Typical Lateral Interactions, but Increased Contrast Sensitivity, in Migraine-With-Aura
Abstract
1. Introduction
1.1. Altered Lateral Interactions in Migraine
1.2. Masking in Migraine
1.3. Global Processing in Migraine
1.4. Current Study
2. Materials and Methods
2.1. Participants
2.2. Apparatus
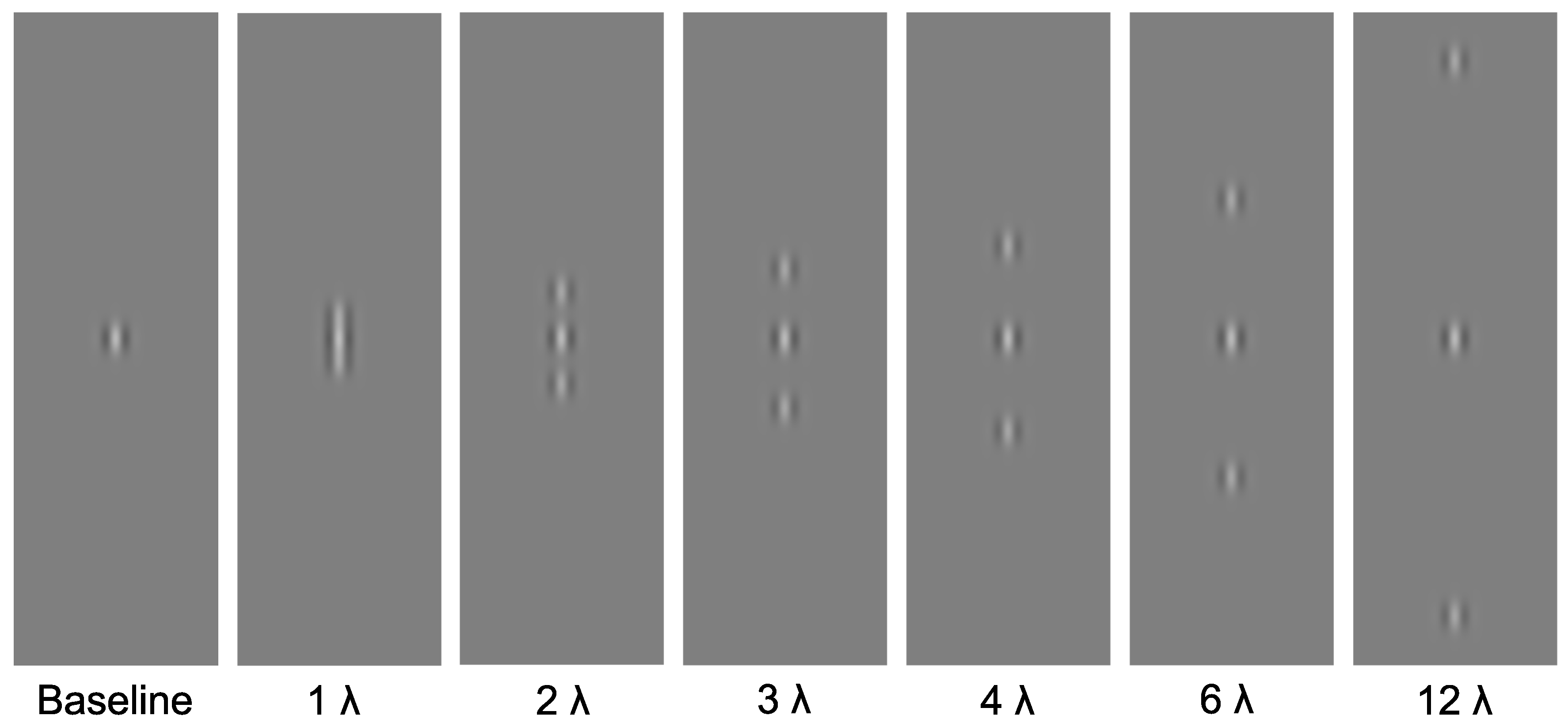
2.3. Stimuli
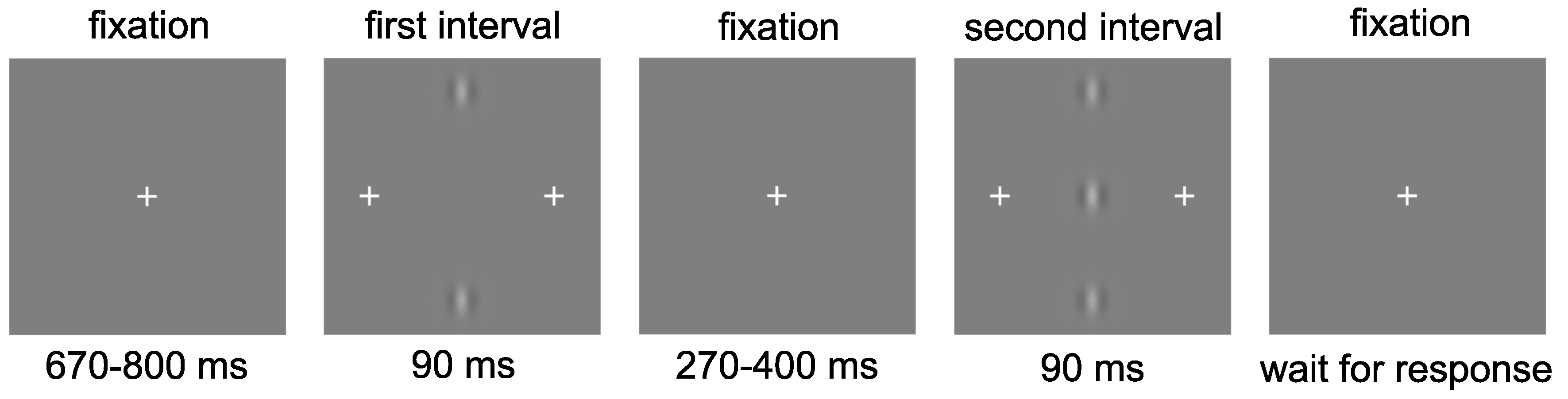
2.4. Procedure
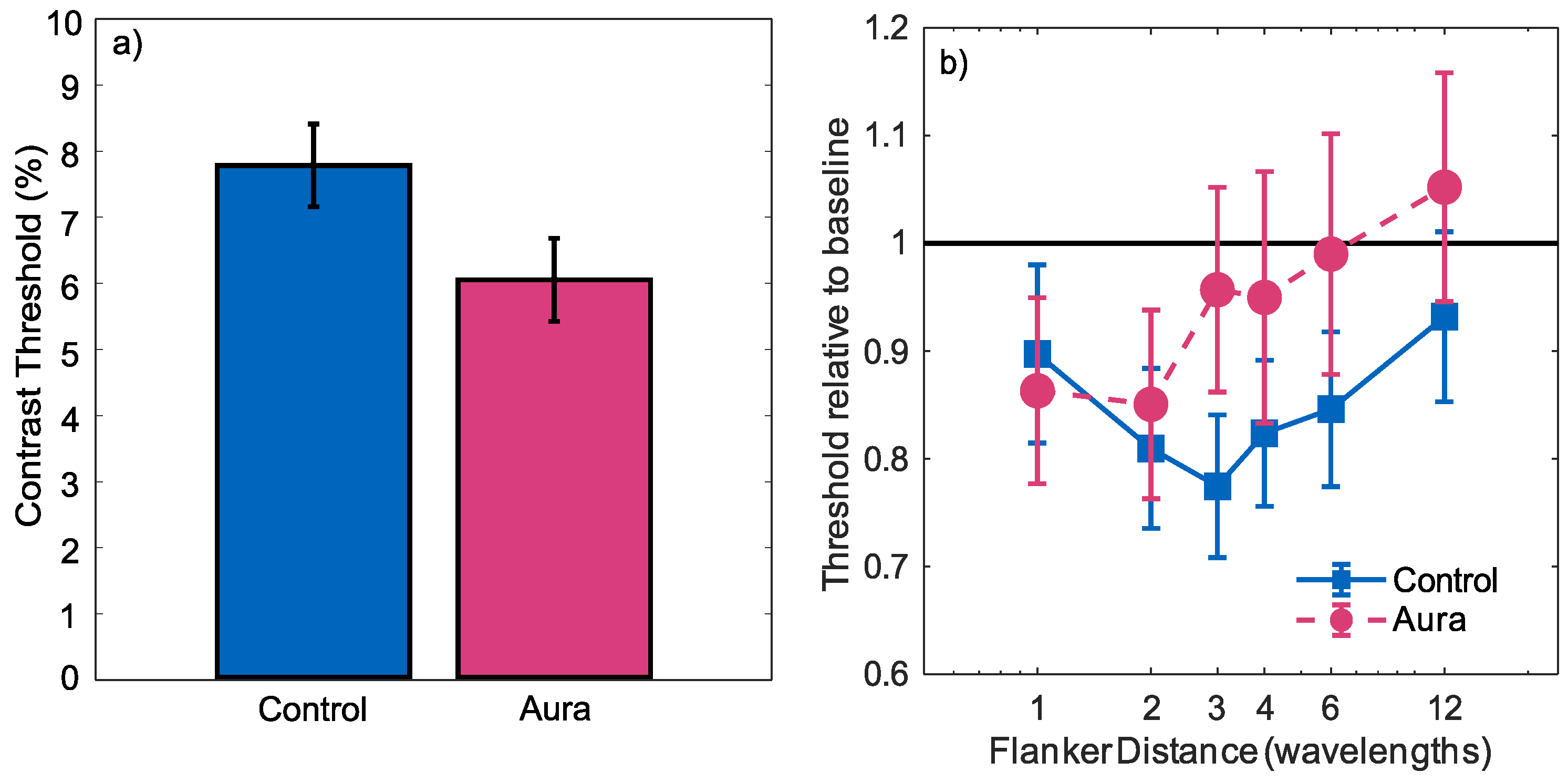
3. Results
4. Discussion
4.1. Contrast Sensitivity
4.2. Inhibition and Lateral Interactions
4.3. Global Processes and External Noise
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| GABA | Gamma-aminobutyric acid |
| 2AFC | Two-alternative-forced-choice |
| V1 | Primary visual cortex |
| VEP | Visual evoked potential |
| EEG | Electroencephalography |
References
- Burch, R.C.; Loder, S.; Loder, E.; Smitherman, T.A. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache 2015, 55, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Andree, C. Prevalence of headache in Europe: A review for the Eurolight project. Headache 2010, 11, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 2013, 33, 629–808. [Google Scholar]
- Queiroz, L.P.; Rapoport, A.M.; Weeks, R.E.; Sheftell, F.D.; Siegel, S.E.; Baskin, S.M. Characteristics of migraine visual aura. Headache 1997, 37, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J. Visual contrast processing in migraine. Cephalalgia 2000, 20, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Battista, J.; Badcock, D.R.; McKendrick, A.M. Migraine increases centre-surround suppression for drifting visual stimuli. PLoS ONE 2011, 6, e18211. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Badcock, D.R.; Gurgone, M. Vernier acuity is normal in migraine, whereas global form and global motion perception are not. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Benedek, K.; Tajti, J.; Janáky, M.; Vécsei, L.; Benedek, G. Spatial contrast sensitivity of migraine without aura. Cephalalgia 2002, 22, 142–145. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Sampson, G.P. Low spatial frequency contrast sensitivity deficits in migraine are not visual pathway selective. Cephalalgia 2009, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.E.; Chronicle, E.P.; Rolan, P.; Mulleners, W.M. Cortical hyperexcitability is cortical under-inhibition: Evidence from a novel functional test of migraine. Cephalalgia 2000, 20, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Temme, J.; Nitsche, M.; Varga, E.; Lang, N.; Paulus, W. Altered motion perception in migraineurs: Evidence for interictal cortical hyperexcitability. Cephalalgia 2005, 25, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, J.A.; McKendrick, A.M.; Badcock, D.R. Processing of global form and motion in migraineurs. Vis. Res. 2006, 46, 141–148. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Vingrys, A.J.; Badcock, D.R.; Heywood, J.T. Visual dysfunction between migraine events. Investig. Ophthalmol. Vis. Sci. 2001, 42, 626–633. [Google Scholar]
- Shepherd, A.J. Increased visual after-effects following pattern adaptation in migraine: A lack of intracortical excitation? Brain 2001, 124, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J. Local and global motion after-effects are both enhanced in migraine, and the underlying mechanisms differ across cortical areas. Brain 2006, 129, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, L.; Hibbard, P.B. Visual processing in migraine. Cephalalgia 2016, 36, 1057–1076. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Chan, Y.M.; Vingrys, A.J.; Turpin, A.; Badcock, D.R. Daily vision testing can expose the prodromal phase of migraine. Cephalalgia 2017. [Google Scholar] [CrossRef] [PubMed]
- McColl, S.L.; Wilkinson, F. Visual contrast gain control in migraine: Measures of visual cortical excitability and inhibition. Cephalalgia 2000, 20, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Pierelli, F.; Schoenen, J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 2007, 27, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.; Wilkinson, F. The brain is hyperexcitable in migraine. Cephalalgia 2007, 27, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Tibber, M.S.; Kelly, M.G.; Jansari, A.; Dakin, S.C.; Shepherd, A.J. An inability to exclude visual noise in migraine. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Parisi, V.; Di Lorenzo, C.; Serrao, M.; Magis, D.; Schoenen, J.; Pierelli, F. Lateral inhibition in visual cortex of migraine between attacks. Headache 2013, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhaoping, L. Understanding Vision: Theory, Models, and Data; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Field, D.J.; Hayes, A.; Hess, R.F. Contour integration by the human visual system: Evidence for a local “association field”. Vis. Res. 1993, 33, 173–193. [Google Scholar] [CrossRef]
- Bridge, H.; Stagg, C.J.; Near, J.; Lau, C.I.; Zisner, A.; Cader, M.Z. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia 2015, 35, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Tibber, M.S.; Guedes, A.; Shepherd, A.J. Orientation discrimination and contrast detection thresholds in migraine for cardinal and oblique angles. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5599–5604. [Google Scholar] [CrossRef] [PubMed]
- Seriès, P.; Latham, P.E.; Pouget, A. Tuning curve sharpening for orientation selectivity: Coding efficiency and the impact of correlations. Nat. Neurosci. 2004, 7, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Edden, R.A.; Muthukumaraswamy, S.D.; Freeman, T.C.; Singh, K.D. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci. 2009, 29, 15721–15726. [Google Scholar] [CrossRef] [PubMed]
- Chubb, C.; Sperling, G.; Solomon, J.A. Texture interactions determine perceived contrast. Proc. Natl. Acad. Sci. USA 1989, 86, 9631–9635. [Google Scholar] [CrossRef] [PubMed]
- Breitmeyer, B.G.; Ogmen, H. Recent models and findings in visual backward masking: A comparison, review, and update. Percept. Psychophys. 2000, 62, 1572–1595. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Wyatt, G.; Tibber, M.S. Visual metacontrast masking in migraine. Cephalalgia 2011, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Manahilov, V.; Gordon, G.E.; Loffler, G. Global shape processing deficits are amplified by temporal masking in migraine. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Polat, U.; Sagi, D. Lateral Interactions between Spatial Channels: Suppression and Facilitation Revealed by Lateral Masking Experiments. Vis. Res. 1993, 33, 993–999. [Google Scholar] [CrossRef]
- Wagner, D.; Manahilov, V.; Loffler, G.; Gordon, G.E.; Dutton, G.N. Visual noise selectively degrades vision in migraine. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Brainard, D.H. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M.; Brainard, D.; Pelli, D.; Ingling, A.; Murray, R.; Broussard, C. What’s new in Psychtoolbox-3. Perception 2007, 36, 1–16. [Google Scholar]
- Pelli, D.G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, A.; Mezzetti, M.; Lacquaniti, F. Modeling psychophysical data at the population-level: The generalized linear mixed model. J. Vis. 2012, 12, 1–17. [Google Scholar]
- Baker, D.H.; Vilidaite, G. Broadband noise masks suppress neural responses to narrowband stimuli. Front. Psychol. 2014, 5, 1–9. [Google Scholar]
- Braunitzer, G.; Rokszin, A.; Kóbor, J.; Benedek, G. Is the development of visual contrast sensitivity impaired in children with migraine? An exploratory study. Cephalalgia 2010, 30, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.C.; Lúcia, M.; Simas, D.B.; Galdino, M.K.C.; Vieira, J.G.; Simas, M.L.d.B.; dos Santos, N.A. Evaluation of contrast sensitivity among with migraine. Psicol. USP 2011, 22, 81–97. [Google Scholar] [CrossRef]
- Shepherd, A.J.; Beaumont, H.M.; Hine, T.J.; Street, M. Motion processing deficits in migraine are related to contrast sensitivity. Cephalalgia 2012, 32, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Hine, T.J.; Beaumont, H.M. Color and spatial frequency are related to visual pattern sensitivity in migraine. Headache 2013, 53, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Nicotra, A.; Wilkins, A.J. Asymmetry of visual function in migraine with aura: Correlation with lateralisation of headache and aura. Cephalalgia 2011, 31, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Mckendrick, A.; Badcock, D. Changes in visual field performance after migraine measured with flickering stimuli. Cephalalgia 2003, 23, 743–744. [Google Scholar]
- Singh, P.; Shepherd, A.J. Enhanced Motion Aftereffects in Migraine Are Related to Contrast Sensitivity: Implications for Models of Differences in Precortical/Cortical FunctionEnhanced Motion Aftereffects in Migraine. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Mather, G.; Verstraten, F.; Anstis, S. The Motion Aftereffect: A Modern Perspective; Mit Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Wilkinson, F.; Crotogino, J. Orientation discrimination thresholds in migraine: A measure of visual cortical inhibition. Cephalalgia 2000, 20, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.F.; Hayes, A.; Field, D.J. Contour integration and cortical processing. J. Physiol. Paris 2003, 97, 105–119. [Google Scholar]
- Dakin, S.C.; Mareschal, I.; Bex, P.J. Local and global limitations on direction integration assessed using equivalent noise analysis. Vis. Res. 2005, 45, 3027–3049. [Google Scholar] [CrossRef] [PubMed]
- Katzner, S.; Busse, L.; Carandini, M. GABAA inhibition controls response gain in visual cortex. J. Neurosci. 2011, 31, 5931–5941. [Google Scholar] [CrossRef] [PubMed]
| Observer | Sex | Age | Age Started | Prior Attack | Frequency per Month |
|---|---|---|---|---|---|
| OB8 | F | 23 | 16 | 1 month | 1–3 |
| OB12 | F | 27 | 10 | 2 weeks | 1–3 |
| OB14 | F | 60 | 19 | 3 months | <1 |
| OB16 | F | 52 | 14 | 5 days | 1–3 |
| OB17 | F | 22 | 17 | 5 days | 1–3 |
| OB20 | M | 30 | 12 | 4 months | 5 or more |
| OB21 | F | 41 | 24 | 9 days | 5 or more |
| OB22 | F | 21 | 10 | 4 days | <1 |
| OB24 | F | 36 | 23 | 7 days | 1–3 |
| OB25 | F | 29 | 3 | 20 days | 1–3 |
| OB28 | F | 30 | 13 | 3 months | <1 |
| OB29 | F | 55 | 40 | 6 days | 1–3 |
| OB30 | F | 25 | 9 | 8 days | 1–3 |
| OB31 | F | 23 | 19 | 1 month | 1–3 |
| OB33 | M | 20 | 12 | 7 days | 1–3 |
| OB48 | F | 18 | NDI | 1 month | 5 or more |
| OB50 | F | 21 | NDI | 7 days | <1 |
| OB63 | F | 18 | 13 | 1 month | 1–3 |
| OB64 | F | 19 | 17 | 7 days | 5 or more |
| OB66 | F | 19 | 16 | A few months | 1–3 |
| OB71 | F | 20 | 11 | > 3 days | <1 |
| OB73 | F | 18 | 15 | 6 days | 5 or more |
| OB75 | F | 19 | 16 | 3 months | <1 |
| OB76 | F | 18 | NDI | > 3 days | NDI |
| Excluded | F | 19 | 16 | 1 day | 1–3 |
| Excluded | F | 18 | 14 | 2 days | 1–3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asher, J.M.; O’Hare, L.; Romei, V.; Hibbard, P.B. Typical Lateral Interactions, but Increased Contrast Sensitivity, in Migraine-With-Aura. Vision 2018, 2, 7. https://doi.org/10.3390/vision2010007
Asher JM, O’Hare L, Romei V, Hibbard PB. Typical Lateral Interactions, but Increased Contrast Sensitivity, in Migraine-With-Aura. Vision. 2018; 2(1):7. https://doi.org/10.3390/vision2010007
Chicago/Turabian StyleAsher, Jordi M., Louise O’Hare, Vincenzo Romei, and Paul B. Hibbard. 2018. "Typical Lateral Interactions, but Increased Contrast Sensitivity, in Migraine-With-Aura" Vision 2, no. 1: 7. https://doi.org/10.3390/vision2010007
APA StyleAsher, J. M., O’Hare, L., Romei, V., & Hibbard, P. B. (2018). Typical Lateral Interactions, but Increased Contrast Sensitivity, in Migraine-With-Aura. Vision, 2(1), 7. https://doi.org/10.3390/vision2010007







