Effect of Vestibular Stimulation on Balance and Gait in Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Inclusion of Studies
2.1.1. Population
2.1.2. Experimental Intervention/Training
2.1.3. Outcome Measures
2.1.4. Study Design
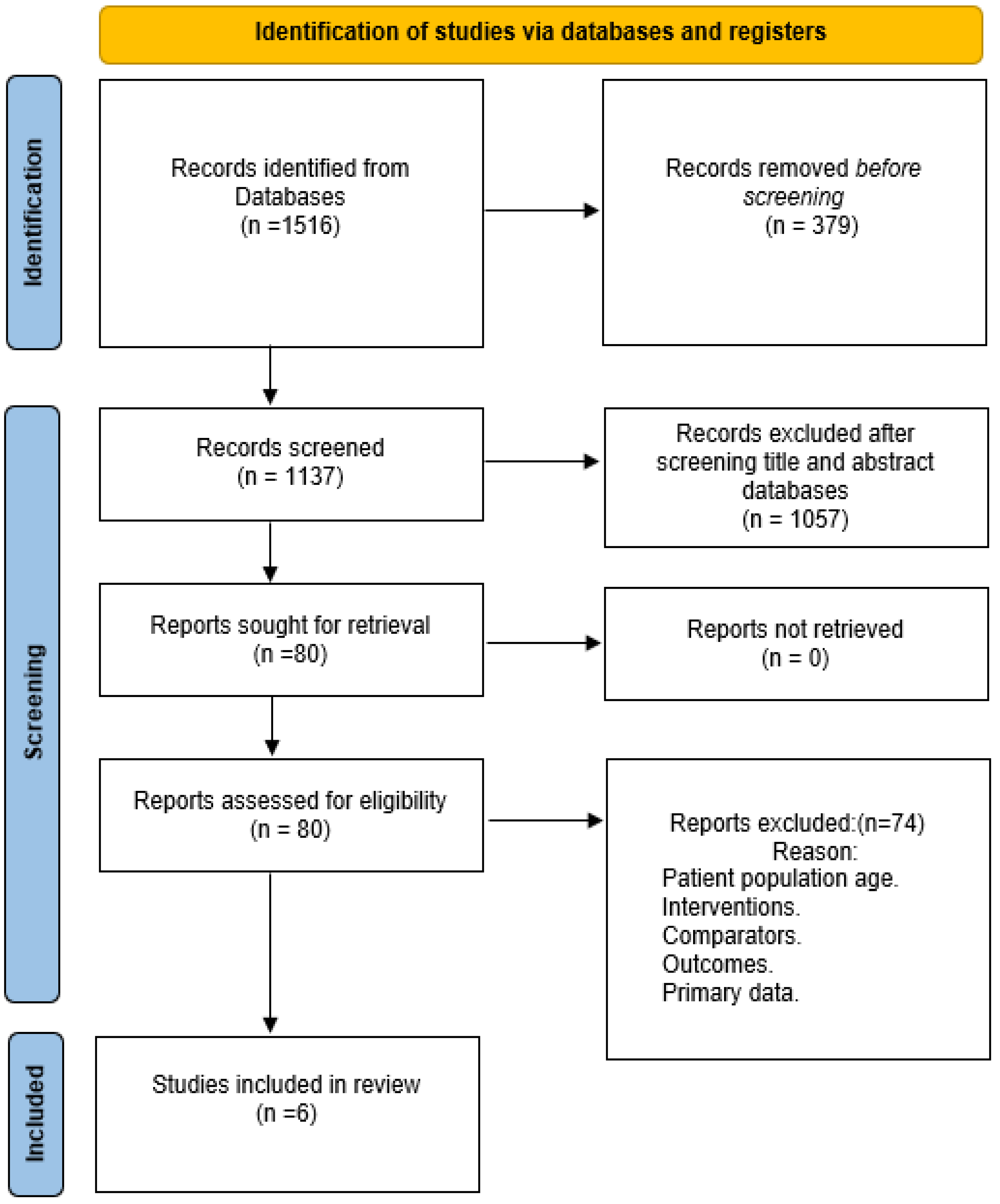
2.2. Search Strategy and Study Selection
2.3. Synthesis and Analysis
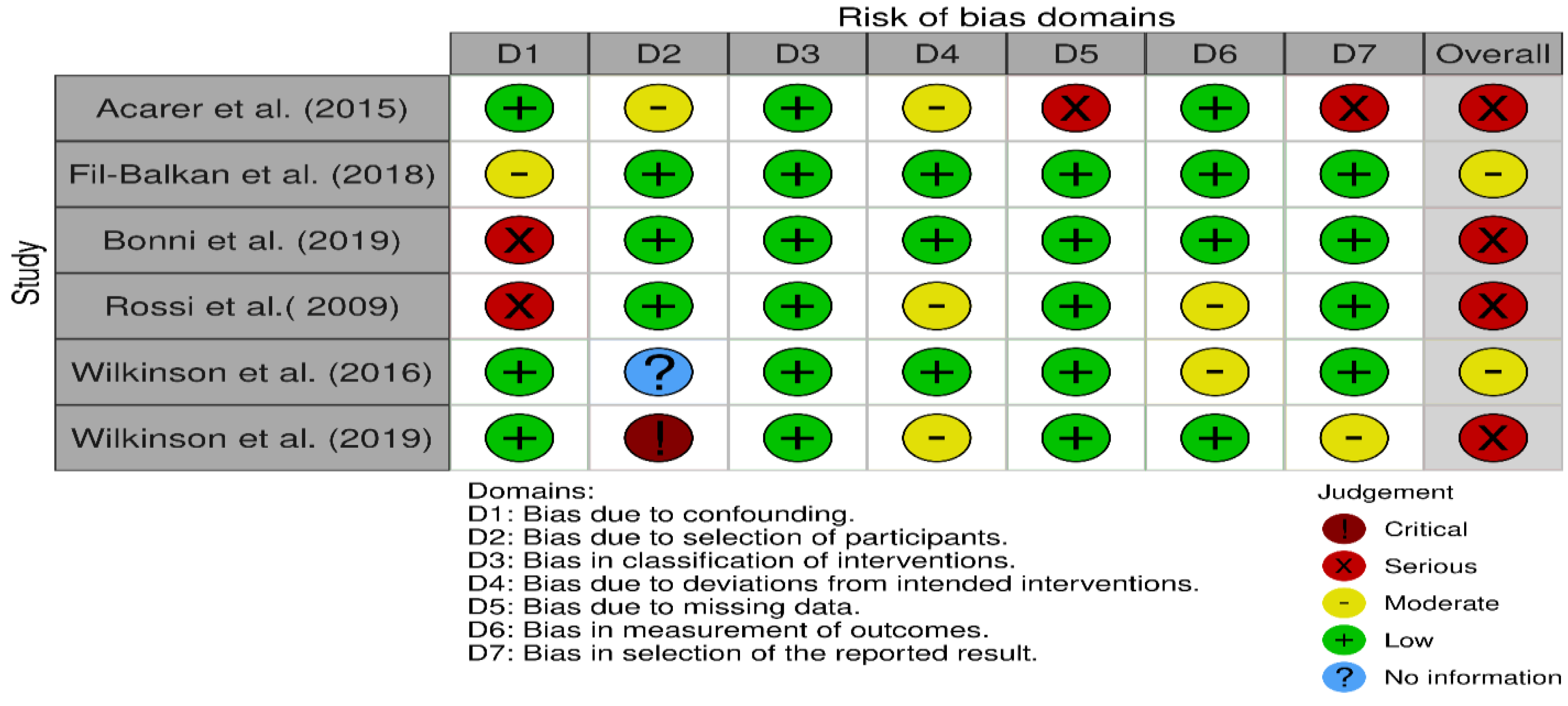
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.H.F.; Ferreira-Peruzzo, S.A.; Ribas, D.I.R.; Franklin, G.L.; Teive, H.A.G. Imbalance and gait impairment in Parkinson’s disease: Discussing postural instability and ataxia. Neurol. Sci. 2024, 45, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism. Neurology 1967, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.A.; Day, B.L.; Marsden, C.D. Vestibular Induced Postural Responses in Parkinson’s Disease. Brain 1993, 116 Pt 5, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Schindlbeck, K.A.; Naumann, W.; Maier, A.; Ehlen, F.; Marzinzik, F.; Klostermann, F. Disturbance of Verticality Perception and Postural Dysfunction in Parkinson’s Disease. Acta Neurol. Scand. 2018, 137, 212–217. [Google Scholar] [CrossRef]
- Bertolini, G.; Wicki, A.; Baumann, C.R.; Straumann, D.; Palla, A. Impaired Tilt Perception in Parkinson’s Disease: A Central Vestibular Integration Failure. PLoS ONE 2015, 10, e0124253. [Google Scholar] [CrossRef]
- Pollak, L.; Prohorov, T.; Kushnir, M.; Clinique, M.R.-N. Vestibulocervical reflexes in idiopathic Parkinson disease. Neurophysiol. Clin. Clin. Neurophysiol. 2009, 39, 235–240. [Google Scholar] [CrossRef]
- Reichert, W.H.; Doolittle, J.; McDowell, F.H. Vestibular Dysfunction in Parkinson Disease. Neurology 1982, 32, 1133–1138. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Horak, F.B. Effects of Deep Brain Stimulation and Levodopa on Postural Sway in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatr. 2002, 73, 267–274. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Koller, W.C.; Glatt, S.; Vetere-Overfield, B.; Hassanein, R. Falls and Parkinson’s Disease. Clin. Neuropharmacol. 1989, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.; Hadi, Z.; Prendergast, M.; Ciocca, M.; Saad, A.R.; Pondeca, Y.; Tai, Y.; Scott, G.; Seemungal, B.M. The Effect of Galvanic Vestibular Stimulation on Postural Balance in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2022, 442, 120414. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.K.; Basumatary, B.; Bansal, R.; Sahani, A.K. Techniques for the Detection and Management of Freezing of Gait in Parkinson’s Disease—A Systematic Review and Future Perspectives. MethodsX 2023, 10, 102106. [Google Scholar] [CrossRef] [PubMed]
- Heumann, R.; Moratalla, R.; Herrero, M.T.; Chakrabarty, K.; Drucker-Colín, R.; Garcia-Montes, J.R.; Simola, N.; Morelli, M. Dyskinesia in Parkinson’s Disease: Mechanisms and Current Non-Pharmacological Interventions. J. Neurochem. 2014, 130, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.P.B.d.Á.; Silva, T.R.; Torres, M.S.; Diniz, M.L.; Tavares, M.C.; Gonçalves, D.U. Galvanic Vestibular Stimulation and Its Applications: A Systematic Review. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S3), 202–211. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Bloch, D.A.; Nelson, L.M. Incidence of Parkin-son’s Disease: Variation by Age, Gender, and Race/Ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, I4919. [Google Scholar] [CrossRef]
- Fil-Balkan, A.; Salci, Y.; Keklicek, H.; Armutlu, K.; Aksoy, S.; Kayihan, H.; Elibol, B. Sensorimotor Integration Training in Parkinson’s Disease. Neurosci. J. 2018, 23, 208–215. [Google Scholar] [CrossRef]
- Cattaneo, D.; Jonsdottir, J.; Zocchi, M.; Regola, A. Effects of balance exercises on people with multiple sclerosis: A pilot study. Clin Rehabil. 2007, 21, 771–781. [Google Scholar] [CrossRef]
- Acarer, A.; Karapolat, H.; Celebisoy, N.; Ozgen, G.; Colakoglu, Z. Is Customized Vestibular Rehabilitation Effective in Patients with Parkinson’s? NeuroRehabilitation 2015, 37, 255–262. [Google Scholar] [CrossRef]
- Wilkinson, D.; Podlewska, A.; Sakel, M. A Durable Gain in Motor and Non-Motor Symptoms of Parkinson’s Disease Following Repeated Caloric Vestibular Stimulation: A Single-Case Study. NeuroRehabilitation 2016, 38, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Podlewska, A.; Banducci, S.E.; Pellat-Higgins, T.; Slade, M.; Bodani, M.; Sakel, M.; Smith, L.; LeWitt, P.; Ade, K. Caloric vestibular stimulation for the management of motor and non-motor symptoms in parkinson’s disease: Intention-to-treat data. Data Brief 2019, 25, 104228. [Google Scholar] [CrossRef] [PubMed]
- Bonnì, S.; Ponzo, V.; Tramontano, M.; Martino Cinnera, A.; Caltagirone, C.; Koch, G.; Peppe, A. Neurophysiological and Clinical Effects of Blindfolded Balance Training (BBT) in Parkinson’s Disease Patients: A Preliminary Study. Eur. J. Phys. Rehabil. Med. 2019, 55, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Izquierdo, M.; Soto-Varela, A.; Santos-Pérez, S.; Sesar-Ignacio, A.; Labella-Caballero, T. Vestibular Rehabilitation with Computerised Dynamic Posturography in Patients with Parkinson’s Disease: Improving Balance Impairment. Disabil. Rehabil. 2009, 31, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; McGregor, H.R.; Hass, C.J.; Pasternak, O.; Seidler, R.D. Sensory System-Specific Associations between Brain Structureand Balance. Neurobiol. Aging 2022, 119, 102–116. [Google Scholar] [CrossRef]
- Lee, S.; Liu, A.; McKeown, M.J. Current Perspectives on Galvanic Vestibular Stimulation in theTreatment of Parkinson’s Disease. Expert Rev. Neurother. 2021, 21, 405–418. [Google Scholar] [CrossRef]
- Bland, M.J.; Altman, D.G. Comparisons against Baseline within Randomised Groups Are Often Used and Can Be Highly Misleading. Trials 2011, 12, 264. [Google Scholar] [CrossRef]
- Tsang, W.W.N. The Effect of Vestibular Stimulation on Eye-Hand Coordination and Postural Control in Elite Basketball Players. Am. J. Sports Sci. 2014, 2, 17. [Google Scholar] [CrossRef]
- Clément, G.; Deguine, O.; Parant, M.; Costes-Salon, M.C.; Vasseur-Clausen, P.; Pavy-Le Traon, A. Effects of Cosmonaut Vestibular Training on Vestibular Function Prior to Spaceflight. Eur. J. Appl. Physiol. 2001, 85, 539–545. [Google Scholar] [CrossRef]
- Monzani, D.; Genovese, E.; Marrara, A.; Presutti, L.; Gherpelli, C.; Panzetti, P.; Forghieri, M. Stimulation of the Cholinergic Neurotransmissions Enhances the Efficacy of Vestibular Rehabilitation. Acta Otorhinolaryngol. Ital. 2010, 30, 11–19. [Google Scholar]
- Gerretsen, P.; Pothier, D.D.; Falls, C.; Armstrong, M.; Balakumar, T.; Uchida, H.; Mamo, D.C.; Pollock, B.G.; Graff-Guerrero, A. Vestibular Stimulation Improves Insight into Illness in Schizophrenia Spectrum Disorders HHS Public Access. Psychiatry Res. 2017, 251, 333–341. [Google Scholar] [CrossRef] [PubMed]

| Database | Syntax |
|---|---|
| PubMed | (“Parkinson Disease”[Mesh] OR Parkinson* OR parkinson’s disease OR parkinson disease OR parkinsons disease OR parkinsons OR parkinsonism) AND (“Vestibular System”[Mesh] OR “Vestibular Stimul*”) AND (Balance OR Stability OR “postural stability” OR instability OR gait OR fall OR “Postural Balance”[Mesh] OR “Gait”[Mesh] OR walking OR locomotion OR mobility OR posture OR posture control OR postural sway) |
| EbscoHost Search (Academic Search Complete, Medline, CINAHL) | (Parkinson* OR parkinson’s disease OR parkinson disease OR parkinsons disease OR parkinsons OR parkinsonism) AND (vestibular N3 stimulat* OR “Vestibular System”) AND (gait OR walking OR locomotion OR mobility OR posture OR postural control OR postural stability OR postural sway OR balance OR instability OR fall) |
| Web of Science | (Parkinson* OR parkinson’s disease OR parkinson disease OR parkinsons disease OR parkinsons OR parkinsonism) AND (vestibular stimulat* OR “Vestibular System”) AND (gait OR walking OR locomotion OR mobility OR posture OR postural control OR postural stability OR postural sway OR balance OR instability OR fall) |
| Google Scholar | Parkinson balance OR fall OR locomotion OR stability OR instability “vestibular stimulation” |
| Cochrance: | 1–((Parkinson* OR parkinson’s disease OR parkinson disease OR parkinsons disease OR parkinsons OR parkinsonism)):ti,ab,kw 2–((vestibular stimulat* OR “Vestibular System”)):ti,ab,kw 3–((gait OR walking OR locomotion OR mobility OR posture OR postural control OR postural stability OR postural sway OR balance OR instability OR fall)):ti,ab,kw #1 AND #2 AND #3 |
| PEDro | (Parkinson*balance) (Parkinson*gait) (Parkinson*fall) (Parkinson*locomotion) (Parkinson*stability) (Parkinson*instability) |
| Authors | Participants | Interventions | Outcome Measure | Notes |
|---|---|---|---|---|
| Fil-Balkan et al. (2018) [18] | 34 participants assessed for eligibility. 30 participants allocated to Study n = 15 Control groups n = 15. 26 participants completed 6-week training: Study group n = 12. Control group n = 14. 24 participants continued to complete and reassessed at the 7th week: Study group n = 12; Control group n = 12. 15 participants completed to follow-up and reassessed at the 12th week: Study group n = 7; Control group n = 8. Inclusion criteria:
Exclusion criteria:
Study group BBS: Mean 40.91 ± 7.91 Control group BBS: Mean 42.17 ± 12.26 p = 0.488 (Mann Whitney U test) Study group TUG: Mean 16.59 ± 8.29 Control group TUG: Mean 16.75 ± 10.45 p = 0.583 (Mann Whitney U test) | Control group: Twice a week 1-h classic physiotherapy for 6 weeks. Classic physiotherapy training included: person-specific flexibility, strengthening, posture, breathing, balance according to Cattaneo et al. (2007) [19], walking exercises, and other functional activities (not defined specifically) intensified with the progress of an individual’s performance, tolerance and needs. Study group: Classic physiotherapy as above + 30 min Sensory Motor Integration Training (SMIT) during each session. SMIT training included: Proprioceptive, visual and vestibular stimulation. A sensorimotor-perceptual integration activity was also designed and used in the form of a “walking trail” to improve motor control components of postural control. | Measurements were made at:
Clinical Measurements:
Statistical analysis: Non-parametric analysis (Wilcoxon Signed Rank Test for intragroup comparisons; Mann-Whitney U test for inter-group comparisons) of change in score (Δscore) between pre-therapy and post-therapy (T1–T0), and pre-therapy and follow-up (T2–T0) was used. Cohen’s d Effect size was calculated. CI was calculated for postural control values. α = 0.05 Results: Study group BBS:
95% CI: [5.03–15.80] Significant improvement.
95% CI: [1.63–21.80] Significant improvement. Control group BBS:
CI: [1.5–6.99] Significant improvement.
CI: [−5.98–8.73] Non-significant improvement. Patients in the Study group showed more improvements compared to those in the Control group (p = 0.027) post treatment and at follow-up (p = 0.037). Study group TUG:
CI: [−6.02–−3.47] Significant improvement.
CI: [−6.97–−2.64] Significant improvement. Control group TUG:
CI: [−3.75–−0.98] Significant improvement.
CI: [−1.71–0.32] Non-significant improvement. Patients in the Study group showed more improvements compared to those in the Control group (p = 0.001) post treatment and at follow-up (p = 0.002). Study group FRT:
CI: [4.53–8.34] Significant improvement.
CI: [1.92–10.36] Significant improvement. Control group FRT:
CI: [1.87–5.16] Significant improvement.
CI: [−0.17–5.42] Non-significant improvement. Patients in the Study group showed more improvements compared to those in the Control group (p = 0.024) post treatment but not at follow up (p = 0.115). | Design: Randomised Controlled Trial Evaluations completed during the “ON” period. Study and Control groups were different at the start of the study for SOT 6th position with the participants in the Control group scoring higher. (Mann Whitney U test) Improvements in the clinical measures after intervention for the Study group. For the Control group, TUG reduced after 6 weeks, too. There is also improvement in vestibular and visual system score for both Study and Control groups (but not supported by the reported values in Table 3 for vestibular system score). 6 weeks follow up assessments shows that improvement was maintained in the Study group even after 6 weeks compared with control group and authors suggested that this positive effect was due to SMIT. Authors contributed improvement in postural control to increasing capacity of the vestibular system. |
| Acarer et al. (2015) [20] | 60 participants were randomized into: Treatment/Group 1, n = 30; Control/Group 2, n = 30. 40 participants completed the study: Rehabilitation Group:
Participants had:
Inclusion criteria:
Exclusion criteria:
| Rehabilitation group: Intervention exercises (hospital and home exercise programs): Hospital exercise program: Once per week for 8 weeks at hospital including: Adaptation exercises (one minute each condition, 3 times a day): Moving the head in a yaw rotation while maintaining gaze on a target in 2 conditions: (A) head rotated while focusing on stationary target; (B) Head and the target both move in opposite direction. Substitution exercises: Physiotherapist trained the patients to substitute a sensory system with the one with lost or poor function. Habituation exercises: Walking with turning the head side to side. Balance exercises: Restoring balance while moving from a static (e.g., standing) position to another dynamic (e.g., walking) position Home exercise program:
Control group: no exercises; received usual care. | Measurements were made at:
Rehabilitation group BBS Before Rehab: 48 (8–56); After Rehab: 53 (21–56); p ˂ 0.05 Control group BBS: Before Rehab: 47 (29–52) After rehab: 44 (7–55); NS Rehabilitation group TUG: Before rehab: 12.2 (9–22) After Rehab: 10 (7–14); p ˂ 0.05 Control group TUG: Before Rehab: 11.2 (77–22) After Rehab: 11.0 (9–14); NS mCTSIB: FIRM EO (After rehab): Rehabilitation group: 0.33 [0.1–0.8] Control group: 0.40 [0.2–1.3] p < 0.05 FOAM EO (After rehab): Rehabilitation group: 0.72 [0.3–1.3] Control group: 0.8 [0.4–2.2] p < 0.05 FIRM EC (After rehab): Rehabilitation group: 0.3 [0.1–1.13] Control group: 0.4 [0.1–1.7] p > 0.05 FOAM EC (After rehab): Rehabilitation group: 1 [0.6–2.6] Control group: 1.6 [0.7–2.5] p > 0.05 | Data in Table 1, Table 2 and Table 3 is poorly reported and it is not clear what they represent. Data in the current table is just a replication of what has been reported by authors. Participants remained on stable dose of PD medication 1 month before and throughout the study. Tests were completed 2 h after receiving medication in “ON” state. Improvement after 8 weeks vestibular rehabilitation on ABC, BBS, DGI, TUG. No improvement on UPDRS-m. participants have reported that their level of confidence improved but fear of fall was reduced. Authors stated an improvement in patients static posturography scores in Control group which was attributed to getting familiar with the test. |
| Wilkinson et al. (2016) [21] | Participant:
symptoms: Hypokinesia, rigidity and memory lost symptoms. Medication: Stalevo 150 mg/25 mg/200 mg Levodopa 100 mg Carbidopa 25 mg Entacapone 200 mg Pramipexole dihydrochlorine 1 mg Remaining unchanged through the study. | Caloric Vestibular stimulator device (Scion Neurostim). One earpiece stimulated ear canal by a cold sawtooth waveform to 17 C every 2 min and the other earpiece stimulated ear canal to 42 C every 1 min and was switched every two days. 5 days a week for 3 months (1-month sham, 2 months active stimulation) Sessions ran for 20 min twice a day, min 4 h gap. CVS delivered with participant inclined in supine position and head flexed at 30 degrees. | Measurements were made at:
Outcome measures:
TUG:
Two-minute walk:
| The greatest improvement was observed after second month. High improvement reported for Mobility and cognition. Patient reported better sleep and less anxious. |
| Wilkinson et al. (2019) [22] | 59 participants screened, 46 recruited. Treatment or active group, n = 23 Control or placebo group, n = 23 33 participants completed treatment period (week 12). Treatment Group: n = 16 Placebo Group: n = 17 31 participants completed follow up period (week 17). Treatment group n = 14 Placebo group n = 17 Treatment group
Placebo Group:
Participants criteria:
| 8 weeks Caloric Vestibular Stimulation (CVS) at home.
| Measurements were made at:
and Just before randomised selection. Week 8 (Midway treatment) Week 12 (End of treatment) Week 17 (5 weeks after end of treatment). Measurements:
TUG:
Active group (Mean): −1.1 Placebo group (Mean): 0.0 Therapeutic Gains: −1.3 95% CI: −3.7 to 0.5 Week 17: Active group (Mean): −0.9 Placebo group (Mean): 0.0 Therapeutic Gains: −0.7 95% CI: −2.7 to 1.1 2-min walk (Distance in meters) Before Rehab: Active group: 73.2 (25.3) Placebo Group: 77.4 (33.1) p = 0.642 Week 12: Active group (Mean): 0.3 Placebo group (Mean): −1.7 Therapeutic Gains: 2.0 95% CI: −7.7 to 11.8 Week 17: Active group (Mean): 4.8 Placebo group (Mean): −3.8 Therapeutic Gains: 8.6 95% CI: 0.4 to 17.5 | Assessments were on medication state. Research partly supported by Scion NeuroStim, LLC. |
| Bonni et al. (2019) [23] | 16 right handed participants (M = 7 F = 9) were randomly assigned to 2 groups. Physiotherapy (PT) group (Control): N = 8: M4/F4 Age (Mean ± SD): 66.6 ±6.9 years Disease duration (Mean ± SD): 7 ± 3.1 years UPDRS score (Mean ± SD): 20 ± 6.8 Blindfolded Balance Training (BBT) group (Experimental): N = 8: M3/F5 Age (Mean ± SD): 71.8 ± 3.1 years Disease duration (Mean ± SD): 5.2 ± 3 years UPDRS score (Mean ± SD): 21 ± 8.5 Inclusion criteria: All treated with Levodopa (600 ± 250 mg), and receiving stable doses for at least 2 weeks before study and during the study. Exclusion criteria: Antidepressant 2 months before study History of pacemaker or brain stimulation History of epilepsy or dementia (Mini Mental status ˂ 24) Serious medical condition or neurological disease | 10 sessions of treatment over 2 weeks: 5 days per week, for 40 min: PT group: conventional physiotherapy including muscle stretching, active and assisted limb mobilization, four limbs coordination exercises, balance training on instable platform and gait training. BBT group: BBT consisting of balance and walking exercises to stimulate dynamic postural control and improve balance reactions. BBT included (1) marching on the spot on a foam cushion blindfolded and turning 90 degrees clockwise every 1 min; and (2) treadmill training during which blindfolded participant walked for 4 min. Initial speed was 1 km/h and increased 0.5 km/h every minute up to 3 km/h. | ANOVA: Time (Pre vs Post) as within-subjects and Group (BBT vs PT) as between-subjects factors; α = 0.05 Stance phase for the more affected body side (MABS) reduced significantly: Time effect (F(1,14) = 19.53; p = 0.0006); Group main effect (F(1,14) = 2.67; p = incorrectly reported in the original article as 12; NS); Time × Group interaction (F(1,14) = 12.92; p = 0.003); Post-hoc: significant reduction of stance phase percentage following BBT (p < 0.0004) Double Stance phase for MABS reduced significantly: Time effect (F(1,14) = 10.43; p = 0.006); Group main effect (F(1,14) = 2.08; p = 0.17); Time × Group interaction (F(1,14) = 8.43; p = 0.01); Post-hoc: significant reduction following BBT (p < 0.0004) Swing phase for MABS increased significantly: Time effect (F(1,14) = 10.15; p = 0.006); Group main effect (F(1,14) = 7.84; p = 0.002); Time × Group interaction (F(1,14) = 10.15; p = 0.006); Post-hoc: significant increase following BBT (p = 0.002) Gait speed for MABS increased significantly: Time effect (F(1,14) = 4.33; p = 0.056); | Authors claimed: Visual deprivation and proprioceptive perturbation may be useful to improve gait and postural control mediated through involvement of the vestibular system. Suggested neural mechanism involved was possibly improved connectivity of the SMA-M1 circuits. Study was a Controlled Randomised Trial Testing session was completed 2 h after first morning drug administration (i.e., in ON condition). |
| Rossi-Izquierdo et al. (2009) [24] | 45 participants who were recruited for a former study were screened, 17 were selected but 7 dropped due to reasons unrelated to the study (death, surgery, lack of transport). Treatment group n = 10 (n = 8 took part in the follow-up; the other 2 changed location and were not accessible) Age: 69.3 years (range 48–80 years) Sex: M = 5, F = 5 Length of illness: 7.15 (4–19) years H&Y score: 6 patients at stage III; 4 patients at stage IV There was no Control Group, but measures of TUG from 20 healthy subjects who were age- and sex-matched was used to determine eligible Treatment group participants. Participants’ characteristics: TUG ˃ 15.90 s No dementia, autonomic disorders, postural instability or hallucinations. No other neurological, cochleovestibular or middle-ear alterations. No wheelchair users Continued their usual medication. | Vestibular rehabilitation via CDP: 9 sessions, half an hour, over 1 month 10 CDP exercises were delivered which were customised according to participant’s deficits. Difficulty of exercises increased throughout training by increasing LOS, transition rate or movement of the posturography platform. Participants took usual medication and were tested and trained in the “on” state. | Measurements: TUG (time, steps, supports, falls, value assigned to the performance of the test) LOS (reaction time, directional control, movement velocity and distance covered (endpoint and maximum excursion) within stability limits) Statistical test: comparing outcome measures before and after training and at 1-year follow-up using Wilcoxon test. Results: TUG: 22.90 s (SD 6.22; range 16–33 s) before vs 16.00 s (SD 6.28; range 11–33 s) after treatment p = 0.004 21.63 s (SD 6.27; range 16–33 s) before vs 17.5 s (SD 4.2; range 14–26 s) at 1-year follow-up p ˂ 0.006 Non-significant difference for: steps, value, support LOS: Significant improvement in reaction time (RT—s), lateral plane movement velocity (MVL—deg/s), lateral plane directional control (DCL—%), and endpoint & maximum excursion after completion of the study and 1-yr follow-up: All ps < 0.05 Significant improvement in all subscales: For emotional p = 0.006 For functional p = 0.037 For physical p = 0.008 | Authors’ conclusion: vestibular rehabilitation is effective for gait velocity, balance and the risk of fall (based on improvement in TUG). Benefits persist over time. Note: no control group Authors claimed rehabilitation can be customised for each individual. |
| Eligibility Criteria | Parkinson’s Disease according to the clinical diagnostic criteria of the UK Parkinson’s Disease Society Brain Bank Age 60 to 65 3 to 7 years from time of diagnosis H&Y score ≥ 3 No history of FoG within the past 12 months Stable dose of PD medication at least 1 month before and during the intervention. Ability to ambulate indoor independently (nonwheelchair-bound). No relevant rehabilitations within the past 6 months. No other Neurological or musculoskeletal disorders. Peripheral vestibular system (PVS) specific: Normal heave (otolith function) and rotational (semicircular canals function) head impulses, no vertical ocular misalignment to suggest otolith imbalance No medical conditions affecting gait & Balance: Blood pressure, Arthritis, Obesity, Vitamin B-12 deficiency, Stroke, Migraine, Head injury, Diabetes, any medications for pain, depression, hallucination, autonomic disorder, cochleovestibular or middle ear alterations). |
| Treatment Strategies | Vestibular stimulation (VS) Physical therapy (PT) Behavioural therapy (BT) Taking VS, PT, BT at the baseline and remained on it during the follow up |
| Assignment procedures | Random allocation to a treatment strategy Blinded assessment |
| Outcomes | Postural stability & balance (TUG, 2-min walk distance, Time for 10-m walk, BBS, FOG) Gait Fatigue severity scale Function and Quality of Life |
| Follow up | Behaviour assessment (e.g., Baseline, Midway, at the end and 5 weeks after treatment) or loss to follow up or death, whichever occurs first Lag time for availability of administration records |
| Causal contrast of interest | Intention to treat effect, per-protocol effects using observational data (effect of Vestibular Stimulation on balance, stability, gait) |
| Statistical methods | Intention to treat analysis to compare the outcomes of the groups assigned to each treatment strategy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iravani-Naeeni, A.; Mohagheghi, A. Effect of Vestibular Stimulation on Balance and Gait in Parkinson’s Disease: A Systematic Review. J. Funct. Morphol. Kinesiol. 2024, 9, 206. https://doi.org/10.3390/jfmk9040206
Iravani-Naeeni A, Mohagheghi A. Effect of Vestibular Stimulation on Balance and Gait in Parkinson’s Disease: A Systematic Review. Journal of Functional Morphology and Kinesiology. 2024; 9(4):206. https://doi.org/10.3390/jfmk9040206
Chicago/Turabian StyleIravani-Naeeni, Ardavan, and Amir Mohagheghi. 2024. "Effect of Vestibular Stimulation on Balance and Gait in Parkinson’s Disease: A Systematic Review" Journal of Functional Morphology and Kinesiology 9, no. 4: 206. https://doi.org/10.3390/jfmk9040206
APA StyleIravani-Naeeni, A., & Mohagheghi, A. (2024). Effect of Vestibular Stimulation on Balance and Gait in Parkinson’s Disease: A Systematic Review. Journal of Functional Morphology and Kinesiology, 9(4), 206. https://doi.org/10.3390/jfmk9040206