The Development and Aging of the Magnocellular and Parvocellular Visual Pathways as Indicated by VEP Recordings between 5 and 84 Years of Age
Abstract
:1. Introduction
2. Results
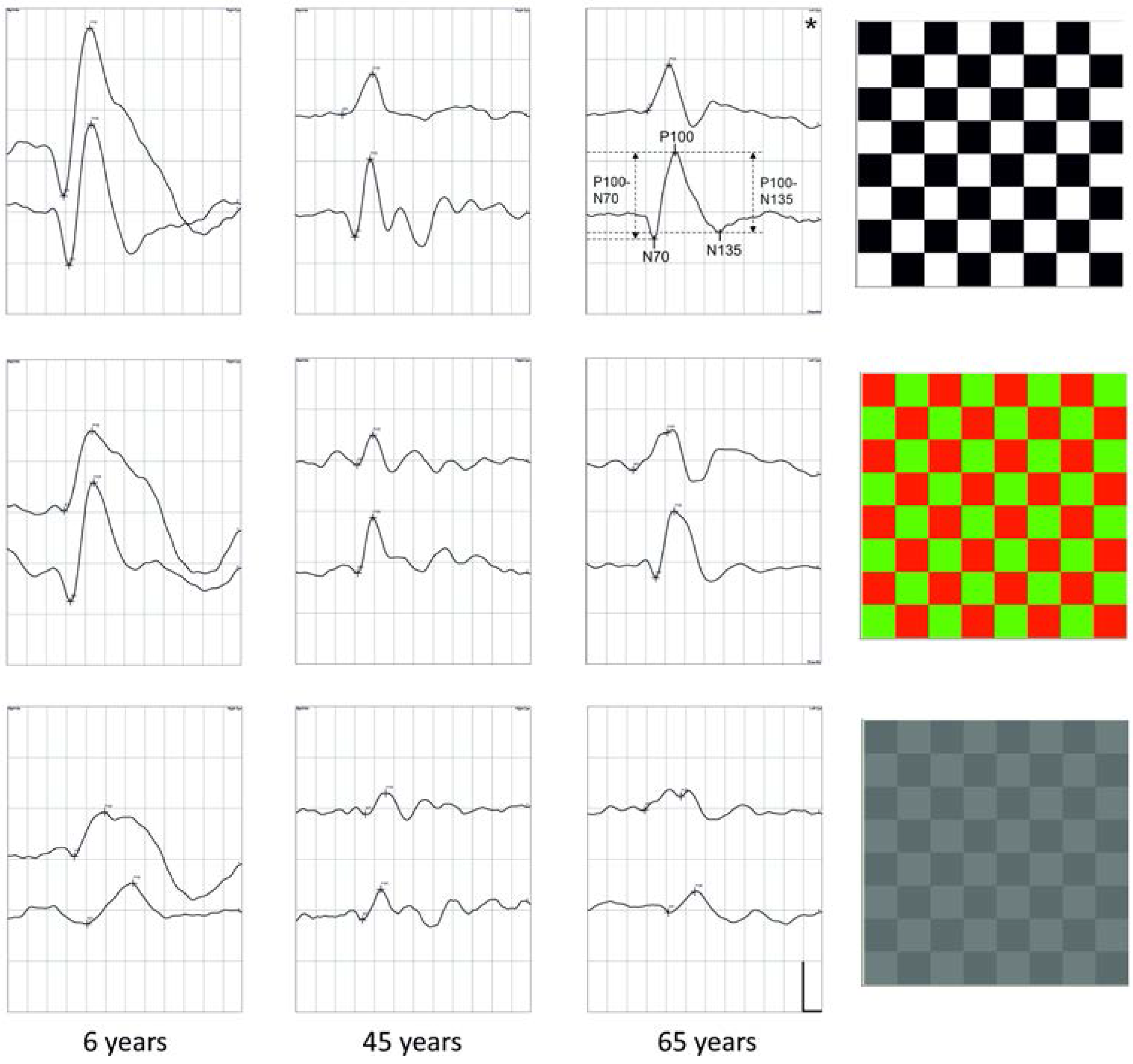
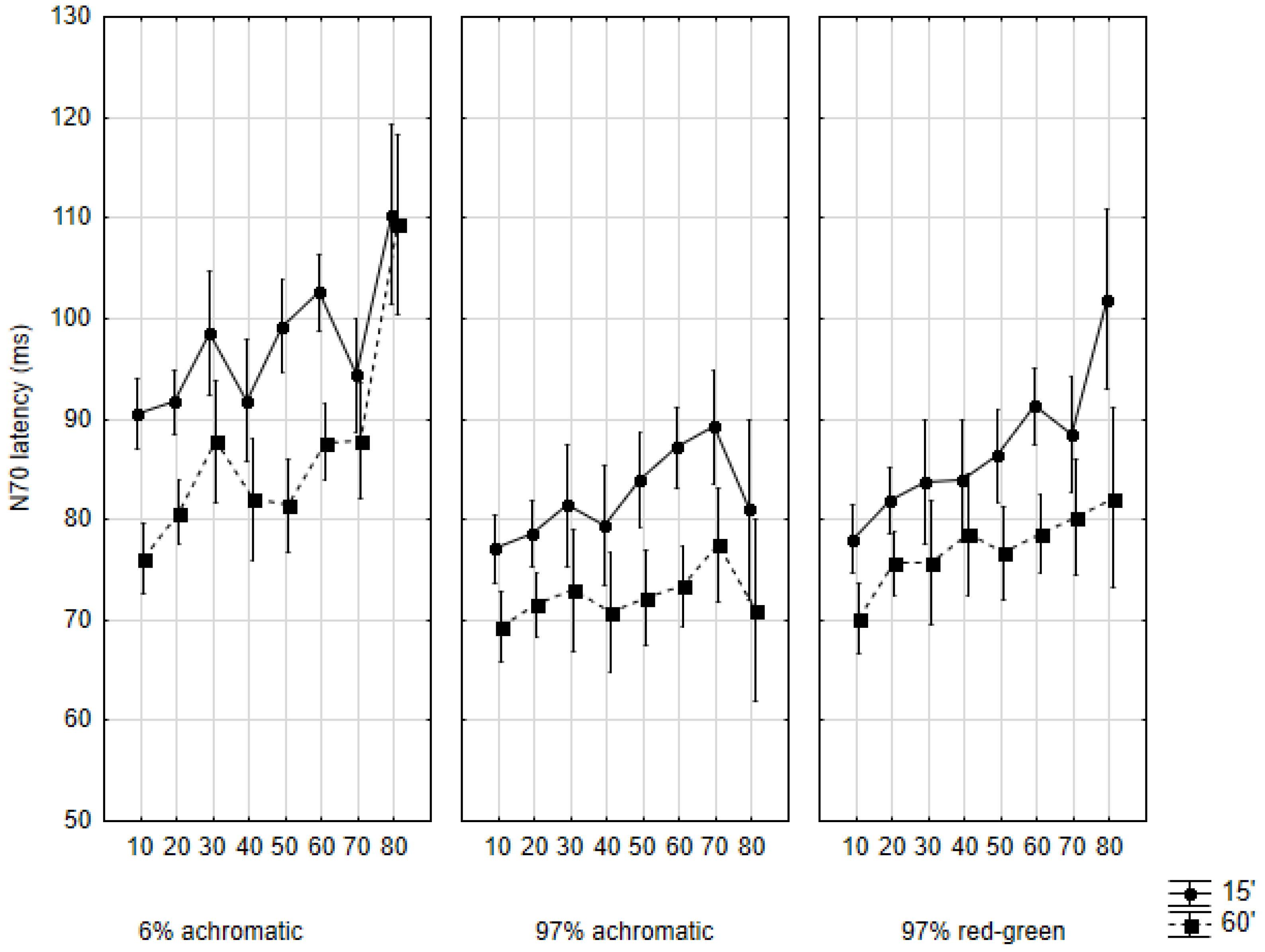
2.1. N70 Latency
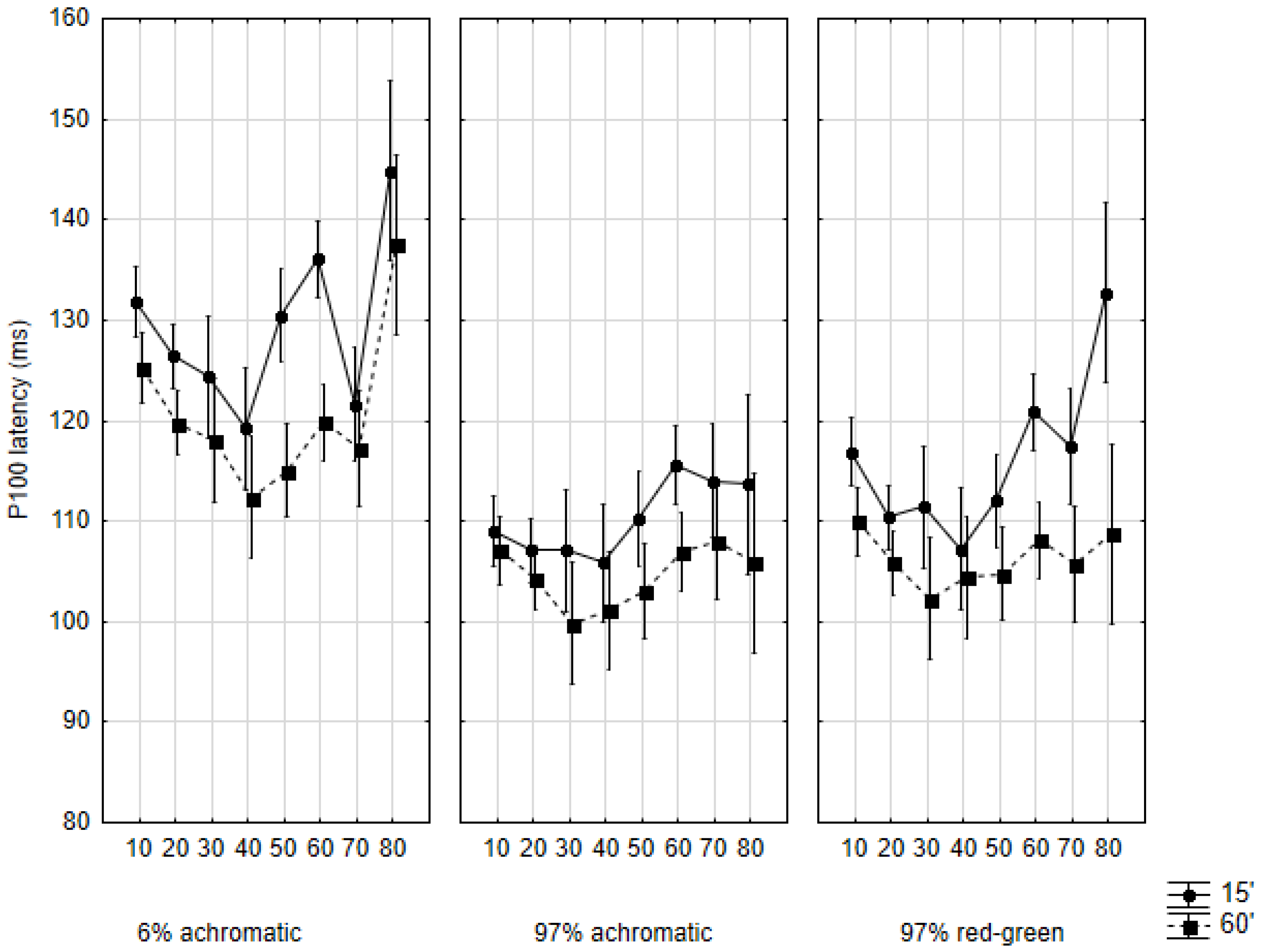
2.2. P100 Latency
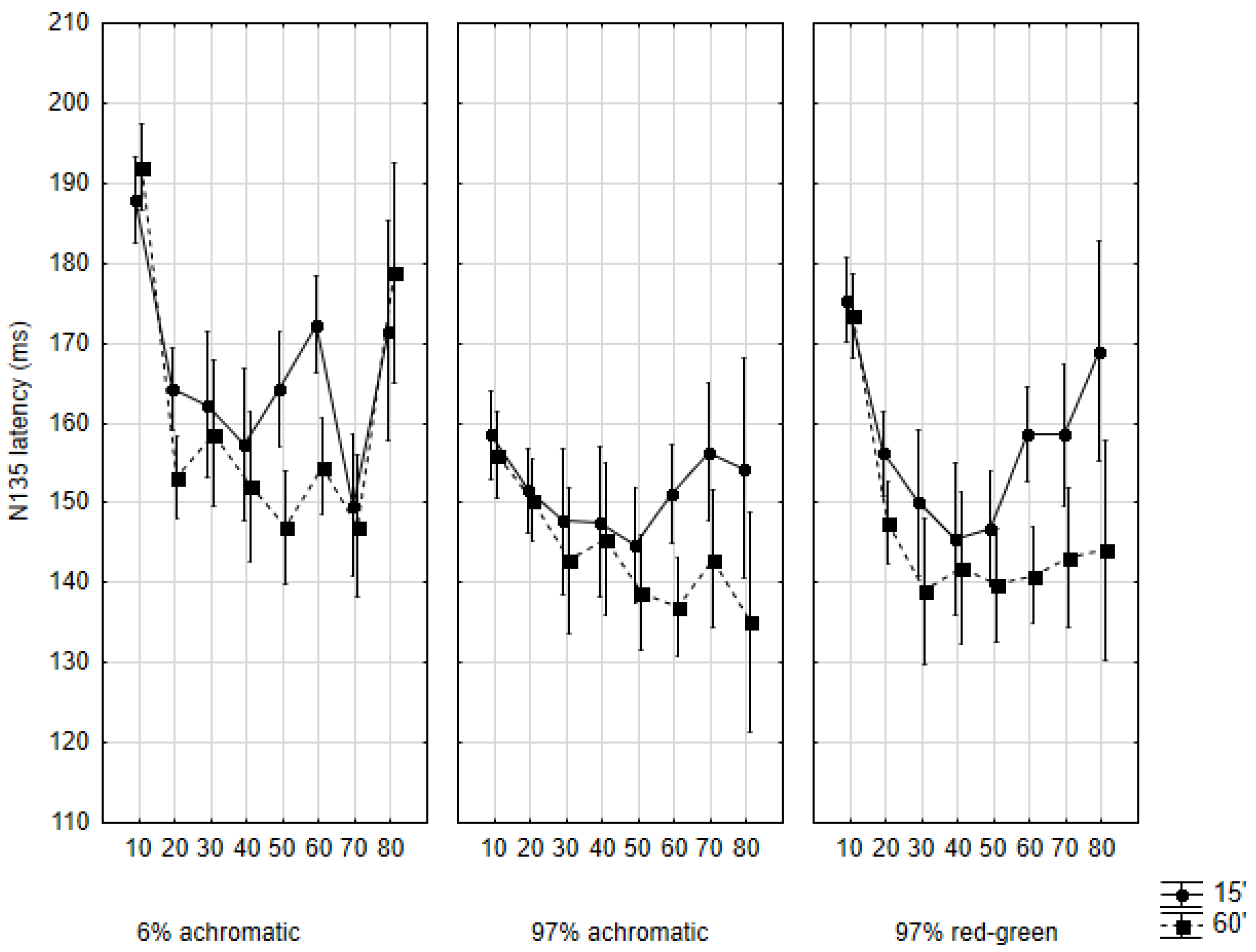
2.3. N135 Latency
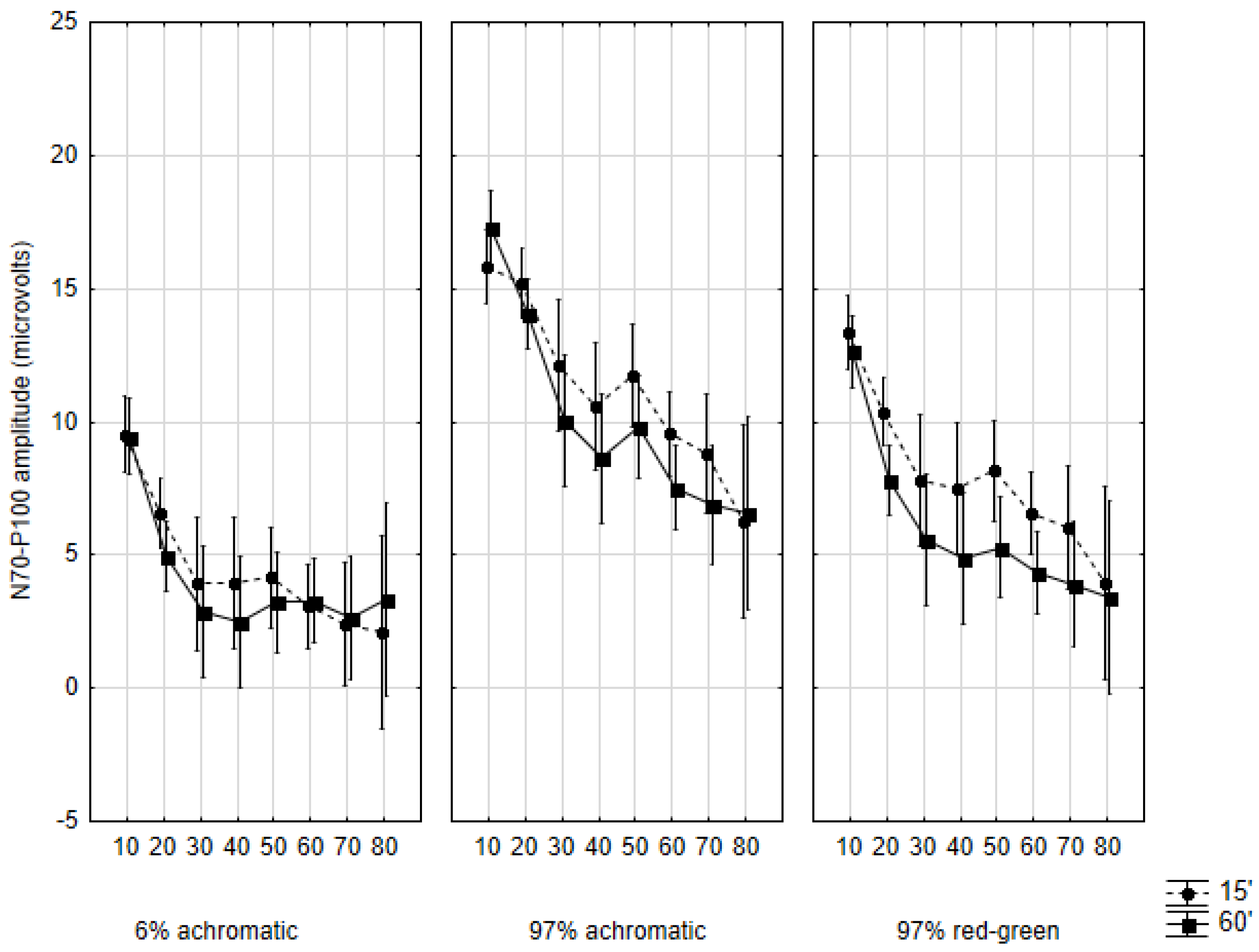
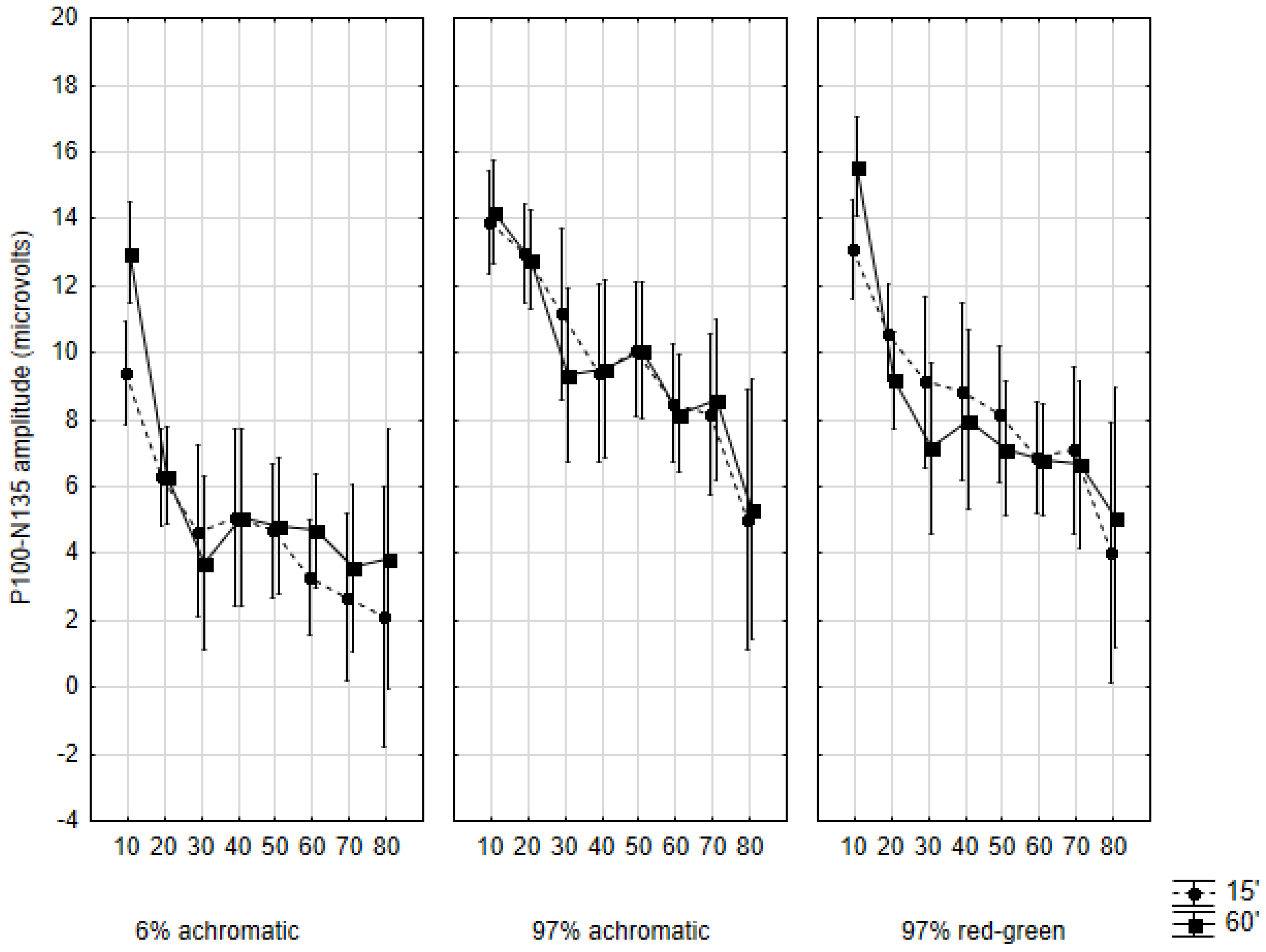
2.4. Amplitudes
3. Discussion
4. Material and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Livingstone, M.; Hubel, D. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science 1988, 240, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Morrone, M.C.; Tosetti, M.; Montanaro, D.; Fiorentini, A.; Cioni, G.; Burr, D.C. A cortical area that responds specifically to optic flow, revealed by fmri. Nat. Neurosci. 2000, 3, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Mascheretti, S.; Giora, E.; Ronconi, L.; Ruffino, M.; Quadrelli, E.; Facoetti, A.; Marino, C. The DCDC2 intron 2 deletion impairs illusory motion perception unveiling the selective role of magnocellular-dorsal stream in reading (dis)ability. Cereb. Cortex 2015, 25, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Derrington, A.M.; Lennie, P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J. Physiol. 1984, 357, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Shapley, R.M. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc. Natl. Acad. Sci. USA 1986, 83, 2755–2757. [Google Scholar] [CrossRef] [PubMed]
- Grunert, U. Anatomical evidence for rod input to the parvocellular pathway in the visual system of the primate. Eur. J. Neurosci. 1997, 9, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Hammarrenger, B.; Lepore, F.; Lippe, S.; Labrosse, M.; Guillemot, J.P.; Roy, M.S. Magnocellular and parvocellular developmental course in infants during the first year of life. Doc. Ophthalmol. 2003, 107, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Dobkins, K.R.; Anderson, C.M.; Lia, B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: Evidence for precocious magnocellular development? Vis. Res. 1999, 39, 3223–3239. [Google Scholar] [CrossRef]
- Parrish, E.E.; Giaschi, D.E.; Boden, C.; Dougherty, R. The maturation of form and motion perception in school age children. Vis. Res. 2005, 45, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Crewther, S.G.; Crewther, D.P.; Klistorner, A.; Kiely, P.M. Development of the magnocellular VEP in children: Implications for reading disability. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 49, 123–128. [Google Scholar] [PubMed]
- Gordon, G.E.; McCulloch, D.L. A VEP investigation of parallel visual pathway development in primary school age children. Doc. Ophthalmol. Adv. Ophthalmol. 1999, 99, 1–10. [Google Scholar] [CrossRef]
- Klaver, P.; Lichtensteiger, J.; Bucher, K.; Dietrich, T.; Loenneker, T.; Martin, E. Dorsal stream development in motion and structure-from-motion perception. NeuroImage 2008, 39, 1815–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Vaegan, A.P.T. ISCEV standard for clinical visual evoked potentials (2009 update). Doc. Ophthalmol. Adv. Ophthalmol. 2010, 120, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.; Kalita, J. Visual evoked potential. In Clinical Neurophysiology: Nerve Conduction, Electromyography, Evoked Potentials; Mishra, U., Kalita, J., Eds.; Reed Elsevier India Private Ltd.: New Delhi, India, 2006; pp. 309–327. [Google Scholar]
- Sakaue, H.; Katsumi, O.; Mehta, M.; Hirose, T. Simultaneous pattern reversal erg and ver recordings. Effect of stimulus field and central scotoma. Investig. Ophthalmol. Vis. Sci. 1990, 31, 506–511. [Google Scholar]
- Schechter, I.; Butler, P.D.; Zemon, V.M.; Revheim, N.; Saperstein, A.M.; Jalbrzikowski, M.; Pasternak, R.; Silipo, G.; Javitt, D.C. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin. Neurophysiol. 2005, 116, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.; Sokol, S. Developmental changes in the human visual system as reflected by the latency of the pattern reversal vep. Electroencephalogr. Clin. Neurophysiol. 1983, 56, 1–15. [Google Scholar] [CrossRef]
- Crognale, M.A. Development, maturation, and aging of chromatic visual pathways: Vep results. J. Vis. 2002, 2, 438–450. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.L.; Skarf, B. Development of the human visual system: Monocular and binocular pattern VEP latency. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2372–2381. [Google Scholar]
- Allison, T.; Wood, C.C.; Goff, W.R. Brain stem auditory, pattern-reversal visual, and short-latency somatosensory evoked potentials: Latencies in relation to age, sex, and brain and body size. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 619–636. [Google Scholar] [CrossRef]
- Brecelj, J.; Strucl, M.; Zidar, I.; Tekavcic-Pompe, M. Pattern ERG and VEP maturation in schoolchildren. Clin. Neurophysiol. 2002, 113, 1764–1770. [Google Scholar] [CrossRef]
- Tomoda, Y.; Tobimatsu, S.; Mitsudome, A. Visual evoked potentials in school children: A comparative study of transient and steady-state methods with pattern reversal and flash stimulation. Clin. Neurophysiol. 1999, 110, 97–102. [Google Scholar] [CrossRef]
- Mahajan, Y.; McArthur, G. Maturation of visual evoked potentials across adolescence. Brain Dev. 2012, 34, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Justino, L.; Kergoat, H.; Kergoat, M.J. Changes in the retinocortical evoked potentials in subjects 75 years of age and older. Clin. Neurophysiol. 2001, 112, 1343–1348. [Google Scholar] [CrossRef]
- Tobimatsu, S.; Kurita-Tashima, S.; Nakayama-Hiromatsu, M.; Akazawa, K.; Kato, M. Age-related changes in pattern visual evoked potentials: Differential effects of luminance, contrast and check size. Electroencephalogr. Clin. Neurophysiol. 1993, 88, 12–19. [Google Scholar] [CrossRef]
- Sokol, S.; Moskowitz, A.; Towle, V.L. Age-related changes in the latency of the visual evoked potential: Influence of check size. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 559–562. [Google Scholar] [CrossRef]
- Snyder, E.W.; Dustman, R.E.; Shearer, D.E. Pattern reversal evoked potential amplitudes: Life span changes. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 429–434. [Google Scholar] [CrossRef]
- Shaw, N.A.; Cant, B.R. Age-dependent changes in the amplitude of the pattern visual evoked potential. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 671–673. [Google Scholar] [CrossRef]
- Shaw, N.A.; Cant, B.R. Age-dependent changes in the latency of the pattern visual evoked potential. Electroencephalogr. Clin. Neurophysiol. 1980, 48, 237–241. [Google Scholar] [CrossRef]
- Atkinson, J.; Braddick, O. The development of visual function. In Scientific Foundations Paediatrics, 2nd ed.; Davis, J., Dobbing, J., Eds.; Heinemann: London, UK, 1981. [Google Scholar]
- Beazley, L.D.; Illingworth, D.J.; Jahn, A.; Greer, D.V. Contrast sensitivity in children and adults. Br. J. Ophthalmol. 1980, 64, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Benedek, K.; Keri, S.; Janaky, M. The scotopic low-frequency spatial contrast sensitivity develops in children between the ages of 5 and 14 years. Neurosci. Lett. 2003, 345, 161–164. [Google Scholar] [CrossRef]
- Kovacs, I.; Kozma, P.; Feher, A.; Benedek, G. Late maturation of visual spatial integration in humans. Proc. Natl. Acad. Sci. USA 1999, 96, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Schrauf, M.; Wist, E.R.; Ehrenstein, W.H. Development of dynamic vision based on motion contrast. Exp. Brain Res. 1999, 124, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Stein, J. The magnocellular theory of developmental dyslexia. Dyslexia 2001, 7, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Dustman, R.E.; Emmerson, R.Y.; Shearer, D.E. Life span changes in electrophysiological measures of inhibition. Brain Cogn. 1996, 30, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.J.; Sekuler, R.; Sekuler, A.B. The effects of aging on motion detection and direction identification. Vis. Res. 2007, 47, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Billino, J.; Bremmer, F.; Gegenfurtner, K.R. Differential aging of motion processing mechanisms: Evidence against general perceptual decline. Vis. Res. 2008, 48, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Habak, C.; Wilkinson, F.; Wilson, H.R. Aging disrupts the neural transformations that link facial identity across views. Vis. Res. 2008, 48, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Billino, J.; Bremmer, F.; Gegenfurtner, K.R. Motion processing at low light levels: Differential effects on the perception of specific motion types. J. Vis. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.; Owsley, C.; Sloane, M.E.; Roenker, D.L.; Bruni, J.R. Visual attention problems as a predictor of vehicle crashes in older drivers. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3110–3123. [Google Scholar]
- Owsley, C.; McGwin, G., Jr. Association between visual attention and mobility in older adults. J. Am. Geriatr. Soc. 2004, 52, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Lovasik, J.V.; Kergoat, M.J.; Justino, L.; Kergoat, H. Neuroretinal basis of visual impairment in the very elderly. Graefes Arch. Clin. Exper. Ophthalmol. 2003, 241, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Balazsi, A.G.; Rootman, J.; Drance, S.M.; Schulzer, M.; Douglas, G.R. The effect of age on the nerve fiber population of the human optic nerve. Am. J. Ophthalmol. 1984, 97, 760–766. [Google Scholar] [CrossRef]
- Curcio, C.A.; Millican, C.L.; Allen, K.A.; Kalina, R.E. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Asselman, P.; Chadwick, D.W.; Marsden, D.C. Visual evoked responses in the diagnosis and management of patients suspected of multiple sclerosis. Brain J. Neurol. 1975, 98, 261–282. [Google Scholar] [CrossRef]
- Celesia, G.G.; Daly, R.F. Effects of aging on visual evoked responses. Arch. Neurol. 1977, 34, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.D. Neural bases of visual deficits during aging. Vis. Res. 1993, 33, 2589–2609. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Z.; Li, G.; Wang, Y.; Zhou, Y.; Leventhal, A.G. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience 2008, 156, 748–757. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Sampson, G.P.; Walland, M.J.; Badcock, D.R. Contrast sensitivity changes due to glaucoma and normal aging: Low-spatial-frequency losses in both magnocellular and parvocellular pathways. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2115–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.F.; Faria, P.; Regateiro, F.S.; Forjaz, V.; Januario, C.; Freire, A.; Castelo-Branco, M. Independent patterns of damage within magno-, parvo- and koniocellular pathways in parkinson’s disease. Brain J. Neurol. 2005, 128, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Schefrin, B.E.; Tregear, S.J.; Harvey, L.O., Jr.; Werner, J.S. Senescent changes in scotopic contrast sensitivity. Vis. Res. 1999, 39, 3728–3736. [Google Scholar] [CrossRef]
- Morrison, J.D.; Reilly, J. The pattern visual evoked cortical response in human ageing. Q. J. Exp. Physiol. 1989, 74, 311–328. [Google Scholar] [CrossRef]
- Rousseff, R.T.; Tzvetanov, P.; Rousseva, M.A. The bifid visual evoked potential-normal variant or a sign of demyelination? Clin. Neurol. Neurosurg. 2005, 107, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Stothart, G.; Tales, A.; Hedge, C.; Kazanina, N. Double peaked P1 visual evoked potentials in healthy ageing. Clin. Neurophysiol. 2014, 125, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, R. Evoked potentials in the elderly. J. Clin. Neurophysiol. 1995, 12, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ossenblok, P.; Spekreijse, H. The extrastriate generators of the EP to checkerboard onset. A source localization approach. Electroencephalogr. Clin. Neurophysiol. 1991, 80, 181–193. [Google Scholar] [CrossRef]
- Di Russo, F.; Pitzalis, S.; Spitoni, G.; Aprile, T.; Patria, F.; Spinelli, D.; Hillyard, S.A. Identification of the neural sources of the pattern-reversal VEP. Neuroimage 2005, 24, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Joshi, S.; Singh, K.D.; Kumar, A. Visual evoked potentials: Normative values and gender differences. J. Clin. Diagn. Res. 2015, 9, CC12–CC15. [Google Scholar] [CrossRef] [PubMed]
- Gregori, B.; Pro, S.; Bombelli, F.; La Riccia, M.; Accornero, N. Vep latency: Sex and head size. Clin. Neurophysiol. 2006, 117, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Zemon, V.; Gordon, J. Luminance-contrast mechanisms in humans: Visual evoked potentials and a nonlinear model. Vis. Res. 2006, 46, 4163–4180. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedek, G.; Horváth, G.; Kéri, S.; Braunitzer, G.; Janáky, M. The Development and Aging of the Magnocellular and Parvocellular Visual Pathways as Indicated by VEP Recordings between 5 and 84 Years of Age. Vision 2017, 1, 7. https://doi.org/10.3390/vision1010007
Benedek G, Horváth G, Kéri S, Braunitzer G, Janáky M. The Development and Aging of the Magnocellular and Parvocellular Visual Pathways as Indicated by VEP Recordings between 5 and 84 Years of Age. Vision. 2017; 1(1):7. https://doi.org/10.3390/vision1010007
Chicago/Turabian StyleBenedek, György, Gyöngyi Horváth, Szabolcs Kéri, Gábor Braunitzer, and Márta Janáky. 2017. "The Development and Aging of the Magnocellular and Parvocellular Visual Pathways as Indicated by VEP Recordings between 5 and 84 Years of Age" Vision 1, no. 1: 7. https://doi.org/10.3390/vision1010007
APA StyleBenedek, G., Horváth, G., Kéri, S., Braunitzer, G., & Janáky, M. (2017). The Development and Aging of the Magnocellular and Parvocellular Visual Pathways as Indicated by VEP Recordings between 5 and 84 Years of Age. Vision, 1(1), 7. https://doi.org/10.3390/vision1010007






