Electrophysiological Studies on The Dynamics of Luminance Adaptation in the Mouse Retina
Abstract
:1. Introduction
2. Results
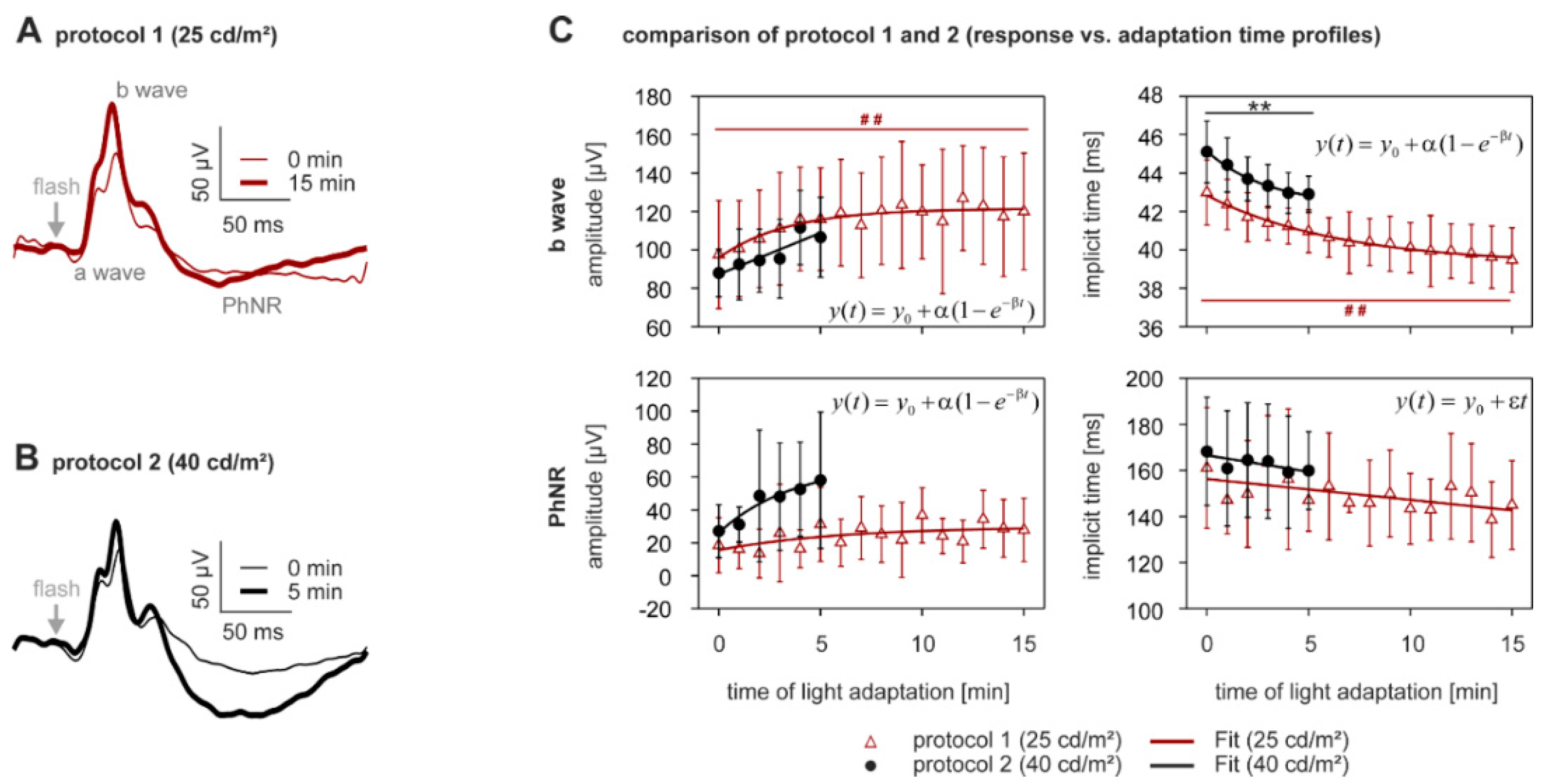
2.1. Adaptation Kinetics of the Flash ERG
2.2. Light Adaptation
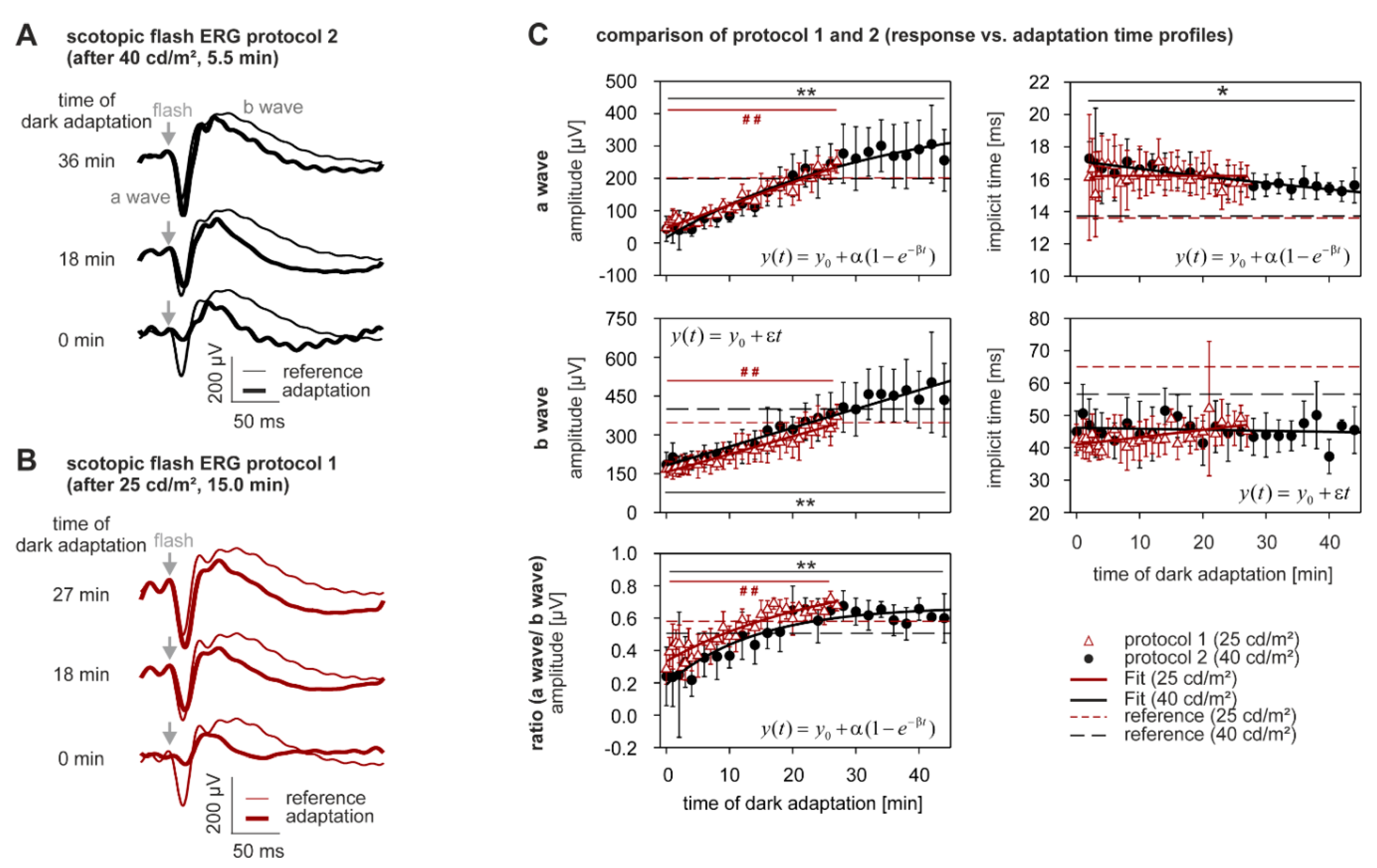
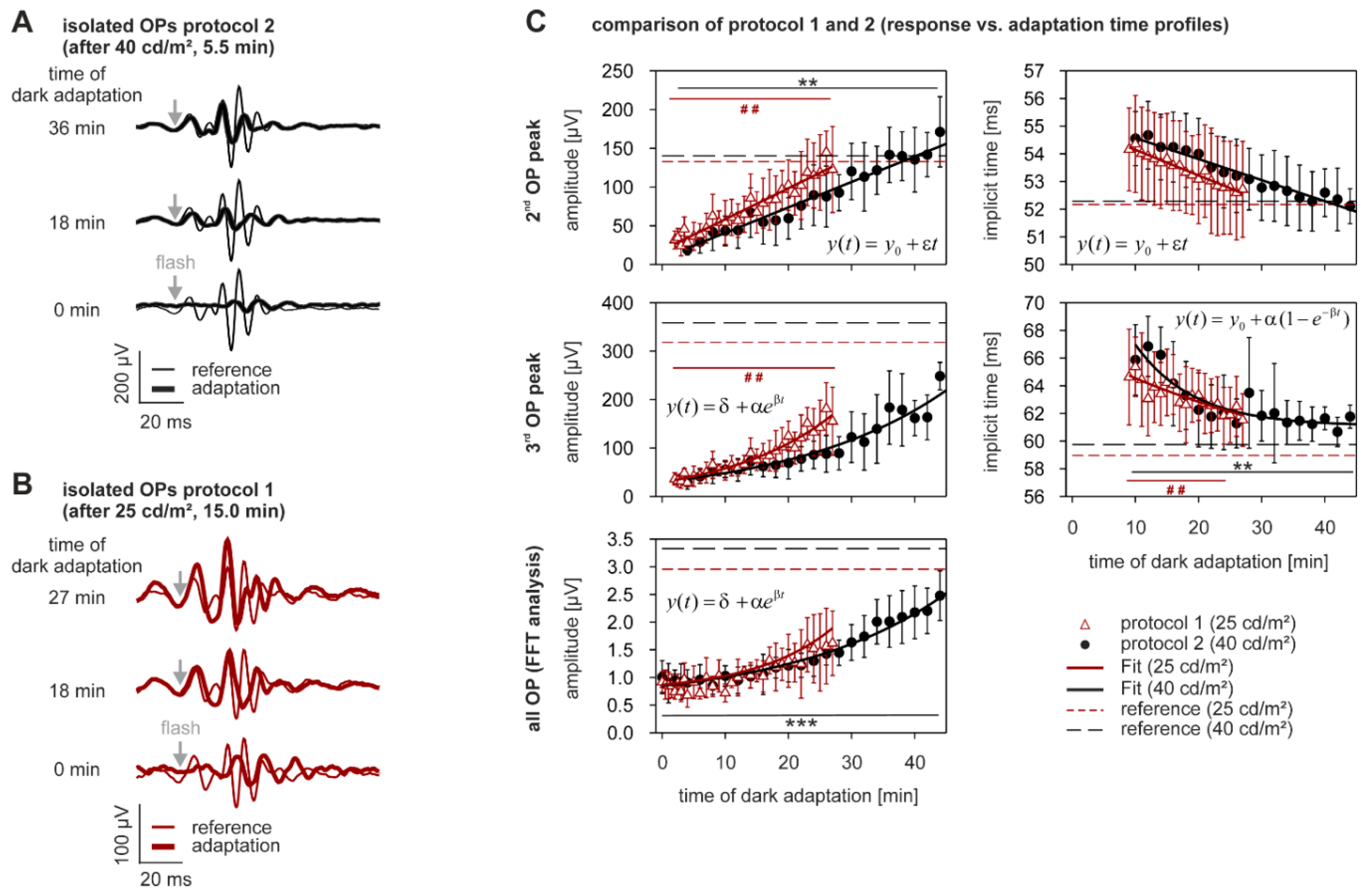
2.3. Dark Adaptation
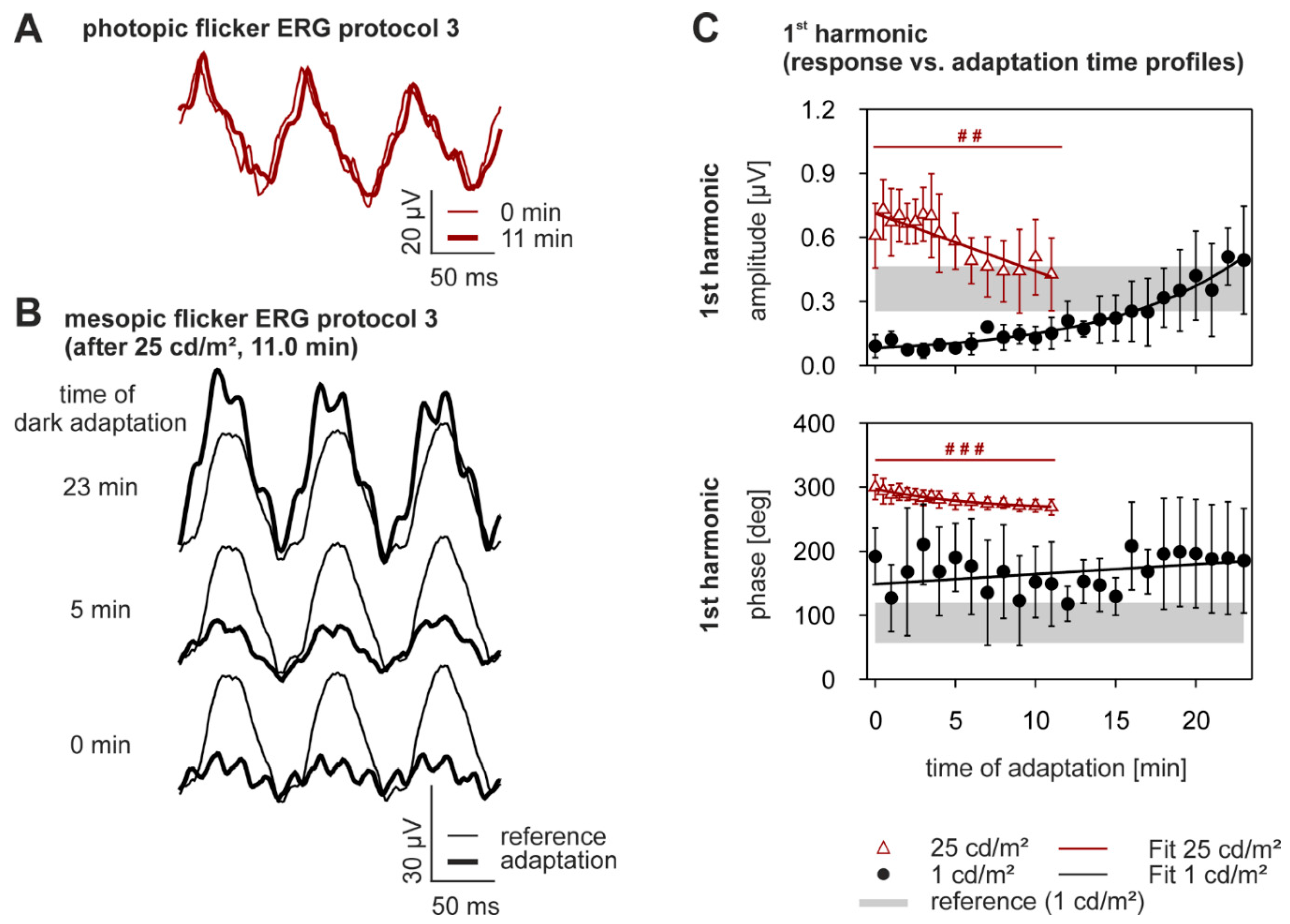
2.4. Dynamics of Adaptation in the Flicker ERG
3. Discussion
3.1. Time Constants of Adaptation
3.2. Amplitudes
3.3. Implicit Times
3.4. A-to-B-Wave Ratio
3.5. Effect of Different Intensities of Adapting Light on the Dynamics of Light Adaptation
3.6. Effect of Different Intensities of Adapting Light on the Dynamics of Dark Adaptation
3.7. Rod and Cone Selectivity of the ERGs
4. Methods
4.1. Experimental Animals
4.2. Preparation
4.3. Visual Stimuli
4.3.1. Flash ERGs
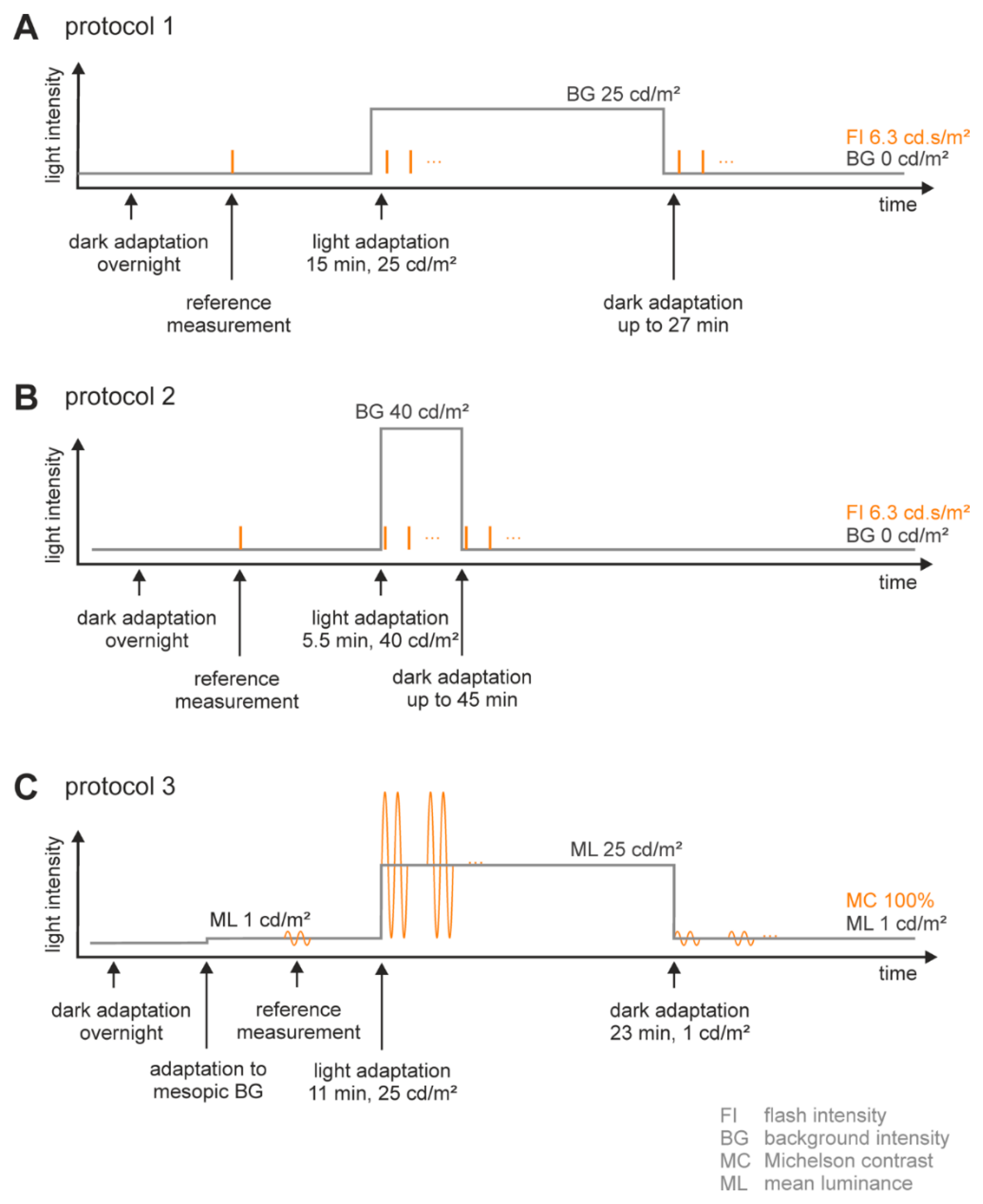
Adaptation Procedure (Figure 7A,B)
Stimulation Protocol (Figure 7A,B)
4.3.2. Flicker ERGs (Figure 7C)
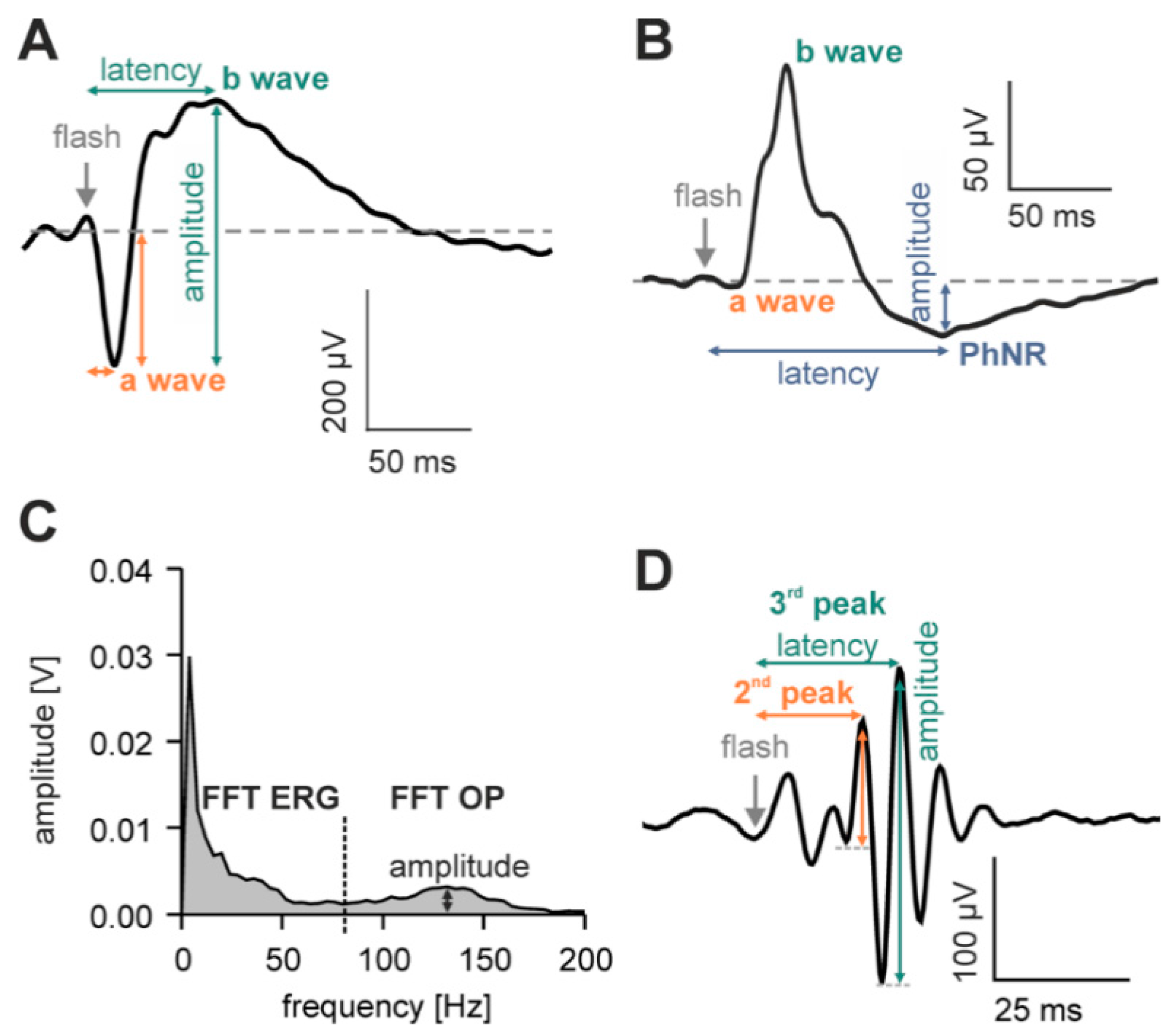
4.4. Signal Acquisition and Analysis
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Tsai, T.I.; Atorf, J.; Neitz, M.; Neitz, J.; Kremers, J. Rod- and cone-driven responses in mice expressing human l-cone pigment. J. Neurophysiol. 2015, 114, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, H.; Vinberg, F.; Pitkänen, M.; Kommonen, B.; Koskelainen, A. Flash Responses of Mouse Rod Photoreceptors in the Isolated Retina and Corneal Electroretinogram: Comparison of Gain and Kinetics. Vis. Neurosci. 2012, 53, 5653–5664. [Google Scholar] [CrossRef] [PubMed]
- Lei, B. Rod-Driven OFF Pathway Responses in the Distal Retina: Dark-Adapted Flicker Electroretinogram in the Mouse. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Gerding, W.M.; Schreiber, S.; Schulte-Middelmann, T.; de Castro Marques, A.; Atorf, J.; Akkad, D.A.; Dekomien, G.; Kremers, J.; Dermietzel, R.; Gal, A.; et al. Ccdc66 null mutation causes retinal degeneration and dysfunction. Hum. Mol. Genet. 2011, 20, 3620–3631. [Google Scholar] [CrossRef] [PubMed]
- Reim, K.; Regus-Leidig, H.; Ammermüller, J.; El-Kordi, A.; Radyushkin, K.; Ehrenreich, H.; Brandstätter, J.H.; Brose, N. Aberrant function and structure of retinal ribbon synapses in the absence of complexin 3 and complexin 4. J. Cell Sci. 2009, 122, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Yao, G.; Zhang, K.; Hofeldt, K.J.; Chang, B. Study of Rod- and Cone-Driven Oscillatory Potentials in Mice. Vis. Neurosci. 2006, 47, 2732–2738. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.; Maeda, H.; Saszik, S.; Frishman, L. In vivo studies of signaling in rod pathways of the mouse using the electroretinogram. Vis. Res. 2004, 44, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Saszik, S.M.; Robson, J.G.; Frishman, L.J. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J. Physiol. 2002, 543, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.L.; Janisch, K.M.; Peshenko, I.V.; Dizhoor, A.M.; Tsang, S.H.; Fain, G.L. Modulation of phosphodiesterase6 turnoff during background illumation in mouse rod photoreceptors. J. Neurosci. 2008, 28, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.V.; Sezate, S.S.; Cao, W.; McGinnis, J.F. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and a-transducin in mouse photoreceptor cells. Mol. Vis. 2004, 10, 672–681. [Google Scholar] [PubMed]
- Sokolov, M.; Lyubarsky, A.; Strissel, K.J.; Savchenko, A.B.; Govardovskii, V.I.; Pugh, E.N., Jr.; Arshavsky, V.Y. Massive Light-Driven Translocation of Transducin between the Two Major Compartments of Rod Cells: A Novel Mechanism of Light Adaptation. Neuron 2002, 33, 95–106. [Google Scholar] [CrossRef]
- Berry, J.; Frederiksen, R.; Yao, Y.; Nymark, S.; Chen, J.; Cornwall, C. Effect of Rhodopsin Physphorylation on Dark Adaptation in Mouse Rods. J. Neurosci. 2016, 36, 6973–6987. [Google Scholar] [CrossRef] [PubMed]
- Babai, N.; Sendelbeck, A.; Regus-Leidig, H.; Fuchs, M.; Mertins, J.; Reim, K.; Brose, N.; Feigenspan, A.; Brandstätter, J.H. Functional Roles of Complexins3 and Complexin4 at Mouse Photoreceptor Ribbon Synapse. J. Neurosci. 2016, 36, 6651–6667. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, H.; Vinberg, F.; Nymark, S.; Koskelainen, A. Mesopic background lights enhance dark-adapted cone ERG flash responses in the intact mouse retina: A possible role for gap junctional decoupling. J. Neurophysiol. 2011, 105, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Mazade, R.; Eggers, E. Light adaptation alters inner retinal inhibition to shape OFF retinal pathway signaling. J. Neurophysiol. 2016, 115, 2761–2778. [Google Scholar] [CrossRef] [PubMed]
- Mazade, R.; Eggers, E. Light adaptation alters the source of inhibition to the mouse retinal OFF pathway. J. Neurophysiol. 2013, 110, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Barlow, H.B.; Fitzhugh, R.; Kuffler, S.W. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J. Physiol. 1957, 137, 338–354. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Xin, D.; Osborne, T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis. Neurosci. 1997, 14, 565–576. [Google Scholar] [CrossRef] [PubMed]
- McAnany, J.; Nolan, P. Changes in the harmonic components of the flicker electroretinogram during light adaptation. Doc. Ophthalmol. 2014, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.R.; Raghuram, A.; Rajagopalan, A.S. Cone phototransduction and growth of the ERG b -wave during light adaptation. Vis. Res. 2006, 46, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Miyake, Y.; Piao, C.; Tanikawa, A.; Horigushi, M.; Terasaki, H. Amplitude Increase of the Multifocal Electroretinogram during Light Adaptation. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2633–2637. [Google Scholar]
- Peachey, N.S.; Alexander, K.R.; Derlacki, D.J.; Fishman, G.A. Light adaptation, rods, and the human cone flicker ERG. Vis. Neurosci. 1992, 8, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Peachey, N.S.; Alexander, K.R.; Fishman, G.A. Visual Adaptation and the Cone Flicker Electroretinogram. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1517–1522. [Google Scholar]
- Gouras, P.; McKay, C.J. Growth in Amplitude of the Human Cone Electroretinogram with Light Adaptation. Investig. Ophthalmol. Vis. Sci. 1989, 30, 625–630. [Google Scholar]
- Maehara, S.; Itoh, Y.; Hoshino, S.; Hayashi, M.; Ito, Y. Dark adaptation time in canine electroretinography using a contact lens electrode with a built-in light source. J. Vet. Med. Sci. 2015, 77, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Peachey, N.S.; Goto, Y.; Al-Ubaidi, M.R.; Naash, M.I. Properties of the mouse cone-mediated electroretinogram during light adaptation. Neurosci. Lett. 1993, 162, 9–11. [Google Scholar] [CrossRef]
- Brown, B.M.; Ramirez, T.; Rife, L.; Craft, C.M. Visual Arrestin 1 Contributes to Cone Photoreceptor Survival and Light Adaptation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.A.; Lucas, R.J. Influence of the rod photoresponse on light adaptation and circadian rhythmicity in the cone ERG. Mol. Vis. 2009, 15, 2209–2216. [Google Scholar] [PubMed]
- Bui, B.V.; Fortune, B. Origin of electroretinogram amplitude growth during light adaptation in pigmented rats. Vis. Neurosci. 2006, 23, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, R.; Nymark, S.; Kolesnikov, A.V.; Berry, J.; Adler, L., IV; Koutalos, Y.; Keflav, V.J.; Cornwall, M.C. Rhodopsin kinase and arrestin binding control the decay of photoactivated rhodopsin and dark adaptation of mouse rods. J. Gen. Physiol. 2016, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nymark, S.; Frederiksen, R.; Estevez, M.E.; Shen, S.Q.; Corbo, J.C.; Cornwall, M.C.; Kefalov, V.J. Chromophore Supply Rate-Limits Mammalian Photoreceptor Dark Adaptation. J. Neurosci. 2014, 34, 11212–11221. [Google Scholar] [CrossRef] [PubMed]
- Hetling, J.R.; Pepperberg, D.R. Sensitivity and kinetics of mouse rod flash responses determined in vivo from paired-flash electroretinograms. J. Physiol. 1999, 516, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fang, Q.; Yu, H. The Shift of ERG B-Wave induced by Hours’ Dark Exposure in Rodents. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Wyszecki, G.; Stiles, W. Color Science: Concepts and Methods, Quantitative Data and Formulas; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Nagaya, M.; Ueno, S.; Kominami, T.; Nakanishi, A.; Koyasu, T.; Kondo, M.; Furukawa, T.; Terasaki, H. Pikachurin Protein Required for Increase of Cone Electroretinogram B-Wave during Light Adaptation. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Wachtmeister, L. Oscillatory Potentials in the Retina: What Do They Reveal. Prog. Retinal Eye Res. 1998, 17, 485–521. [Google Scholar] [CrossRef]
- Liu, D.M.; Zhou, S.; Chen, J.M.; Peng, S.Y.; Xia, W.T. The Intoxicating Effects of Methanol and Formic Acid on Rat Retina Function. J. Ophthalmol. 2016, 2016, 408709. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.T.; Stockman, A. Rod pathways: The importance of seeing nothing. Trends Neurosci. 1999, 22, 497–504. [Google Scholar] [CrossRef]
- Sharpe, L.T.; Stockman, A. Dual rod pathways. In From Pigments to Perception: Advances in Understanding Visual Processes; Valberg, A., Lee, B.B., Eds.; Plenum Press: New York, NY, USA; London, UK, 1991; pp. 53–66. [Google Scholar]
- Tsai, I.T.; Joachimsthaler, A.; Kremers, J. Mesopic and Photopic Rod and Cone Photoreceptor-Driven Visual Processes in Mice With Long-Wavelength Shifted Cone Pigments. IOVS 2017, 58, 5177–5187. [Google Scholar]
- Robson, J.; Saszik, S.; Ahmed, J.; Frishman, L. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J. Physiol. 2003, 547, 509–530. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, T.; Kondo, M.; Miyata, K.; Ueno, S.; Miyata, T.; Nishizawa, Y.; Terasaki, H. Photopic Electroretinograms of mGluR6-Deficient Mice. Curr. Eye Res. 2008, 33, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Shiells, R.A.; Falk, G. Contribution of rod, on-bipolar, and horizontal cell light responses to the ERG of dogfish retina. Vis. Neurosci. 1999, 16, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.; Jiang, M.; Wang, T.L.; Lyubarsky, A.; Savchenko, A.; Bar-Yehuda, T.; Sterling, P.; Birnbaumer, L.; Vardi, N. Light Response of Retinal ON Bipolar Cells Requires a Specific splice Variant of Ga0. J. Neurosci. 2000, 22, 4878–4884. [Google Scholar]
- Robson, J.G.; Frishman, L.J. Dissecting the dark-adapted electroretinogram. Doc. Ophthalmol. 1999, 95, 187–215. [Google Scholar] [CrossRef]
- Calvert, P.D.; Krasnoperova, N.V.; Lyubarsky, A.L.; Isayama, T.; Nicoló, M.; Kosaras, B.; Wong, G.; Gannon, K.S.; Margolskee, R.F.; Sidman, R.L.; et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin a-subunit. PNAS 2000, 97, 13913–13918. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Morris, L.; Xu, J.; Ma, H.; Michalakis, S.; Biel, M.; Ding, X.-Q. Endoplasmatic Reticulus Stress-associated Cone Photoreceptor Degeneration in Cyclic Nucleotide-gated Channel Deficency. J. Biol. Chem. 2012, 287, 18018–18029. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.H.; Williams, G.A.; Cahill, H.; Nathans, J. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science 2007, 315, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, P.M.; Olveczky, B.P.; Williams, G.L.; Jacobs, G.H.; Reese, B.E.; Meister, M.; Nathans, J. Genetically engineered mice with an additional class of cone photoreceptors: implications for the evolution of color vision. Proc. Natl. Acad. Sci. USA 2003, 100, 11706–11711. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, S.H.; Kuchenbecker, J.A.; Roberson, D.K.; Neitz, M.; Neitz, J. S-opsin knockout mice with the endogenous M-opsin gene replaced by an l-opsin variant. Vis. Neurosci. 2014, 31, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Harazny, J.; Scholz, M.; Buder, T.; Lausen, B.; Kremers, J. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc. Ophthalmol. 2009, 119, 181–197. [Google Scholar] [CrossRef] [PubMed]


| ERG Component | Protocol | % Change During Light Adaptation Mean ± SD | p-Value | |
|---|---|---|---|---|
| b-wave | amplitude [µV] | 25 cd/m2 | +21.8 ±18.8 | <0.0005 |
| 40 cd/m2 | +22.3 ± 22.4 | 0.007 1 | ||
| implicit time [ms] | 25 cd/m2 | −7.7 ± 4.2 | <0.0005 | |
| 40 cd/m2 | −4.8 ± 1.8 | <0.0005 | ||
| PhNR | amplitude [µV] | 25 cd/m2 | +85.2 ± 116.0 | 0.007 1 |
| 40 cd/m2 | +47.5 ± 228.1 | 0.766 | ||
| implicit time [ms] | 25 cd/m2 | −8.5 ± 12.5 | 0.722 | |
| 40 cd/m2 | +5.6 ± 13.3 | 0.722 | ||
| 2nd oscillatory potential (OP) peak | amplitude [µV] | 25 cd/m2 | +101.6 ± 103.9 | 0.453 |
| 40 cd/m2 | +133.9 ± 184.2 | 0.066 | ||
| implicit time [ms] | 25 cd/m2 | −1.7 ± 1.7 | 0.002 | |
| 40 cd/m2 | −1.2 ± 2.2 | 0.140 | ||
| 3rd OP peak | amplitude [µV] | 25 cd/m2 | +70.6 ± 57.9 | <0.0005 |
| 40 cd/m2 | +102.9 ± 127.7 | 0.017 1 | ||
| implicit time [ms] | 25 cd/m2 | −5.3 ± 1.8 | <0.0005 | |
| 40 cd/m2 | +0.7 ± 2.5 | 0.002 | ||
| all OP (FFT analysis) | amplitude [µV] | 25 cd/m2 | +40.8 ± 47.4 | 0.008 1 |
| 40 cd/m2 | +29.2 ± 25.1 | 0.131 | ||
| ERG Component | Protocol | % Change during Light Adaptation Mean ± SD | p-Value | |
|---|---|---|---|---|
| a-wave | amplitude [µV] | 25 cd/m2 | +309.3 ± 50.6 | <0.0005 |
| 40 cd/m2 | +840.4 ± 571.0 | <0.0005 | ||
| implicit time [ms] | 25 cd/m2 | +9.2 ± 6.6 | 0.328 | |
| 40 cd/m2 | −7.0 ± 14.2 | 0.004 | ||
| b-wave | amplitude [µV] | 25 cd/m2 | +100.0 ± 38.7 | <0.0005 |
| 40 cd/m2 | +126.8 ± 64.3 | <0.0005 | ||
| implicit time [ms] | 25 cd/m2 | +12.2 ± 9.2 | 0.867 | |
| 40 cd/m2 | −17.0 ± 13.7 | 0.52 | ||
| a-to-b ratio | 25 cd/m2 | +114.3 ± 45.6 | <0.0005 | |
| 40 cd/m2 | +301.9 ± 277.2 | <0.0005 | ||
| 2nd OP peak | amplitude [µV] | 25 cd/m2 | +321.9 ± 76.2 | <0.0005 |
| 40 cd/m2 | +588.2 ± 240.0 | <0.0005 | ||
| implicit time [ms] | 25 cd/m2 | +0.1 ± 8.3 | 0.006 1 | |
| 40 cd/m2 | −2.8 ± 3.2 | 0.0061 | ||
| 3rd OP peak | amplitude [µV] | 25 cd/m2 | +272.2 ± 85.5 | <0.0005 |
| 40 cd/m2 | +435.0 ± 170.6 | 0.0061 | ||
| implicit time [ms] | 25 cd/m2 | −2.5 ± 4.6 | <0.0005 | |
| 40 cd/m2 | +1.7 ± 4.4 | <0.0005 | ||
| all OP | amplitude [µV] | 25 cd/m2 | +78.9 ± 55.7 | 0.008 1 |
| (FFT analysis) | 40 cd/m2 | +124.4 ± 57.5 | <0.0005 | |
| 1st Harmonic Parameter | Mean Luminance | % change During Light Adaptation Mean ± SD | p-Value | Mean Luminance | %-Change During Dark Adaptation Mean ± SD |
|---|---|---|---|---|---|
| amplitude [µV] | 25 cd/m2 | −5.8 ± 39.3 | 0.002 | 1 cd/m2 | +438.1 ± 304.4 |
| phase [deg] | 25 cd/m2 | −8.7 ± 7.2 | <0.0005 | 1 cd/m2 | +51.1 ± 159.2 |
| Phot cd/m2 | Log Phot cd/m2 | Scot cd/m2 | Log Scot cd/m2 | ||
|---|---|---|---|---|---|
| Background light | 1.0 | 0.0 | 1.6 | 0.2 | |
| 25.0 | 1.4 | 40.6 | 1.6 | ||
| 40.0 | 1.6 | 65.0 | 1.8 | ||
| Phot cd·s/m2 | Log Phot cd·s/m2 | Scot cd·s/m2 | Log Scot cd·s/m2 | ||
| Flash | 6.3 | 0.8 | 10.2 | 1.0 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joachimsthaler, A.; Tsai, T.I.; Kremers, J. Electrophysiological Studies on The Dynamics of Luminance Adaptation in the Mouse Retina. Vision 2017, 1, 23. https://doi.org/10.3390/vision1040023
Joachimsthaler A, Tsai TI, Kremers J. Electrophysiological Studies on The Dynamics of Luminance Adaptation in the Mouse Retina. Vision. 2017; 1(4):23. https://doi.org/10.3390/vision1040023
Chicago/Turabian StyleJoachimsthaler, Anneka, Tina I. Tsai, and Jan Kremers. 2017. "Electrophysiological Studies on The Dynamics of Luminance Adaptation in the Mouse Retina" Vision 1, no. 4: 23. https://doi.org/10.3390/vision1040023
APA StyleJoachimsthaler, A., Tsai, T. I., & Kremers, J. (2017). Electrophysiological Studies on The Dynamics of Luminance Adaptation in the Mouse Retina. Vision, 1(4), 23. https://doi.org/10.3390/vision1040023






