114–1124. [Google Scholar] [CrossRef]
- Cichy, R.M.; Pantazis, D.; Oliva, A. Resolving human object recognition in space and time. Nat. Neurosci. 2014, 17, 455–462. [Google Scholar] [CrossRef]
- Cichy, R.M.; Pantazis, D.; Oliva, A. Similarity-Based Fusion of MEG and fMRI Reveals Spatio-Temporal Dynamics in Human Cortex During Visual Object Recognition. Cereb. Cortex 2016, 26, 3563–3579. [Google Scholar] [CrossRef]
- Dale, A.M.; Halgren, E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr. Opin. Neurobiol. 2001, 11, 202–208. [Google Scholar] [CrossRef]
- Debener, S.; Ullsperger, M.; Siegel, M.; Engel, A.K. Single-trial EEG–fMRI reveals the dynamics of cognitive function. Trends Cogn. Sci. 2006, 10, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.J.; Daunizeau, J.; Friston, K.J. EEG-fMRI Integration: A Critical Review of Biophysical Modeling and Data Analysis Approaches. J. Integr. Neurosci. 2010, 09, 453–476. [Google Scholar] [CrossRef]
- Huster, R.J.; Debener, S.; Eichele, T.; Herrmann, C.S. Methods for Simultaneous EEG-fMRI: An Introductory Review. J. Neurosci. 2012, 32, 6053–6060. [Google Scholar] [CrossRef] [PubMed]
- Jorge, J.; van der Zwaag, W.; Figueiredo, P. EEG–fMRI integration for the study of human brain function. NeuroImage 2014, 102, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Adali, T.; Yu, Q.; Chen, J.; Calhoun, V.D. A review of multivariate methods for multimodal fusion of brain imaging data. J. Neurosci. Methods 2012, 204, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Khaligh-Razavi, S.-M.; Cichy, R.M.; Pantazis, D.; Oliva, A. Tracking the Spatiotemporal Neural Dynamics of Real-world Object Size and Animacy in the Human Brain. J. Cogn. Neurosci. 2018, 30, 1559–1576. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, Y.; Qin, S.; Cichy, R.M.; Pantazis, D. Ultra-Rapid serial visual presentation reveals dynamics of feedforward and feedback processes in the ventral visual pathway. eLife 2018, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Hebart, M.N.; Bankson, B.B.; Harel, A.; Baker, C.I.; Cichy, R.M. The representational dynamics of task and object processing in humans. eLife 2018, 7, 1–22. [Google Scholar] [CrossRef]
- Thorpe, S.; Fize, D.; Marlot, C. Speed of processing in the human visual system. Nature 1996, 381, 520–522. [Google Scholar] [CrossRef]
- VanRullen, R.; Thorpe, S.J. The Time Course of Visual Processing: From Early Perception to Decision-Making. J. Cogn. Neurosci. 2001, 13, 454–461. [Google Scholar] [CrossRef]
- Mormann, F.; Kornblith, S.; Quiroga, R.Q.; Kraskov, A.; Cerf, M.; Fried, I.; Koch, C. Latency and Selectivity of Single Neurons Indicate Hierarchical Processing in the Human Medial Temporal Lobe. J. Neurosci. 2008, 28, 8865–8872. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Agam, Y.; Madsen, J.R.; Kreiman, G. Timing, Timing, Timing: Fast Decoding of Object Information from Intracranial Field Potentials in Human Visual Cortex. Neuron 2009, 62, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Isik, L.; Meyers, E.M.; Leibo, J.Z.; Poggio, T. The dynamics of invariant object recognition in the human visual system. J. Neurophysiol. 2014, 111, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.; Tovar, D.A.; Alink, A.; Kriegeskorte, N. Representational dynamics of object vision: The first 1000 ms. J. Vis. 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Contini, E.W.; Wardle, S.G.; Carlson, T.A. Decoding the time-course of object recognition in the human brain: From visual features to categorical decisions. Neuropsychologia 2017, 105, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.X.; Rajsic, J.; Ferber, S.; Walther, D.B. Discriminating scene categories from brain activity within 100 milliseconds. Cortex 2018, 106, 275–287. [Google Scholar] [CrossRef]
- Simmons, J.P.; Nelson, L.D.; Simonsohn, U. False-Positive Psychology: Undisclosed Flexibility in Data Collection and Analysis Allows Presenting Anything as Significant. Psychol. Sci. 2011, 22, 1359–1366. [Google Scholar] [CrossRef]
- Pashler, H.; Harris, C.R. Is the Replicability Crisis Overblown? Three Arguments Examined. Perspect. Psychol. Sci. 2012, 7, 531–536. [Google Scholar] [CrossRef]
- Bennett, C.M.; Miller, M.B. fMRI reliability: Influences of task and experimental design. Cogn. Affect. Behav. Neurosci. 2013, 13, 690–702. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef]
- Epstein, R.; Harris, A.; Stanley, D.; Kanwisher, N. The Parahippocampal Place Area: Recognition, Navigation, or Encoding? Neuron 1999, 23, 115–125. [Google Scholar] [CrossRef]
- Epstein, R.; Kanwisher, N. A cortical representation of the local visual environment. Nature 1998, 392, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Downing, P.E. A Cortical Area Selective for Visual Processing of the Human Body. Science 2001, 293, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Grill-Spector, K.; Kushnir, T.; Edelman, S.; Avidan, G.; Itzchak, Y.; Malach, R. Differential Processing of Objects under Various Viewing Conditions in the Human Lateral Occipital Complex. Neuron 1999, 24, 187–203. [Google Scholar] [CrossRef]
- Dilks, D.D.; Julian, J.B.; Paunov, A.M.; Kanwisher, N. The Occipital Place Area Is Causally and Selectively Involved in Scene Perception. J. Neurosci. 2013, 33, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Khosla, A.; Raju, A.S.; Torralba, A.; Oliva, A. Understanding and Predicting Image Memorability at a Large Scale. In Proceedings of the 2015 IEEE International Conference on Computer Vision (ICCV), Santiago, Chile, 7–13 December 2015; pp. 2390–2398. [Google Scholar]
- Torralba, A.; Oliva, A. Statistics of natural image categories. Netw. Comput. Neural Syst. 2003, 14, 391–412. [Google Scholar] [CrossRef]
- Bainbridge, W.A.; Oliva, A. A toolbox and sample object perception data for equalization of natural images. Data Brief 2015, 5, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Konkle, T.; Oliva, A. Parametric Coding of the Size and Clutter of Natural Scenes in the Human Brain. Cereb. Cortex 2015, 25, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Dong, W.; Socher, R.; Li, L.-J.; Li, K.; Fei-Fei, L. ImageNet: A Large-Scale Hierarchical Image Database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009. [Google Scholar]
- Kriegeskorte, N. Representational similarity analysis—Connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008, 2, 4. [Google Scholar] [CrossRef]
- Kriegeskorte, N.; Kievit, R.A. Representational geometry: Integrating cognition, computation, and the brain. Trends Cogn. Sci. 2013, 17, 401–412. [Google Scholar] [CrossRef]
- John, L.K.; Loewenstein, G.; Prelec, D. Measuring the Prevalence of Questionable Research Practices With Incentives for Truth Telling. Psychol. Sci. 2012, 23, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.E.; Lau, M.Y.; Howard, G.S. Is psychology suffering from a replication crisis? What does “failure to replicate” really mean? Am. Psychol. 2015, 70, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Munafò, M.R.; Nosek, B.A.; Bishop, D.V.M.; Button, K.S.; Chambers, C.D.; Percie du Sert, N.; Simonsohn, U.; Wagenmakers, E.-J.; Ware, J.J.; Ioannidis, J.P.A. A manifesto for reproducible science. Nat. Hum. Behav. 2017, 1, 0021. [Google Scholar] [CrossRef]
- Haynes, J.-D.; Rees, G. Decoding mental states from brain activity in humans. Nat. Rev. Neurosci. 2006, 7, 523–534. [Google Scholar] [CrossRef]
- Grill-Spector, K.; Malach, R. THE HUMAN VISUAL CORTEX. Annu. Rev. Neurosci. 2004, 27, 649–677. [Google Scholar] [CrossRef] [PubMed]
- Op de Beeck, H.P.; Haushofer, J.; Kanwisher, N.G. Interpreting fMRI data: Maps, modules and dimensions. Nat. Rev. Neurosci. 2008, 9, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Amano, K. Estimation of the Timing of Human Visual Perception from Magnetoencephalography. J. Neurosci. 2006, 26, 3981–3991. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Why Most Published Research Findings Are False. PLoS Med. 2005, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Riesenhuber, M.; Poggio, T. Neural mechanisms of object recognition. Curr. Opin. Neurobiol. 2002, 12, 162–168. [Google Scholar] [CrossRef]
- Serre, T.; Oliva, A.; Poggio, T. A feedforward architecture accounts for rapid categorization. Proc. Natl. Acad. Sci. USA 2007, 104, 6424–6429. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.; Hall, D.L.; Zelaya, F.O.; Williams, S.C.R.; Mehta, M.A. Measuring fMRI reliability with the intra-class correlation coefficient. NeuroImage 2009, 45, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Van Horn, J.D. Individual variability in brain activations associated with episodic retrieval: A role for large-scale databases. Int. J. Psychophysiol. 2007, 63, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Taulu, S.; Kajola, M.; Simola, J. Suppression of Interference and Artifacts by the Signal Space Separation Method. Brain Topogr. 2003, 16, 269–275. [Google Scholar] [CrossRef]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kriegeskorte, N.; Goebel, R.; Bandettini, P. Information-based functional brain mapping. Proc. Natl. Acad. Sci. USA 2006, 103, 3863–3868. [Google Scholar] [CrossRef]
- Haynes, J.-D.; Sakai, K.; Rees, G.; Gilbert, S.; Frith, C.; Passingham, R.E. Reading Hidden Intentions in the Human Brain. Curr. Biol. 2007, 17, 323–328. [Google Scholar] [CrossRef]
- Wang, L.; Mruczek, R.E.B.; Arcaro, M.J.; Kastner, S. Probabilistic Maps of Visual Topography in Human Cortex. Cereb. Cortex 2015, 25, 3911–3931. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Pantazis, D.; Nichols, T.E.; Baillet, S.; Leahy, R.M. A comparison of random field theory and permutation methods for the statistical analysis of MEG data. NeuroImage 2005, 25, 383–394. [Google Scholar] [CrossRef] [PubMed]
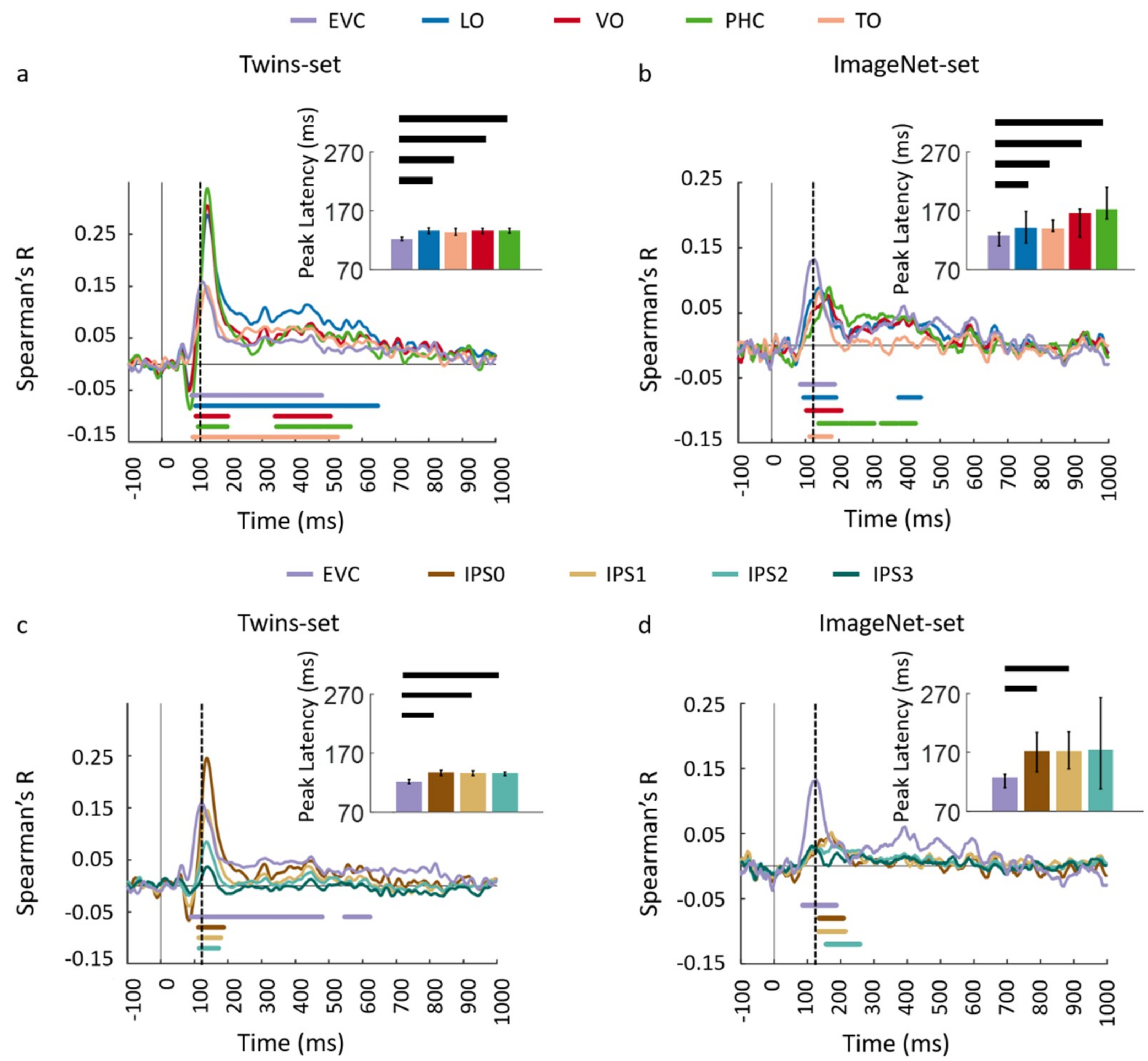
| Region of Interest | Twin-Set 1 | Twin-Set 2 | ||
|---|---|---|---|---|
| Peak latency (ms) | Onset latency (ms) | Peak latency (ms) | Onset latency (ms) | |
| EVC | 120 (117–137) | 92 (85–99) | 123 (118–126) | 90 (51–94) |
| Dorsal regions | ||||
| IPS0 | 134 (131–139) | 107 (97–116) | 136 (131–143) | 113 (107–118) |
| IPS1 | 134 (128–141) | 113 (102–123) | 135 (130–138) | 111 (105–116) |
| IPS2 | 134 (131–142) | 118 (95–156) | 136 (130–138) | 116 (107–126) |
| Ventral regions | ||||
| LO | 136 (131–141) | 101 (95–107) | 134 (129–138) | 105 (100–108) |
| VO | 134 (130–139) | 100 (95–105) | 135 (130–138) | 104 (99–111) |
| TO | 133 (130–141) | 104 (92–111) | 131 (122–138) | 85 (73–101) |
| PHC | 134 (130–138) | 106 (99–113) | 135 (132–139) | 112 (107–116) |
| Region of Interest | Twins-Set | ImageNet-Set | ||
|---|---|---|---|---|
| Peak latency (ms) | Onset latency (ms) | Peak latency (ms) | Onset latency (ms) | |
| EVC | 122 (119–125) | 89 (56–95) | 127 (110–133) | 84 (71–91) |
| Dorsal regions | ||||
| IPS0 | 137 (131–141) | 111 (102–117) | 172 (137–204) | 133 (99–156) |
| IPS1 | 136 (132–140) | 111 (103–119) | 172 (142–205) | 137 (99–158) |
| IPS2 | 136 (132–138) | 112 (100–119) | 174 (108–263) | 103 (90–186) |
| Ventral regions | ||||
| LO | 136 (131–141) | 102 (97–107) | 141 (115–169) | 96 (87–118) |
| VO | 136 (131–139) | 102 (95–109) | 166 (125–173) | 103 (95–125) |
| TO | 134 (128–140) | 90 (77–100) | 139 (135–154) | 115 (95–126) |
| PHC | 136 (132–139) | 109 (102–115) | 172 (156–210) | 121 (99–159) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsenzadeh, Y.; Mullin, C.; Lahner, B.; Cichy, R.M.; Oliva, A. Reliability and Generalizability of Similarity-Based Fusion of MEG and fMRI Data in Human Ventral and Dorsal Visual Streams. Vision 2019, 3, 8. https://doi.org/10.3390/vision3010008
Mohsenzadeh Y, Mullin C, Lahner B, Cichy RM, Oliva A. Reliability and Generalizability of Similarity-Based Fusion of MEG and fMRI Data in Human Ventral and Dorsal Visual Streams. Vision. 2019; 3(1):8. https://doi.org/10.3390/vision3010008
Chicago/Turabian StyleMohsenzadeh, Yalda, Caitlin Mullin, Benjamin Lahner, Radoslaw Martin Cichy, and Aude Oliva. 2019. "Reliability and Generalizability of Similarity-Based Fusion of MEG and fMRI Data in Human Ventral and Dorsal Visual Streams" Vision 3, no. 1: 8. https://doi.org/10.3390/vision3010008
APA StyleMohsenzadeh, Y., Mullin, C., Lahner, B., Cichy, R. M., & Oliva, A. (2019). Reliability and Generalizability of Similarity-Based Fusion of MEG and fMRI Data in Human Ventral and Dorsal Visual Streams. Vision, 3(1), 8. https://doi.org/10.3390/vision3010008





