Covert Exogenous Cross-Modality Orienting between Audition and Vision †
Abstract
:1. Introduction
2. Part I: Visual Orienting toward the Source of Auditory Stimulation
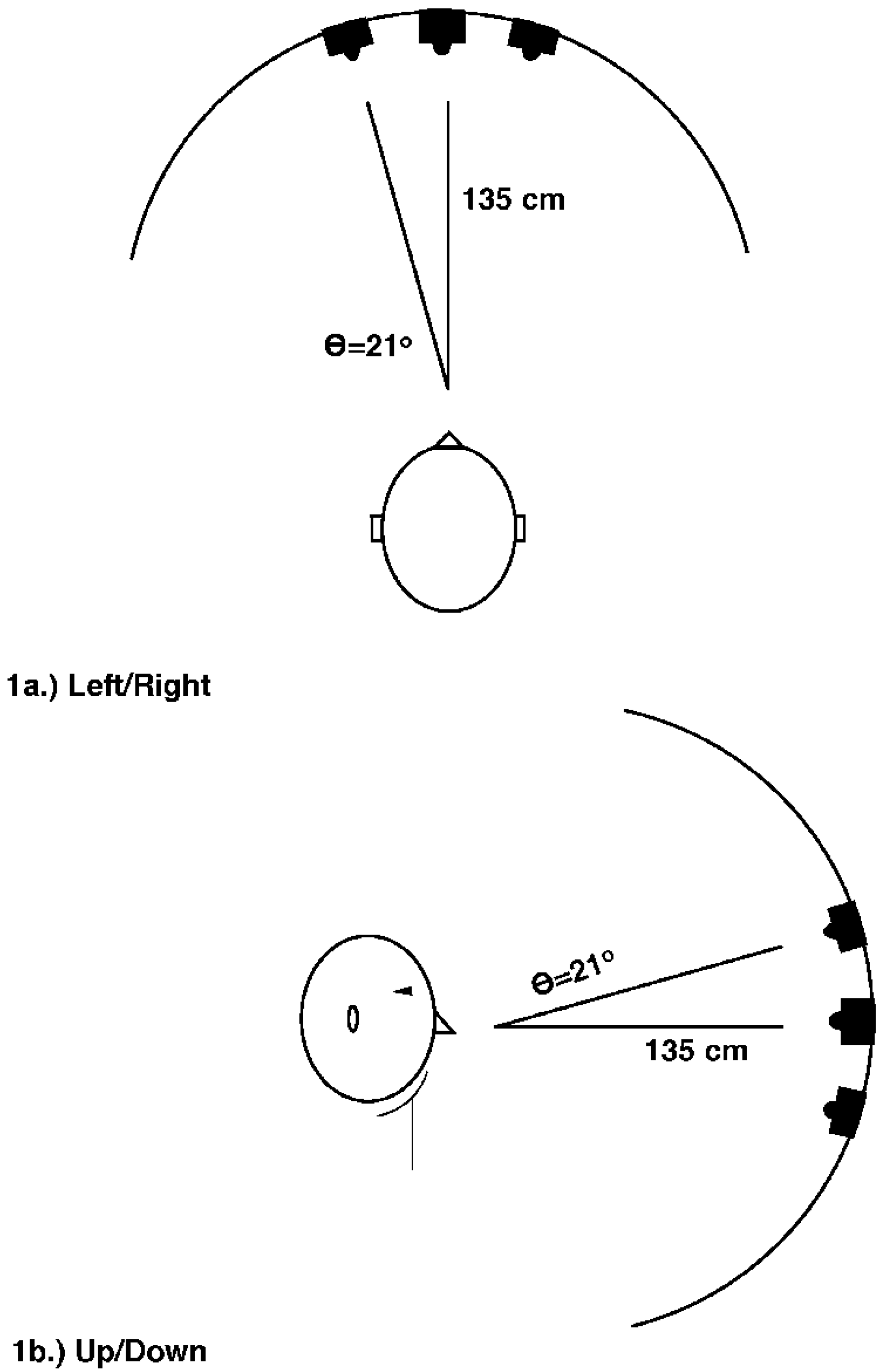
2.1. Experiment 1
2.1.1. Methods
2.1.2. Results and Discussion
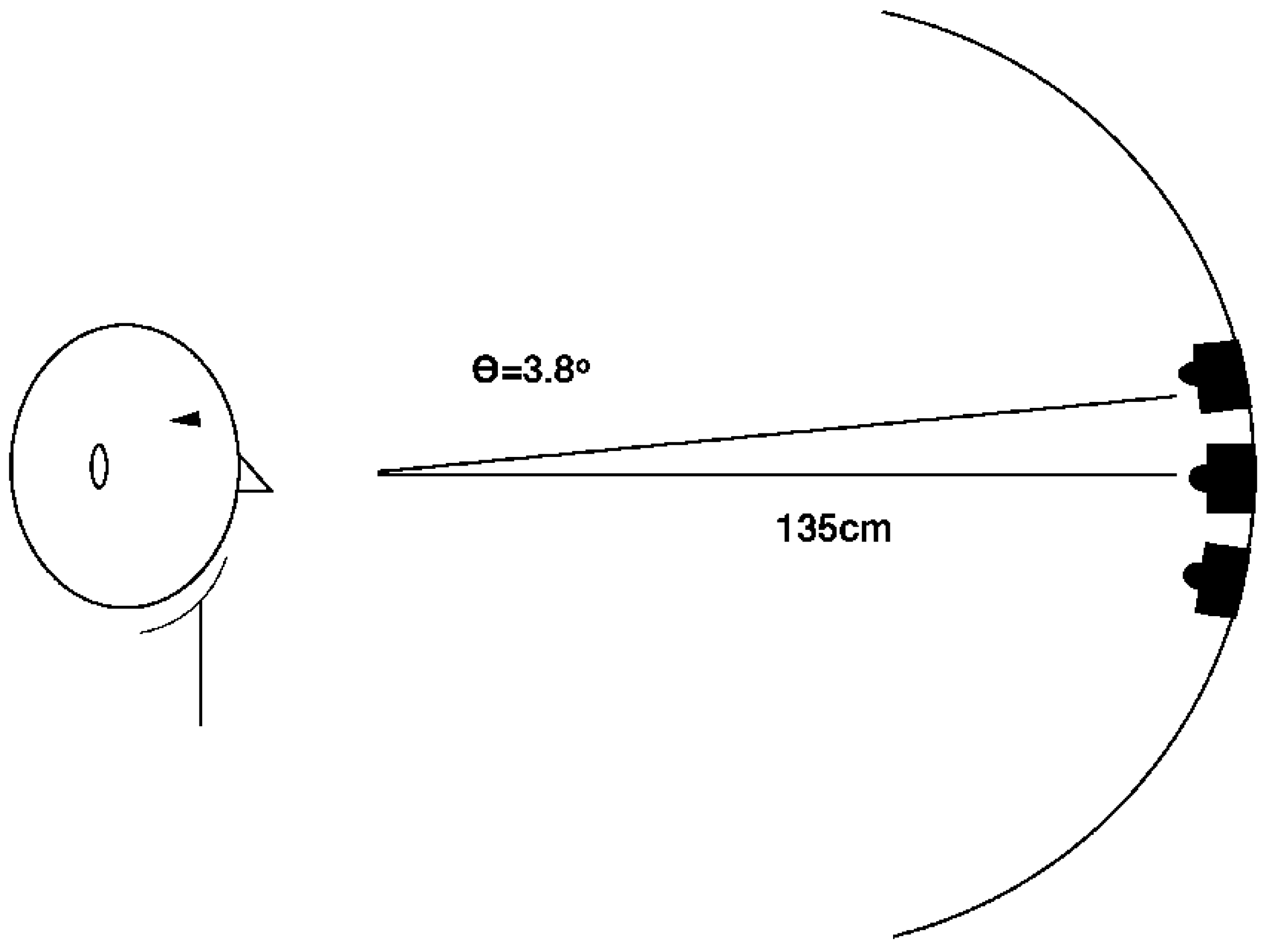
2.2. Experiment 2
2.2.1. Method
2.2.2. Results and Discussion
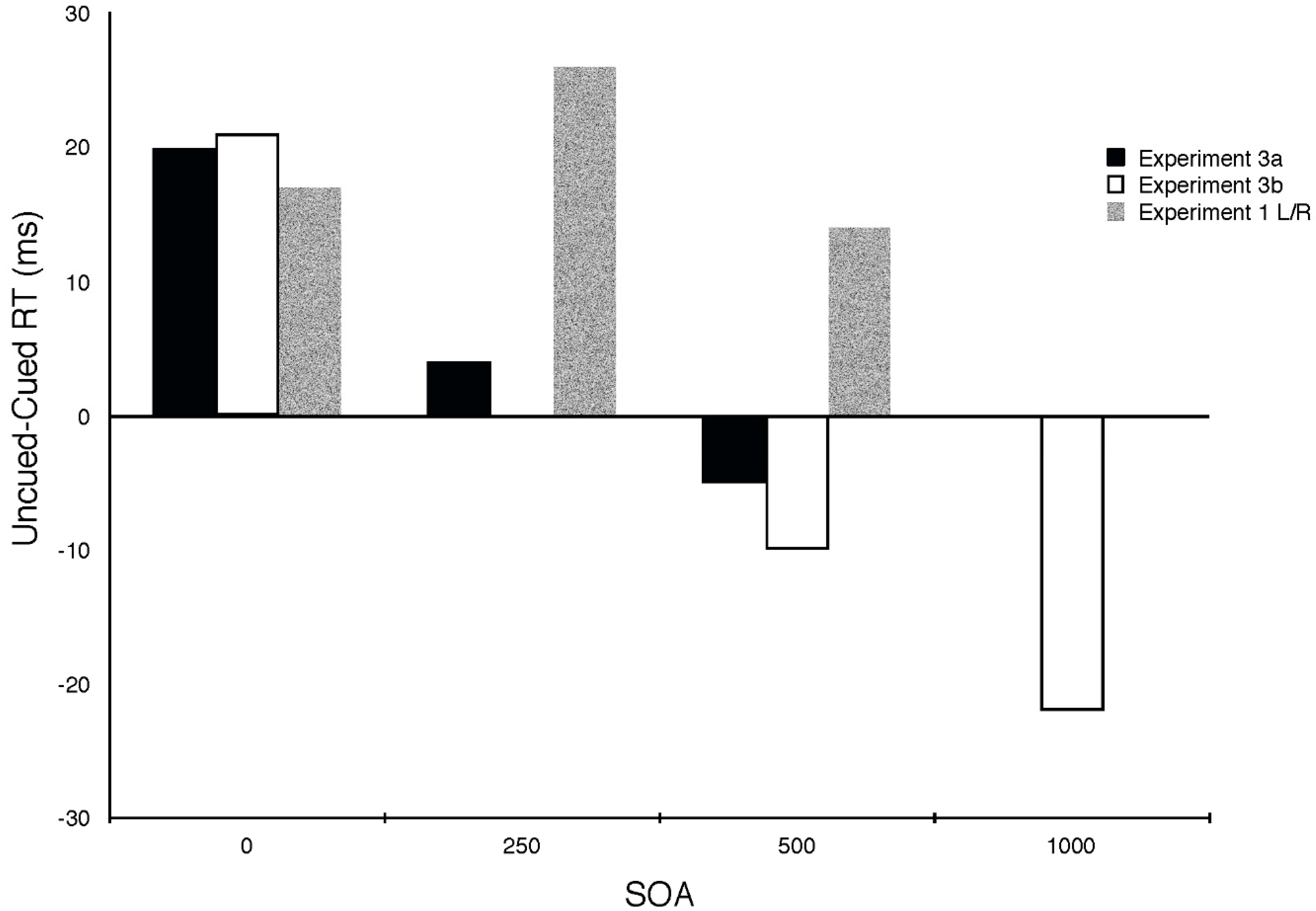
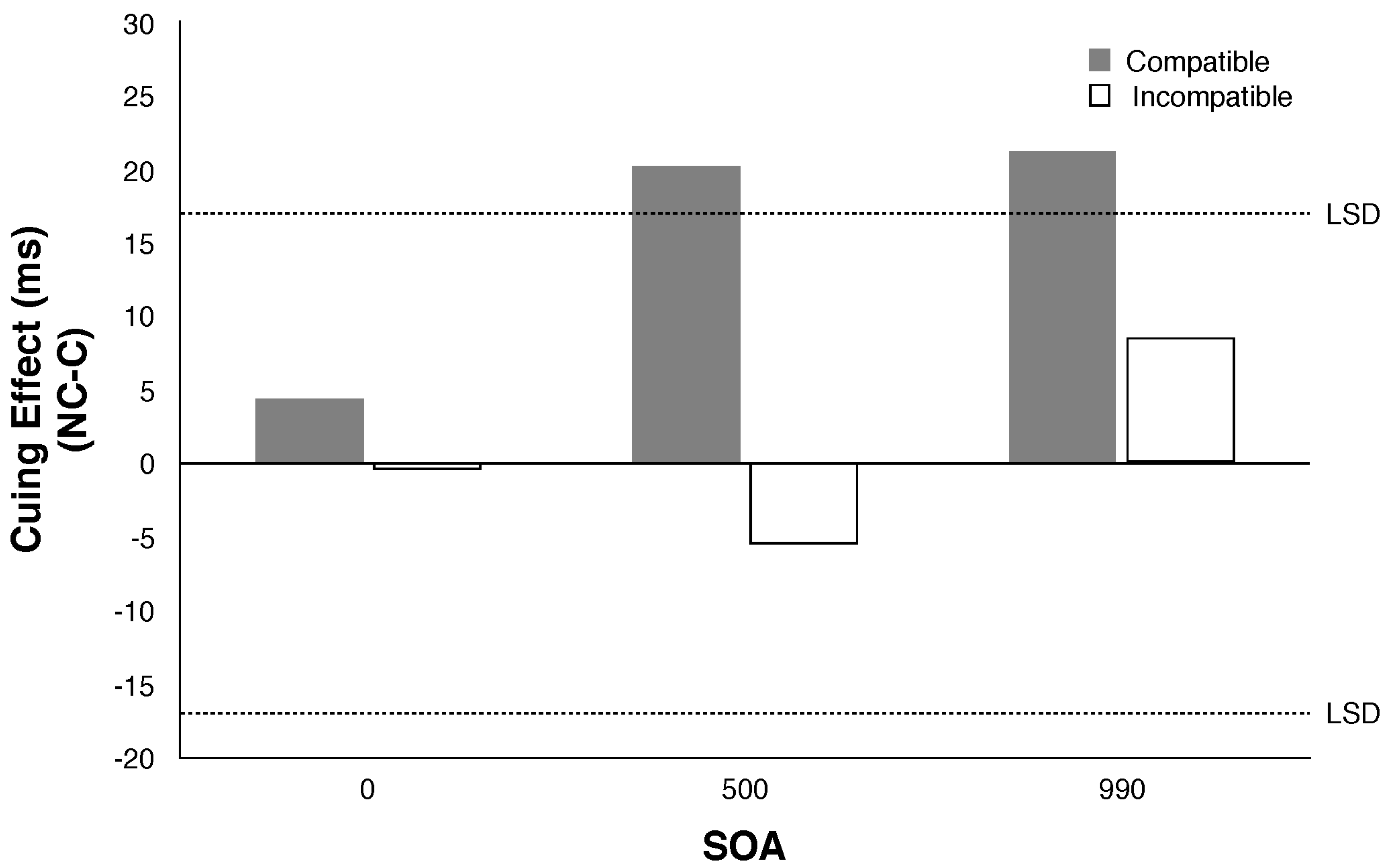
2.3. Experiment 3
2.3.1. Methods
2.3.2. Results and Discussion
2.4. Experiment 4
2.4.1. Methods
2.4.2. Results and Discussion
2.5. Experiment 5
2.5.1. Methods
2.5.2. Results and Discussion
2.6. Conclusions
3. Part II: Frequency Glides and Endogenous Cross-Modal Orienting of Attention
3.1. Experiment 6
3.1.1. Methods
3.1.2. Results
3.2. Experiment 7
3.2.1. Methods
3.2.2. Results
3.3. Conclusions
4. Part III: Putting Old Findings about Covert Cross-Modality Orienting into a Contemporary Context
4.1. Localizable Auditory Stimuli Generate an Exogenous Shift of Covert Visual Attention
4.2. Such Cross-Modality Cuing Effects Are Relatively Automatic
4.3. Localizable Visual Stimuli Do Not Generate an Exogenous Shift of Covert Auditory Attention
4.4. Neither the Frequency Nor the Direction of a Frequency Glide Generates an Exogenous Shift of Covert Visual Attention
4.5. The Endogenous Use of Pitch Glides Is Influenced by Their Natural Meanings
5. Epilogue
Conflicts of Interest
References and Notes
- Posner, M.I. Chronometric Explorations of Mind; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1978. [Google Scholar]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.M. On the role of endogenous orienting in the inhibitory aftermath of exogenous orienting. In Developing Individuality in the Human Brain: A Tribute to Michael I. Posner; Mayr, U., Awh, E., Keele, S., Eds.; APA Books: Washington, DC, USA, 2005; pp. 45–64. [Google Scholar]
- Meredith, M.A.; Stein, B.E. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J. Neurophysiol. 1986, 56, 640–662. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.M.; Brennan, M.; Gilani, A. Covert cross-modality orienting of attention in space. In Proceedings of the Paper Presented at the Annual Meeting of the Psychonomic Society, Seattle, DC, USA, 6–8 November 1987. [Google Scholar]
- Klein, R.; Brennan, M.; D’Aloisio, A.; D’Entremont, B.; Gilani, A. Covert crossmodality orienting of attention. 1987; in press. [Google Scholar]
- Klein, R.M.; Juckes, T. Can auditory frequency control the direction of visual attention. In Proceedings of the Paper Presented at the Canadian Acoustic Association, Halifax, NS, Canada, 16–19 October 1989. [Google Scholar]
- Buchtel, H.A.; Butter, C.M. Spatial attentional shifts: Implications for the role of poly sensory mechanisms. Neuropsychologia 1988, 26, 499–509. [Google Scholar] [CrossRef]
- Jonides, J.; Mack, R. On the cost and benefit of cost and benefit. Psychol. Bull. 1984, 96, 2444. [Google Scholar] [CrossRef]
- Mudd, S.A. Spatial stereotyps of four dimensions of pure tone. J. Exp. Psychol. 1963, 66, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J. How melodies influence the meaning of dynamic visual displays. Can. Psychol. 1989, 30, 343. [Google Scholar]
- Bernstein, I.H.; Edelstein, B.A. Effects of some variations in auditory input upon visual choice reaction time. J. Exp. Psychol. 1971, 87, 241–247. [Google Scholar] [CrossRef] [PubMed]
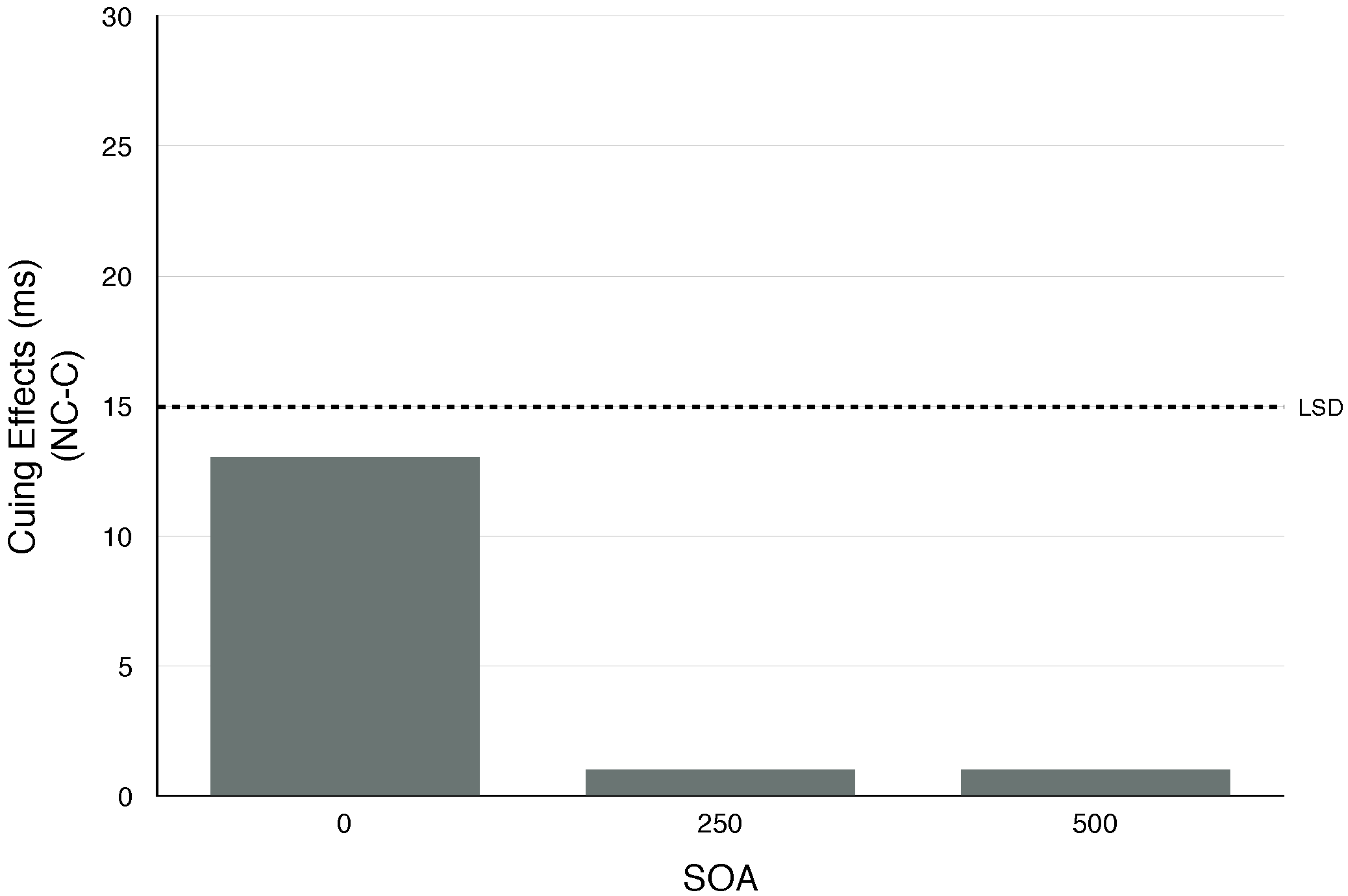
- Six subjects were tested in a single session using pure tones with the same pitch as those used to signal the top (2500 Hz), middle (650 Hz) and bottom (250 Hz) row in Sperling’s (1960) [43] classic paper on visual short term memory. Two SOAS (O and 500 ms) were each tested in three blocks. Uncued minus cued RT was −1 and 3 ms in the 0 and 500 ms SOA conditions, respectively.
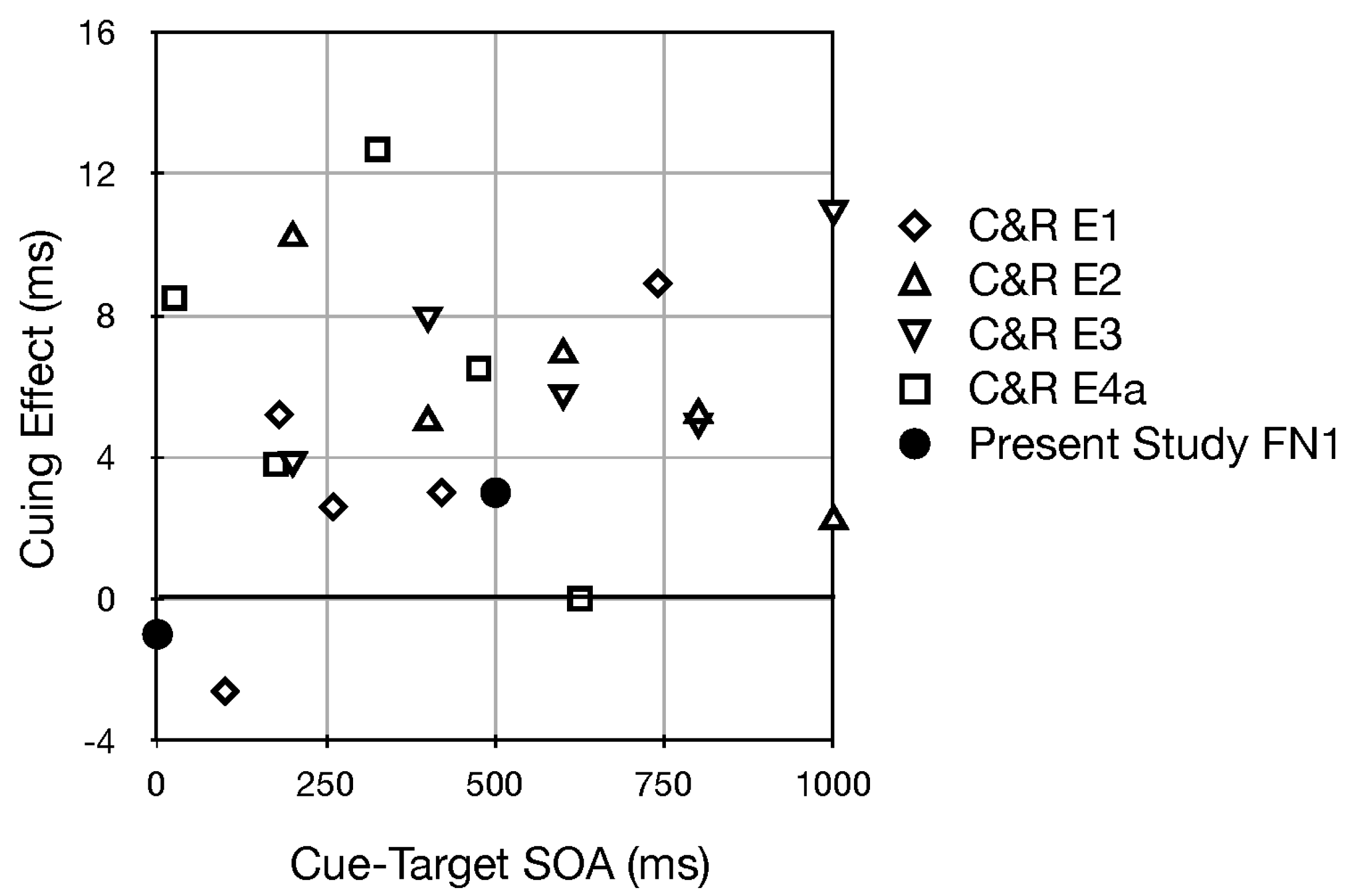
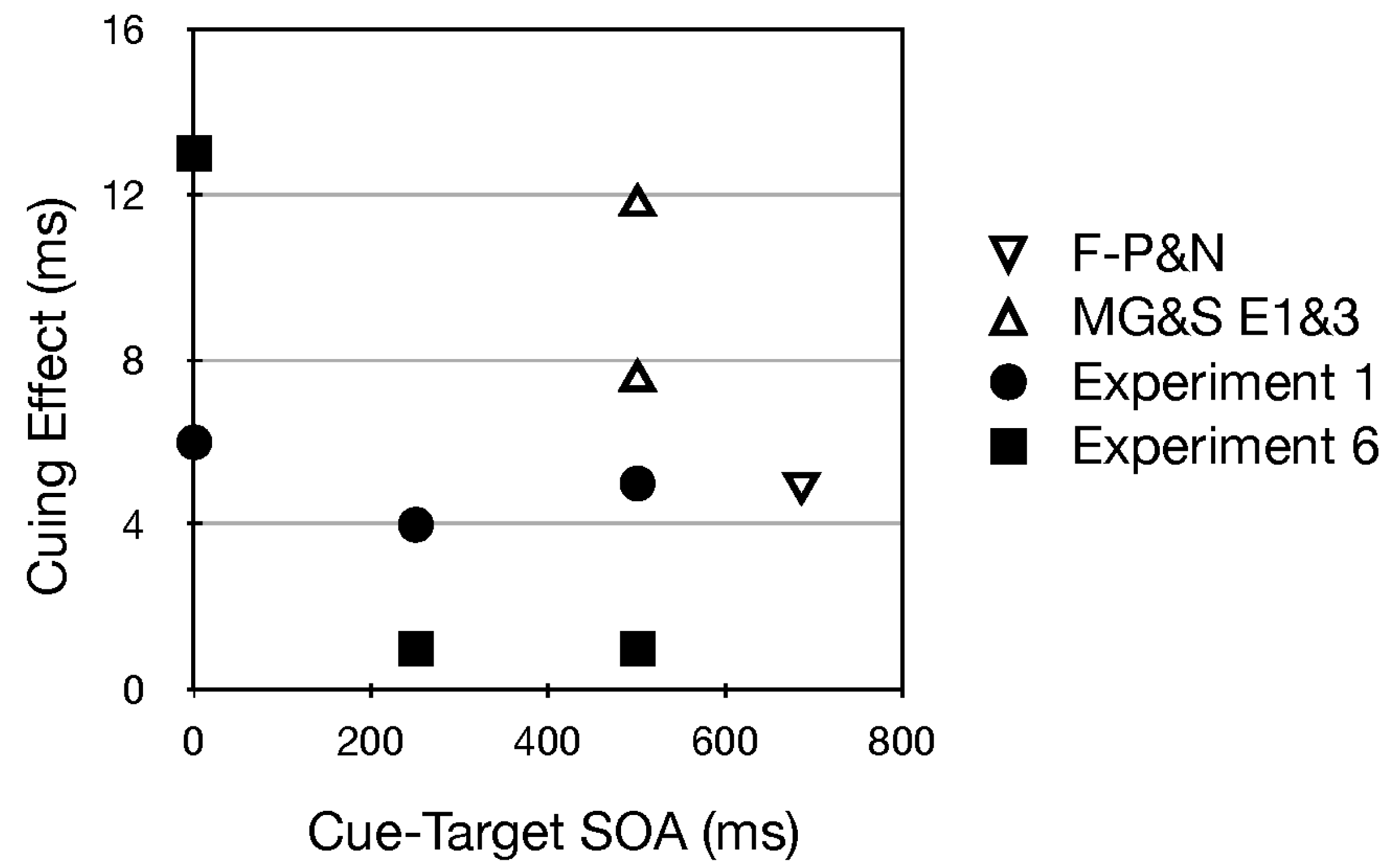
- Interestingly, among all studies using uninformative pitch and pitch glide cues, those reported here (in footnote 1 and Experiments 1 and 6) were the only ones with a single, visible source (see Figure 1b). In Fernández-Prieto & Navarra (2017) [38] and Chiou and Rich (2012) [36] the auditory stimuli were presented through two loudspeakers positioned to the left and right of the computer screen. Certainly the speakers were visible, but in contrast to our methods they did not serve as the central fixation stimulus. In Mossbridge et al. (2011) [39] the auditory stimuli were presented over headphones and hence were not localized in the environment. If the attention of a participant is “engaged” upon an auditory stimulus emanating from a fixated and visible source, perhaps it is difficult to disengage from it. If the effects of pitch and pitch glides in our studies were smaller than in these other studies, such a disengagement difficulty could be the reason.
- Klein, R. Attention and visual dominance: A chronometric analysis. J. Exp. Psychol. Hum. Percept. Perfom. 1977, 3, 365–378. [Google Scholar] [CrossRef]
- Whittington, D.A.; Hepp-Reymond, M.C.; Flood, W. Eye and head movements to auditory targets. Exp. Brain Res. 1981, 41, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Snyder, C.R.R.; Davidson, B.J. Attention and the detection of Signals. J. Exp. Psychol. Gen. 1980, 109, 160–174. [Google Scholar] [CrossRef]
- Mangun, G.R.; Hansen, J.C.; Hillyard, S.A. The spatial orienting of attention: Sensory facilitation or response bias. In Proceedings of the Paper Presented at the Eighth International Conference on Event-Related Potentials of the Brain, Stanford, CA, USA, 28 June 1986. [Google Scholar]
- Sperling, G. A unified theory of attention and signal detection. In Varieties of Attention; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Duncan, J. Directing attention in the visual field. Percept. Psychophys. 1981, 30, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.L. Identifying attentional and decision-making components in information processing. In Attnetion and Performance VIII; Nickerson, R.S., Ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1980. [Google Scholar]
- Muller, H.J.; Findlay, J.M. Sensitivity and criterion effects in the spatial cuing of visual attention. Percept. Psychophys. 1987, 42, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Nissen, M.J.; Klein, R. Visual dominance: An information Processing account of its origins and significance. Psychol. Rev. 1976, 83, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Jonides, J. Voluntary vs. automatic control over the mind’s eye’s movement. In Attention and Performance; Long, J., Baddeley, A., Eds.; Erlbaum: Hillsdale, NJ, USA, 1981. [Google Scholar]
- While such a linkage is possible, if not probable with exogenous orienting, Klein (1980) [44] has shown that such a linkage probably does not underlie endogenous orienting.
- Posner, M.I.; Nissen, M.J.; Ogden, W.C. Attended and unattended processing modes: Role of set for spatial location. In Modes of Perceiving and Processing Information; Pick, H., Saltzman, E., Eds.; Erlbaum: Hillsdale, NJ, USA, 1978. [Google Scholar]
- Marks, L.E. On cross-modal similarity: Auditory-visual interactions in speeded discrimination. J. Exp. Psychol. Hum. Percept. Perform. 1987, 13, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.M.; Posner, M.I. Attention to visual and kinesthetic components of skills. Brain Res. 1974, 71, 401–412. [Google Scholar] [CrossRef]
- Spence, C.; Driver, J. Audiovisual links in exogenous covert spatial orienting. Atten. Percept. Psychophys. 1997, 59, 1–22. [Google Scholar] [CrossRef]
- Lee, J.; Spence, C. On the spatial specificity of audiovisual crossmodal exogenous cuing effects. Acta Psychol. 2017, 177, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Snyder, C.R.R. Attention and cognitive control. In Information Processing and Cognition: The Loyola Symposium; Solso, R.L., Ed.; Erlbaum: Hillsdale, NJ, USA, 1975; pp. 55–85. [Google Scholar]
- Folk, C.L.; Remington, R.W.; Johnston, J.C. Involuntary covert orienting is contingent on attentional control settings. J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 1030. [Google Scholar] [CrossRef] [PubMed]
- It must be pointed out that such counter-informative cues, if they are to be used, cannot be ignored because they must be processed in order to know where the visual target is likely to be presented. This is in contrast to truly uninformative cues that, in principle, could be completely ignored.
- Spence, C.; Lloyd, D.; McGlone, F.; Nicholls, M.E.; Driver, J. Inhibition of return is supramodal: A demonstration between all possible pairings of vision, touch, and audition. Exp. Brain Res. 2000, 134, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Spence, C.; Deroy, O. How automatic are crossmodal correspondences? Conscious. Cognit. 2013, 22, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Chiou, R.; Rich, A.N. Cross-modality correspondence between pitch and spatial location modulates attentional orienting. Perception 2012, 41, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Prieto, I.; Vera-Constán, F.; García-Morera, J.; Navarra, J. Spatial recoding of sound: Pitch-varying auditory cues modulate up/down visual spatial attention. See. Perceiv. 2012, 25, 150–151. [Google Scholar] [CrossRef]
- Fernández-Prieto, I.; Navarra, J. The higher the pitch the larger its crossmodal influence on visuospatial processing. Psychol. Music 2017. [Google Scholar] [CrossRef]
- Mossbridge, J.A.; Grabowecky, M.; Suzuki, S. Changes in auditory frequency guide visual-spatial attention. Cognition 2011, 121, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.M.; Redden, R.S. How “Inhibition of return” biases orienting. In Spatial Biases in Cognition; Hubbard, T., Ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Tversky, A.; Kahneman, D. Belief in the law of small numbers. Psychol. Bull. 1971, 76, 105–110. [Google Scholar] [CrossRef]
- Klein, R.M. On the belief that the cognitive exercise associated with the acquisition of a second language enhances extra-linguistic cognitive functions: Is Type-I incompetence at work here? Cortex 2015, 73, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Sperling, G. The information available in brief visual displays. Psychol. Monogr. 1960, 74, 498. [Google Scholar] [CrossRef]
- Klein, R.M. Does oculomotor readiness mediate cognitive control of visual attention? In Attention and Performance; Erlbaum: Hillsdale, NJ, USA, 1980. [Google Scholar]
| SOA | |||
|---|---|---|---|
| Left/Right | 0 | 250 | 500 |
| Cued | 305 | 288 | 303 |
| Neutral | 319 | 306 | 316 |
| Uncued | 322 | 314 | 317 |
| Cuing Effect | 17 | 26 | 14 |
| Rising/Falling | 0 | 250 | 500 |
| Valid | 325 | 316 | 319 |
| Neutral | 331 | 320 | 325 |
| Uncued | 331 | 320 | 324 |
| Cuing Effect | 6 | 4 | 5 |
| SOA | |||
|---|---|---|---|
| 0 | 250 | 500 | |
| Valid | 331 | 311 | 336 |
| Neutral | 335 | 323 | 342 |
| Uncued | 337 | 334 | 351 |
| Cuing Effect | 6 | 23 | 15 |
| SOA | |||
|---|---|---|---|
| Experiment 3a | 0 | 250 | 500 |
| Cued | 330 | 342 | 366 |
| Neutral | 350 | 354 | 372 |
| Uncued | 350 | 346 | 361 |
| Cuing Effect | 20 | 4 | −5 |
| Experiment 3b | 0 | 500 | 1000 |
| Cued | 330 | 373 | 391 |
| Neutral | 351 | 373 | 369 |
| Uncued | 351 | 353 | 369 |
| Cuing Effect | 21 | −10 | −22 |
| RT | % Error | |
|---|---|---|
| Cued | 612 | 20.0 |
| Neutral | 629 | 19.3 |
| Uncued | 651 | 18.0 |
| SOA | |||
|---|---|---|---|
| 0 | 250 | 500 | |
| Cued | 321 | 284 | 294 |
| Neutral | 326 | 304 | 319 |
| Uncued | 316 | 276 | 303 |
| Cuing Effect | −5 | −8 | 9 |
| SOA | |||
|---|---|---|---|
| 0 | 250 | 500 | |
| Corresponding | 356 | 330 | 364 |
| Neutral | 362 | 345 | 375 |
| Non-Corresponding | 369 | 331 | 365 |
| Cuing Effect | 13 | 1 | 1 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, R.M. Covert Exogenous Cross-Modality Orienting between Audition and Vision. Vision 2018, 2, 8. https://doi.org/10.3390/vision2010008
Klein RM. Covert Exogenous Cross-Modality Orienting between Audition and Vision. Vision. 2018; 2(1):8. https://doi.org/10.3390/vision2010008
Chicago/Turabian StyleKlein, Raymond M. 2018. "Covert Exogenous Cross-Modality Orienting between Audition and Vision" Vision 2, no. 1: 8. https://doi.org/10.3390/vision2010008
APA StyleKlein, R. M. (2018). Covert Exogenous Cross-Modality Orienting between Audition and Vision. Vision, 2(1), 8. https://doi.org/10.3390/vision2010008




