Abstract
Background/Objectives: Parkinson’s Disease (PD) can be associated with balance and gait impairments leading to increased risk of falls. Several studies have reported positive effects of various forms of vestibular stimulation (VS) for improving balance and stability in people with PD (PwP). The purpose of present study was to synthesise the current evidence on the effectiveness of VS, highlighting its potential benefits in improving postural stability and reducing gait impairments in people with Parkinson’s Disease. Method: A systematic search was conducted across databases Cochrane, Medline, PEDro, PubMed, Web of Science, and Google Scholar. Studies were included if they involved PwP at stages 3 or 4 of the Hoehn and Yahr scale, aged 60 years or older. The Risk of Bias (RoB) was assessed using the ROBINS-I tool. The review followed the PRISMA guidelines and the protocol was registered with PROSPERO (CRD42022283898). Results: demonstrated that various forms of VS have shown promise in mitigating symptoms of vestibular dysfunction and improving gait and balance in PwP. However, the overall RoB ranged from moderate to critical, with variations across different domains. Conclusions: While VS appears to offer potential benefits in improving balance and gait in PwP, the presence of biases in the reviewed studies necessitate caution in interpreting the results. Further research should focus on addressing these biases to confirm the therapeutic potential of VS in PD.
1. Introduction
Parkinson’s Disease (PD) is mainly diagnosed based on specific motor symptoms: tremor, rigidity, slowness of movement, and postural instability [1]. Among these symptoms postural instability present significant challenges for People with Parkinson (PwP), primarily due to impaired mechanisms for maintaining posture [2], but patients do not usually experience balance-related symptoms earlier than stage 3 according to H&Y scale [3]. Varied pathophysiological mechanisms for postural instability in PwP are proposed. One such factor is the deficits of the (peripheral and/or central) vestibular system.
Pastor et al. investigated the role of vestibular dysfunction as a primary cause of postural instability in PD and reported no significant differences between patients and healthy controls in body sway responses induced by galvanic vestibular stimulation [4]. However, since then several studies have suggested vestibular deficits in PwP and linked these with balance dysfunction. For instance, Schindlbeck et al. reported that PwP who had balance impairments also had a relative inability to perceive vertical position [5], suggesting impairment of the vestibular system at both the peripheral and central levels. Bertolini et al. reported that a vestibular deficit, likely attributed to higher-order sensory integration centers including the basal ganglia, was present in PD patients [6]. Pollak et al. [7] reported significantly higher number of abnormal Vestibular Evoked Myogenic Potentials (VEMP) responses in PwP compared to the control group. The study concluded that an abnormal vestibulocollic reflex (VCR) along with an impaired vestibulo-ocular reflex (VOR) might contribute to postural instability in PD patients. Reichert et al. [8] reported absent caloric responses from the vestibular system in PwP, suggesting that impaired peripheral vestibular function could contribute to postural instability and balance.
Evidence for the effect of pharmacological interventions on postural instability and gait in PD is limited. Rocchi et al. concluded that abnormalities in postural sway in patients with Parkinson who took levodopa increased and there was no significant improvement in balance with levodopa treatment [9]. They suggested that these findings might be the result of the effect of levodopa on reducing stiffness without improving postural control. Curtze et al. reported very small gait improvement with levodopa, but worsening of balance and increase in the risk of falls. They concluded that manipulation of the neurotransmitters in the cortical and brainstem circuits rather than dopamine replacement therapy may be needed to improve the condition [10].
Different approaches to improving balance may be adopted based on the underlying pathophysiological mechanism [11,12]. There is evidence for the potential benefit of stimulating the vestibular nerve via Galvanic Vestibular Stimulation (GVS) or vestibular receptors via temperature-induced movement of the endolymph through Caloric Vestibular Stimulation (CVS), which require minimal active participation from the patients, on balance and gait in PD [13]. Possible mechanisms for the effect of therapeutic vestibular stimulation using GVS and CVS for improving balance and locomotion may include enhancing neural plasticity in other brain structures that receive vestibular input. This may help with the central processing of the vestibular information in the process of sensory reweighting, or may enhance sensory sensitivity to the incoming vestibular afferents in basal ganglia [14]. Therefore, the remaining striatal cells may work more efficiently which consequently improve balance and coordination of movement. Pires et al. [15] also found evidence for the potential benefit of direct stimulation of the vestibular system to improve gait and reduce risk of fall in patients with PD. They attributed this effect to the enhanced integration of vestibular, visual and proprioceptive inputs and neuroplastic changes in the brain, helping to rewire neural pathways involved in the control of balance and movement.
Beneficial effects of vestibular stimulation (VS) on balance and gait are consistently reported, but there is an argument that small sample size, methods of presentation of data, inconsistent protocols for the effective use of vestibular stimulation, and baseline differences between the control and the experimental groups may have affected reported results.
The aim of this systematic review was to examine how well vestibular stimulation (VS) can improve balance, postural stability and gait in PwP who were at later stages of the disease according to Hoehn and Yahr (H&Y) scale by reviewing the effect of GVS, CVS and exercises (including vestibular rehabilitation techniques) that naturally stimulate vestibular system (NVS) on these measures.
2. Materials and Methods
The protocol of the study was registered with PROSPERO (CRD42022283898). Scope of the review was decided following a PICO model, and PRISMA guidance and checklist (PRISMA 2020) were used for reporting relevant items of the methodology.
2.1. Criteria for Inclusion of Studies
2.1.1. Population
Studies were eligible for inclusion if they involved people with PD at stages 3 or 4 of H&Y scale, and aged 60 years or older. Reasons for these criteria were that only 4% of people with PD are below 50 years of age and the incidence of PD rapidly increases over the age of 60 years [16], and that loss of balance and slowness of movements appear at stage 3 on H&Y scale.
2.1.2. Experimental Intervention/Training
Studies that tested the effects of the following various forms of VS were included: Galvanic Vestibular Stimulation, Caloric Vestibular Stimulation, and Natural Vestibular Stimulation (natural movements with a vestibular specific component).
2.1.3. Outcome Measures
All studies that investigated the effect of VS on balance and gait outcomes were eligible for inclusion regardless of the presence of retention test. This included biomechanical outcome measures of (i) postural stability/balance related to the behavior of center of pressure (COP) and (ii) spatiotemporal characteristics of gait. Clinical measurements were limited to Berg Balance Score (BBS), Functional Reach Test (FRT), Timed-Up and Go (TUG), Four Square Step Test (FSST), Limits Of Stability (LOS), Biodex Balance System (Static and Dynamic), modified Clinical Test of Sensory Organisation on Balance (mCTSIB), Freezing of gait (FoG) and Anticipatory Postural Adjustment (APA) deficiency.
2.1.4. Study Design
Experimental groups were compared against Control groups consisting of PwP who were “on” or “off” medication and received either no intervention (passive control) or alternative therapeutic interventions (active control).
2.2. Search Strategy and Study Selection
Cochrane, Medline, PEDro, PubMed, Web of Science and Google Scholar databases were searched to find relevant artciles. Four main keywords, i.e., Parkinson’s Disease, Vestibular Stimulation, Gait, and Balance were used to identify records within databases. Keywords of Parkinson’s Disease (Parkinson), vestibular stimulation, balance (balance, stability, postural stability, locomotion, instability, gait and fall) were also identified. Search strategies developed using those terms for each database are shown (see Table 1). Searches were restricted to English language and from 1971 through 8 May 2022. The final search syntax which were reviewed and agreed upon by two researchers (AI and AM) are included in Table 1.

Table 1.
Search syntax employed for different databases.
Retrieved articles from all databases were merged, with duplicates removed. Two independent reviewers (AI and AM) conducted screening of Titles and Abstracts. Full text screening of potentially relevant articles was conducted by AI and checked by AM. The reference lists of eligible studies were hand searched to identify additional publications. Data extraction was conducted by (AI) and another reviewer (AM) checked all data entry. Corresponding authors of individual studies were contacted via email and additional data relevant to the present study inclusion criteria was requested if, for example, these studies included participants who were younger than 60 or had H&Y scores other than 3 or 4.
2.3. Synthesis and Analysis
The ROBINS-I tool [17] was used to assess Risk of Bias (RoB) and appraise articles strength and weaknesses. The tool evaluates seven domains of bias. The first two domains, are related to confounding parameters and selection of participants into the study and bias that might have happened before the start of the interventions. The third domain addresses classification of the interventions and the other four domains address bias introduced after the start of the intervention. RoB for each domain was classified as low, moderate, serious, critical, or having no information and the final overall evaluation for each study was based on the finding from each domain.
3. Results
Out of 1516 studies which were initially retrieved, 6 were qualified for inclusion in the review. Figure 1 illustrates process of identification of the qualified studies.
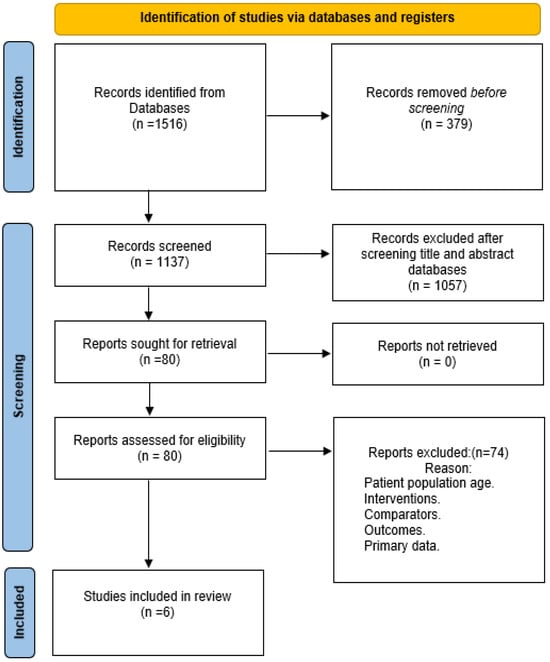
Figure 1.
Process of identification of qualified studies.
Table 2 includes detailed characteristics of the qualified studies. Qualified studies demonstrated improvements in the selected outcome measures following vestibular stimulation employing a range of different interventions.

Table 2.
Study characteristics of the qualified studies.
Reduction in TUG was reported by Wilkinson et al. [21] and Wilkinson et al. [22] who utilised different caloric vestibular stimulation devices. Rossi-Izquierdo et al. [24] applied rehabilitation exercises with computerised dynamic posturography (CDP) to induce vestibular stimulation. Fil-Balkan et al. [18] and Acarer et al. [20] interventions were purely exercise based, but similarly led to significant reduction in TUG with training.
Bonni et al. [23] reported an increased relative duration of the single-legged stance and double-support phases, as well as a reduced duration of the swing phase, after blindfolded balance training compared to routine physiotherapy. This was despite the latter intervention including balance training on an unstable platform and gait training.
Improved BBS scores were reported in the studies by Acarer et al. [20] and Fil-Balkan et al. [18]. Fil-Balkan et al. also noted improved BBS scores in their control group who only received classic physiotherapy for 6 weeks. However, the improvement in the study group, who received sensory motor integration training in addition to classic physiotherapy, was reported to be significantly higher both after the completion of the intervention (6 weeks) and at follow-up (12 weeks). Results for the FRT were similar.
Only one study [22] reported the results of vestibular rehabilitation on Limits of Stability using CDP. The difficulty of training was customised and increased throughout the program, resulting in a significant increase in all LOS related outcome measures after training, which persisted for at least one year.
Changes in the mCTSIB following customised rehabilitation intervention was reported by Acarer et al. [20]. Data reported in their Table 3 (p. 259) suggests decreased sway speed in the rehabilitation group for the eyes open condition on both firm and foam surfaces, indicative of improvement in balance abilities.

Table 3.
Protocol considerations for Target trial.
Protocol consideration for the “Target trial” against which assessment of RoB was completed is presented in Table 3.
Figure 2 shows ROB in different domains across qualified studies, and Figure 3 is a graphical summary of the overall assessment of ROB. Overall and across all domains, 4 studies had serious RoB, and 2 had a moderate RoB.
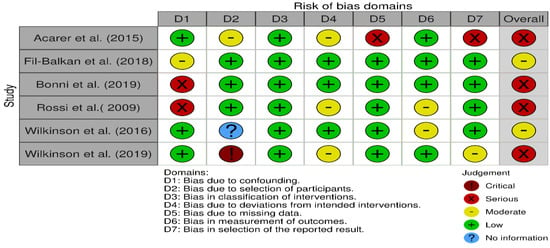
Figure 2.
Risk of Bias in different domains in the qualified studies [18,20,21,22,23,24].
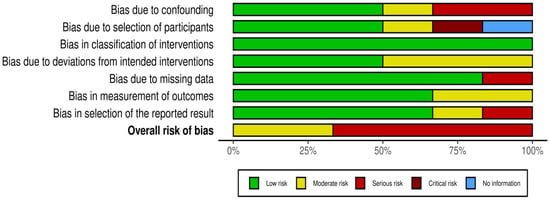
Figure 3.
Graphical summary of the overall RoB.
4. Discussion
This systematic review examined evidence from studies which investigated the effectiveness of different forms of Vestibular Simulation interventions on selected measures of balance and gait in patients with Parkinson’s Disease. Interventions for stimulating vestibular system, which included purposeful movements and exercises (collectively examined under natural vestibular stimulation), or stimulation using external devices (Galvanic and Caloric Vestibular Stimulation) were reported to positively affect different aspects of walking and postural abilities in PwP. Possible mechanisms for the effect of therapeutic vestibular stimulation using GVS and CVS for improving balance and locomotion may include enhancing neural plasticity in other brain structures, to where direct vestibular inputs project. This may help with the central processing of the vestibular information in the process of sensory reweighting [25]. Furthermore, vestibular stimulation may enhance sensory sensitivity to the incoming vestibular afferents in basal ganglia [26]. Therefore, the remaining cells can work with more efficiency which may consequently improve balance and coordination of movement. Consistency of the reported findings, regardless of the methodology employed, suggest that vestibular stimulation can be an effective intervention for enhancing balance and postural steadiness in PD, and it had a positive effect on motor performance after being delivered for minimum two weeks and frequency of 10 sessions for minimum 20 min.
There were methodological issues concerning statistical approaches to analysing the results. Not all studies [18] analysed differences between an experimental and control group at baseline with differences after completion of the study and/or follow-up [27] excluded.
The practice can potentially ignore natural changes over time and regression towards the mean in the outcome measures.
Despite reported overall positive findings, we argue that the presence of biases in the qualified studies, might have also contributed to the observed outcomes. We identified biases in all domains, apart from Domain 3 (bias in the classification of interventions). Addressing causes of bias will be necessary in any future study designs which investigate effect of vestibular stimulation on improving balance or gait.
Domain 5 (bias due to missing data), placed one of the qualified studies [20] at serious RoB. Proportions of participants for whom data was available for the analysis differed substantially across intervention (n = 29) and control groups (n = 11) in the Acarer et al. study. In Fil-Balkan et al. study [18], from patients who started the study (n = 34), only 44% (n = 15) were assessed at the end of follow-up period. Although reasons for patients drop out were not reported, there is a possibility that interventions had adverse effects or were intolerable to participants. Particularly, lost to follow up was more noticeable in studies where participants had to commit to physical activities such as those in Acarer et al. and Fil-Balkan et al. [18,20].
Participants’ general characteristics should be assessed within every study because of their association with balance impairment and falls. For example, effects of high Blood Pressure, Arthritis, Obesity, Vitamin B-12 Deficiency, Stroke, Migraine, Head injury, Diabetes, and any medications for pain should be accounted in all studies related to the assessment of balance and risk of fall as they may confound variables being assessed or show significant differences between (experimental and control) groups at baseline. Three of the reviewed studies [18,23,24] did not consider one or more of these characteristics and were considered as having moderate or severe RoB according to Domain 1 (bias due to confounding) in ROBINS-I. As this domain may affect both interventions and outcomes of interest, we suggest researchers to recognise all known confounding variables, report any differences between groups and control/adjust for these variables, where possible. We have suggested a protocol of considerations for Target Trial that may be adopted and used to this end (Table 3).
Three studies [20,22,24] had moderate RoB (Figure 2) due to departures from intended interventions (Domain 4). In Acarer et al. interventions were intentionally personalised and it may be argued that did not introduce bias in outcomes. However, in the design of observational studies, it is ideal to standardise intervention protocols and avoid co-interventions.
With regard to bias in the measurement of outcomes (Domain 6), majority of studies (4 out of 6) were classified as having low RoB (Figure 2), with only 2 considered at moderate level. Rossi- Izquierdo et al. study was considered as moderate for this domain, because the assessor could not be blinded. Ideally for this domain, assessors should be blinded to interventions status of collected data.
Domain 7 (Bias in selection of the reported results) was difficult to assess for two studies [20,22] as authors did not fully report their protocols.
Finally, bias due to selection of participants into the study (Domain 2) in the overall assessment of RoB, was judged at serious based on one [22] out of 6 studies. The bias could have arisen due to inclusion of prevalent users and/or not including results of all participants after initiation of the intervention.
5. Conclusions
To conclude, this review suggests potential positive effects on aspects of balance and gait by vestibular stimulation in patients with Parkinson’s Disease, but these results should be interpreted with caution. It is evident from the limited number of studies qualified for this review (six) that published data on this topic is extremely scarce. Importantly, these studies employed various interventions for vestibular stimulation, making it difficult to reach firm conclusions about the best therapeutic approach, identify potential “best” responders to vestibular stimulation, or understand the underlying mechanisms of action. Although the results suggest improvements in gait, gait control, balance, postural control, and mobility [18,20,21,22,23,24], drawing definitive conclusions remains challenging. Consequently, we argue that there is a need for further high-quality research to better understand the effectiveness of vestibular stimulation for improving balance and gait, and specific conditions under which and populations in whom vestibular-based interventions are most effective. Long-term sustainability of the observed benefits should also be examined. Following guidelines of ROBINS-I tool, and recommended protocol for creating a target trial [17], the proposed target trial (Table 3) can be considered for designing studies in which the effect of vestibular stimulation for improving aspects of gait and locomotion are examined.
As our understanding of the vestibular system and its interactions with other sensory systems expands, new opportunities for use of vestibular stimulation emerge. Researchers are currently exploring its potential in areas such as sports performance enhancement [28], motion sickness prevention [29], cognitive rehabilitation [30], and treatment of mental health [31]. This systematic review provides evidence supporting the effectiveness of vestibular stimulation interventions in reducing risk of falls by affecting measures which indirectly show improvement in balance and walking abilities, although RoB in different domains were present and should be considered alongside reported results. Vestibular stimulation intervention offers a promising approach to traditional therapeutic modalities and may be considered as an addition to treatment plans in clinical and community settings. Future research should focus on strengthening the evidence and optimising protocols for the implementation of vestibular stimulation as part of patients’ therapeutic interventions.
Author Contributions
Conceptualization, A.M. and A.I.-N.; methodology, A.M. and A.I.-N.; formal analysis, A.M. and A.I.-N.; writing—original draft preparation, A.I.-N.; writing—review and editing, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.H.F.; Ferreira-Peruzzo, S.A.; Ribas, D.I.R.; Franklin, G.L.; Teive, H.A.G. Imbalance and gait impairment in Parkinson’s disease: Discussing postural instability and ataxia. Neurol. Sci. 2024, 45, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism. Neurology 1967, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.A.; Day, B.L.; Marsden, C.D. Vestibular Induced Postural Responses in Parkinson’s Disease. Brain 1993, 116 Pt 5, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Schindlbeck, K.A.; Naumann, W.; Maier, A.; Ehlen, F.; Marzinzik, F.; Klostermann, F. Disturbance of Verticality Perception and Postural Dysfunction in Parkinson’s Disease. Acta Neurol. Scand. 2018, 137, 212–217. [Google Scholar] [CrossRef]
- Bertolini, G.; Wicki, A.; Baumann, C.R.; Straumann, D.; Palla, A. Impaired Tilt Perception in Parkinson’s Disease: A Central Vestibular Integration Failure. PLoS ONE 2015, 10, e0124253. [Google Scholar] [CrossRef]
- Pollak, L.; Prohorov, T.; Kushnir, M.; Clinique, M.R.-N. Vestibulocervical reflexes in idiopathic Parkinson disease. Neurophysiol. Clin. Clin. Neurophysiol. 2009, 39, 235–240. [Google Scholar] [CrossRef]
- Reichert, W.H.; Doolittle, J.; McDowell, F.H. Vestibular Dysfunction in Parkinson Disease. Neurology 1982, 32, 1133–1138. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Horak, F.B. Effects of Deep Brain Stimulation and Levodopa on Postural Sway in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatr. 2002, 73, 267–274. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Koller, W.C.; Glatt, S.; Vetere-Overfield, B.; Hassanein, R. Falls and Parkinson’s Disease. Clin. Neuropharmacol. 1989, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.; Hadi, Z.; Prendergast, M.; Ciocca, M.; Saad, A.R.; Pondeca, Y.; Tai, Y.; Scott, G.; Seemungal, B.M. The Effect of Galvanic Vestibular Stimulation on Postural Balance in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2022, 442, 120414. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.K.; Basumatary, B.; Bansal, R.; Sahani, A.K. Techniques for the Detection and Management of Freezing of Gait in Parkinson’s Disease—A Systematic Review and Future Perspectives. MethodsX 2023, 10, 102106. [Google Scholar] [CrossRef] [PubMed]
- Heumann, R.; Moratalla, R.; Herrero, M.T.; Chakrabarty, K.; Drucker-Colín, R.; Garcia-Montes, J.R.; Simola, N.; Morelli, M. Dyskinesia in Parkinson’s Disease: Mechanisms and Current Non-Pharmacological Interventions. J. Neurochem. 2014, 130, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.P.B.d.Á.; Silva, T.R.; Torres, M.S.; Diniz, M.L.; Tavares, M.C.; Gonçalves, D.U. Galvanic Vestibular Stimulation and Its Applications: A Systematic Review. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S3), 202–211. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Bloch, D.A.; Nelson, L.M. Incidence of Parkin-son’s Disease: Variation by Age, Gender, and Race/Ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, I4919. [Google Scholar] [CrossRef]
- Fil-Balkan, A.; Salci, Y.; Keklicek, H.; Armutlu, K.; Aksoy, S.; Kayihan, H.; Elibol, B. Sensorimotor Integration Training in Parkinson’s Disease. Neurosci. J. 2018, 23, 208–215. [Google Scholar] [CrossRef]
- Cattaneo, D.; Jonsdottir, J.; Zocchi, M.; Regola, A. Effects of balance exercises on people with multiple sclerosis: A pilot study. Clin Rehabil. 2007, 21, 771–781. [Google Scholar] [CrossRef]
- Acarer, A.; Karapolat, H.; Celebisoy, N.; Ozgen, G.; Colakoglu, Z. Is Customized Vestibular Rehabilitation Effective in Patients with Parkinson’s? NeuroRehabilitation 2015, 37, 255–262. [Google Scholar] [CrossRef]
- Wilkinson, D.; Podlewska, A.; Sakel, M. A Durable Gain in Motor and Non-Motor Symptoms of Parkinson’s Disease Following Repeated Caloric Vestibular Stimulation: A Single-Case Study. NeuroRehabilitation 2016, 38, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Podlewska, A.; Banducci, S.E.; Pellat-Higgins, T.; Slade, M.; Bodani, M.; Sakel, M.; Smith, L.; LeWitt, P.; Ade, K. Caloric vestibular stimulation for the management of motor and non-motor symptoms in parkinson’s disease: Intention-to-treat data. Data Brief 2019, 25, 104228. [Google Scholar] [CrossRef] [PubMed]
- Bonnì, S.; Ponzo, V.; Tramontano, M.; Martino Cinnera, A.; Caltagirone, C.; Koch, G.; Peppe, A. Neurophysiological and Clinical Effects of Blindfolded Balance Training (BBT) in Parkinson’s Disease Patients: A Preliminary Study. Eur. J. Phys. Rehabil. Med. 2019, 55, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Izquierdo, M.; Soto-Varela, A.; Santos-Pérez, S.; Sesar-Ignacio, A.; Labella-Caballero, T. Vestibular Rehabilitation with Computerised Dynamic Posturography in Patients with Parkinson’s Disease: Improving Balance Impairment. Disabil. Rehabil. 2009, 31, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; McGregor, H.R.; Hass, C.J.; Pasternak, O.; Seidler, R.D. Sensory System-Specific Associations between Brain Structureand Balance. Neurobiol. Aging 2022, 119, 102–116. [Google Scholar] [CrossRef]
- Lee, S.; Liu, A.; McKeown, M.J. Current Perspectives on Galvanic Vestibular Stimulation in theTreatment of Parkinson’s Disease. Expert Rev. Neurother. 2021, 21, 405–418. [Google Scholar] [CrossRef]
- Bland, M.J.; Altman, D.G. Comparisons against Baseline within Randomised Groups Are Often Used and Can Be Highly Misleading. Trials 2011, 12, 264. [Google Scholar] [CrossRef]
- Tsang, W.W.N. The Effect of Vestibular Stimulation on Eye-Hand Coordination and Postural Control in Elite Basketball Players. Am. J. Sports Sci. 2014, 2, 17. [Google Scholar] [CrossRef]
- Clément, G.; Deguine, O.; Parant, M.; Costes-Salon, M.C.; Vasseur-Clausen, P.; Pavy-Le Traon, A. Effects of Cosmonaut Vestibular Training on Vestibular Function Prior to Spaceflight. Eur. J. Appl. Physiol. 2001, 85, 539–545. [Google Scholar] [CrossRef]
- Monzani, D.; Genovese, E.; Marrara, A.; Presutti, L.; Gherpelli, C.; Panzetti, P.; Forghieri, M. Stimulation of the Cholinergic Neurotransmissions Enhances the Efficacy of Vestibular Rehabilitation. Acta Otorhinolaryngol. Ital. 2010, 30, 11–19. [Google Scholar]
- Gerretsen, P.; Pothier, D.D.; Falls, C.; Armstrong, M.; Balakumar, T.; Uchida, H.; Mamo, D.C.; Pollock, B.G.; Graff-Guerrero, A. Vestibular Stimulation Improves Insight into Illness in Schizophrenia Spectrum Disorders HHS Public Access. Psychiatry Res. 2017, 251, 333–341. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).