Heart Rate Variability Applications in Strength and Conditioning: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Heart Rate Variability Measurement
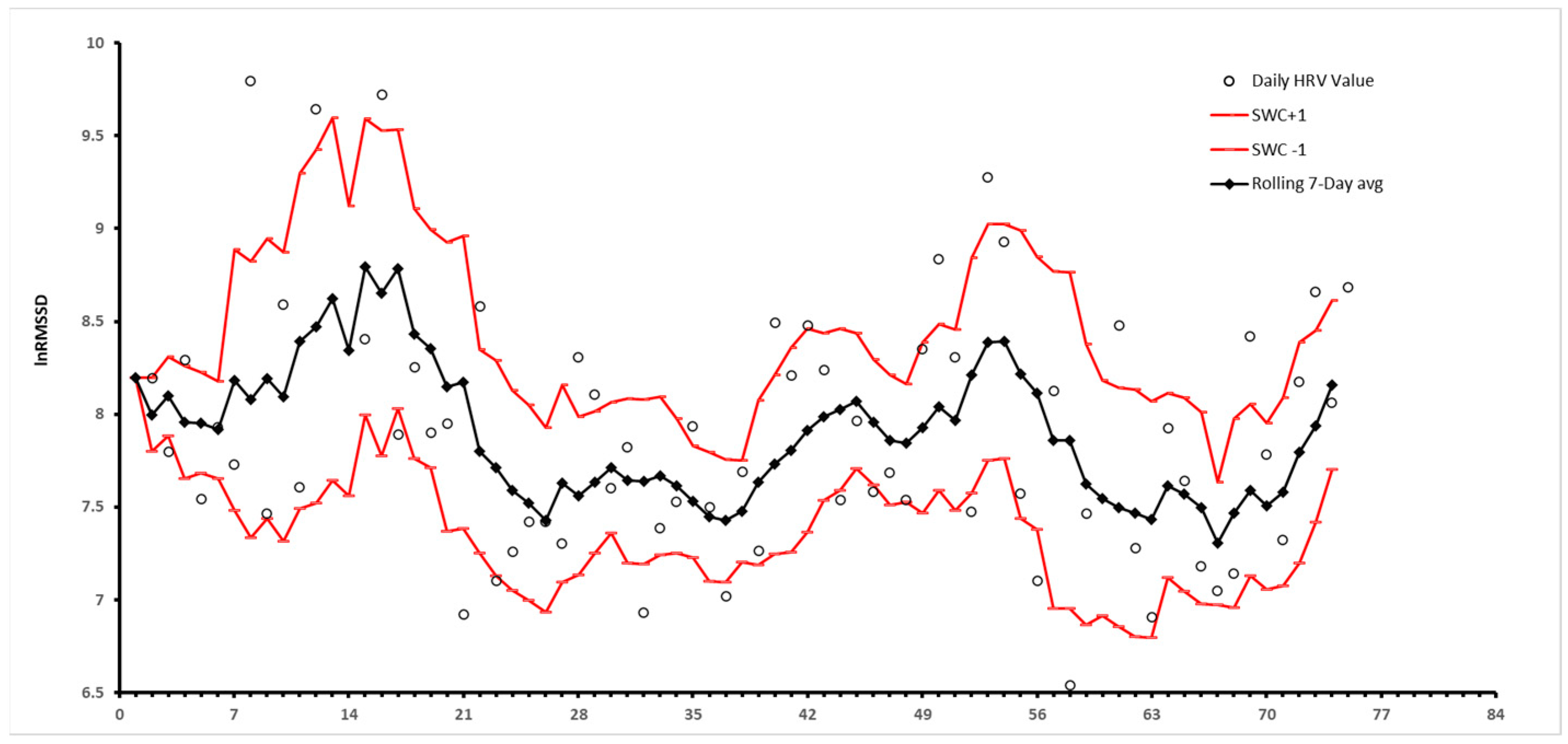
Measurement Considerations
4. Heart Rate Variability and Training Adaptations
4.1. Aerobic/Endurance-Trained Athletes
4.2. Strength/Resistance-Trained Athletes
4.3. Untrained or Moderately Trained Individuals
5. HRV and Overreaching/Overtraining
5.1. Aerobic/Endurance-Trained Athletes
5.2. Strength/Resistance-Trained Athletes
5.3. Untrained or Moderately Trained Individuals
6. Heart Rate Variability-Guided Programming
6.1. Aerobic/Endurance-Trained Athletes
6.2. Strength/Resistance-Trained Athletes
6.3. Untrained or Moderately Trained Individuals
7. Factors Interacting with Heart Rate Variability
8. Preliminary Guidelines
- In the scope of strength and conditioning, relative differences to an individual’s own baseline are the best interpretive level. Interpreting further may have limited use.
- HRV is a helpful metric to assess training status, adaptability, and recovery of trained athletes in a variety of sports and fitness activities as well as untrained individuals. Coaches can utilize this information to evaluate an athlete’s preparedness for a difficult training day. Regardless of a high or low HRV, stability of HRV within the smallest worthwhile change window (SWC) is considered a long-term goal of training.
- Barring all other contextual factors, decreasing HRV may be a sign of overreaching and/or overtraining syndrome. In aerobic athletes, HRV metrics may not be as sensitive to overreaching and/or overtraining syndrome and may be best utilized in addition to secondary markers. Further investigations are needed to fully understand the autonomic response to overreaching and overtraining syndrome and which measures are best to predict or identify these changes.
- There is utility to HRV-guided programming compared to predefined programming in several sports and athletic activities, especially in training programs with an aerobic or endurance component.
- There are many factors that influence an individual’s HRV, including but not limited to various physiological and psychological disorders. Professionals interpreting HRV data should ensure they only act within their appropriate scope of practice and refer to an appropriate healthcare professional as needed.
9. Limitations and Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef]
- Lundstrom, C.J.; Foreman, N.A.; Biltz, G. Practices and Applications of Heart Rate Variability Monitoring in Endurance Athletes. Int. J. Sports Med. 2023, 44, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Vega-Martinez, G.; Ramos-Becerril, F.J.; Mirabent-Amor, D.; Franco-Sánchez, J.G.; Vera-Hernández, A.; Alvarado-Serrano, A.; Leija-Salas, L. Analysis of heart rate variability and its application in sports medicine: A review. In Proceedings of the 2018 Global Medical Engineering Physics Exchanges/Pan American Health Care Exchanges (GMEPE/PAHCE), Porto, Portugal, 19–24 March 2018. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public. Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Ernst, G. Heart-Rate Variability-More than Heart Beats? Front. Public. Health 2017, 5, 240. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R. Evaluating Individual Training Adaptation With Smartphone-Derived Heart Rate Variability in a Collegiate Female Soccer Team. J. Strength. Cond. Res. 2016, 30, 378–385. [Google Scholar] [CrossRef]
- Altini, M.; Berk, S.; Jansen, T.W. Heart rate variability during the first week of an altitude training camp is representative of individual training adaptation at the end of the camp in elite triathletes. Sports Perform. Sci. Rep. 2020, 125, 60. [Google Scholar]
- Prinsloo, G.E.; Rauch, H.G.; Derman, W.E. A brief review and clinical application of heart rate variability biofeedback in sports, exercise, and rehabilitation medicine. Phys. Sportsmed. 2014, 42, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Plews, D.; Froelicher, V. Heart Rate Variability: An Old Metric with New Meaning in the Era of using mHealth Technologies for Health and Exercise Training Guidance. Part One: Physiology and Methods. Arrhythm. Electrophysiol. Rev. 2018, 7, 193–198. [Google Scholar] [CrossRef]
- Saul, J.P.; Albrecht, P.; Berger, R.D.; Cohen, R.J. Analysis of long term heart rate variability: Methods, 1/f scaling and implications. Comput. Cardiol. 1988, 14, 419–422. [Google Scholar]
- Sherman, S.R.; Holmes, C.J.; Hornikel, B.; MacDonald, H.V.; Fedewa, M.V.; Esco, M.R. Heart-Rate Variability Recording Time and Performance in Collegiate Female Rowers. Int. J. Sports Physiol. Perform. 2021, 16, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Bentho, O.; Park, M.Y.; Sharabi, Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 2011, 96, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Liu, R.; Shen, Z. Effects of slow breathing rate on blood pressure and heart rate variabilities. Int. J. Cardiol. 2013, 169, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Nunan, D.; Sandercock, G.R.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Fatisson, J.; Oswald, V.; Lalonde, F. Influence diagram of physiological and environmental factors affecting heart rate variability: An extended literature overview. Heart Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef] [PubMed]
- Soares-Miranda, L.; Negrao, C.E.; Antunes-Correa, L.M.; Nobre, T.S.; Silva, P.; Santos, R.; Vale, S.; Mota, J. High levels of C-reactive protein are associated with reduced vagal modulation and low physical activity in young adults. Scand. J. Med. Sci. Sports 2012, 22, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, A.M.; Hautala, A.J.; Kinnunen, H.; Tulppo, M.P. Endurance training guided individually by daily heart rate variability measurements. Eur. J. Appl. Physiol. 2007, 101, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, A.M.; Hautala, A.J.; Kinnunen, H.; Nissila, J.; Virtanen, P.; Karjalainen, J.; Tulppo, M.P. Daily exercise prescription on the basis of HR variability among men and women. Med. Sci. Sports Exerc. 2010, 42, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Evaluating training adaptation with heart-rate measures: A methodological comparison. Int. J. Sports Physiol. Perform. 2013, 8, 688–691. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Heart rate variability in elite triathletes, is variation in variability the key to effective training? A case comparison. Eur. J. Appl. Physiol. 2012, 112, 3729–3741. [Google Scholar] [CrossRef]
- Schafer, A.; Vagedes, J. How accurate is pulse rate variability as an estimate of heart rate variability? A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Scott, B.; Altini, M.; Wood, M.; Kilding, A.E.; Laursen, P.B. Comparison of Heart-Rate-Variability Recording With Smartphone Photoplethysmography, Polar H7 Chest Strap, and Electrocardiography. Int. J. Sports Physiol. Perform. 2017, 12, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Hailu, R. Fitbits and Other Wearables May Not Accurately Track Heart Rates in People of Color. STAT. 2019. Available online: https://www.statnews.com/2019/07/24/fitbit-accuracy-dark-skin/ (accessed on 30 January 2024).
- Boullosa, D.A.; Abreu, L.; Nakamura, F.Y.; Munoz, V.E.; Dominguez, E.; Leicht, A.S. Cardiac autonomic adaptations in elite Spanish soccer players during preseason. Int. J. Sports Physiol. Perform. 2013, 8, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Vesterinen, V.; Hakkinen, K.; Hynynen, E.; Mikkola, J.; Hokka, L.; Nummela, A. Heart rate variability in prediction of individual adaptation to endurance training in recreational endurance runners. Scand. J. Med. Sci. Sports 2013, 23, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Fuller, J.T.; Thomson, R.L.; Davison, K.; Robertson, E.Y.; Buckley, J.D. Monitoring Athletic Training Status Through Autonomic Heart Rate Regulation: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1461–1486. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Haugen, O.; Kuffel, E. Autonomic recovery after exercise in trained athletes: Intensity and duration effects. Med. Sci. Sports Exerc. 2007, 39, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Hebisz, R.; Hebisz, P.; Danek, N.; Michalik, K.; Zaton, M. Predicting Changes in Maximal Oxygen Uptake in Response to Polarized Training (Sprint Interval Training, High-Intensity Interval Training, and Endurance Training) in Mountain Bike Cyclists. J. Strength. Cond. Res. 2022, 36, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
- Chalencon, S.; Busso, T.; Lacour, J.R.; Garet, M.; Pichot, V.; Connes, P.; Gabel, C.P.; Roche, F.; Barthelemy, J.C. A model for the training effects in swimming demonstrates a strong relationship between parasympathetic activity, performance and index of fatigue. PLoS ONE 2012, 7, e52636. [Google Scholar] [CrossRef] [PubMed]
- Garet, M.; Tournaire, N.; Roche, F.; Laurent, R.; Lacour, J.R.; Barthelemy, J.C.; Pichot, V. Individual Interdependence between nocturnal ANS activity and performance in swimmers. Med. Sci. Sports Exerc. 2004, 36, 2112–2118. [Google Scholar] [CrossRef]
- Maggioni, M.A.; Rundfeldt, L.C.; Gunga, H.C.; Joerres, M.; Merati, G.; Steinach, M. The Advantage of Supine and Standing Heart Rate Variability Analysis to Assess Training Status and Performance in a Walking Ultramarathon. Front. Physiol. 2020, 11, 731. [Google Scholar] [CrossRef]
- Flatt, A.A.; Hornikel, B.; Nakamura, F.Y.; Esco, M.R. Effect of Competitive Status and Experience on Heart Rate Variability Profiles in Collegiate Sprint-Swimmers. J. Strength. Cond. Res. 2022, 36, 2898–2904. [Google Scholar] [CrossRef]
- Santos, I.F.; Lemos, L.K.; Biral, T.M.; de Cavina, A.P.S.; Pizzo Junior, E.; Teixeira Filho, C.A.T.; Vendrame, J.W.; Vanderlei, F.M. Relationship between heart rate variability and performance in eccentric training with blood flow restriction. Clin. Physiol. Funct. Imaging 2022, 42, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Lucini, D.; Volterrani, M.; Casasco, M.; Salvati, A.; Gianfelici, A.; Di Gianfrancesco, A.; Urso, A.; Manzi, V. Autonomic nervous system responses to strength training in top-level weight lifters. Physiol. Rep. 2019, 7, e14233. [Google Scholar] [CrossRef] [PubMed]
- Tornberg, J.; Ikaheimo, T.M.; Kiviniemi, A.; Pyky, R.; Hautala, A.; Mantysaari, M.; Jamsa, T.; Korpelainen, R. Physical activity is associated with cardiac autonomic function in adolescent men. PLoS ONE 2019, 14, e0222121. [Google Scholar] [CrossRef] [PubMed]
- Fohr, T.; Pietila, J.; Helander, E.; Myllymaki, T.; Lindholm, H.; Rusko, H.; Kujala, U.M. Physical activity, body mass index and heart rate variability-based stress and recovery in 16 275 Finnish employees: A cross-sectional study. BMC Public. Health 2016, 16, 701. [Google Scholar] [CrossRef] [PubMed]
- Pope, Z.C.; Gabriel, K.P.; Whitaker, K.M.; Chen, L.Y.; Schreiner, P.J.; Jacobs, D.R., Jr.; Sternfeld, B.; Carr, J.J.; Lloyd-Jones, D.M.; Pereira, M.A. Association between Objective Activity Intensity and Heart Rate Variability: Cardiovascular Disease Risk Factor Mediation (CARDIA). Med. Sci. Sports Exerc. 2020, 52, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Tulppo, M.P.; Makikallio, T.H.; Seppanen, T.; Laukkanen, R.T.; Huikuri, H.V. Vagal modulation of heart rate during exercise: Effects of age and physical fitness. Am. J. Physiol. 1998, 274, H424–H429. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L.; Bouhaddi, M.; Perrey, S.; Cappelle, S.; Henriet, M.T.; Wolf, J.P.; Rouillon, J.D.; Regnard, J. Decrease in heart rate variability with overtraining: Assessment by the Poincare plot analysis. Clin. Physiol. Funct. Imaging 2004, 24, 10–18. [Google Scholar] [CrossRef]
- Dupuy, O.; Bherer, L.; Audiffren, M.; Bosquet, L. Night and postexercise cardiac autonomic control in functional overreaching. Appl. Physiol. Nutr. Metab. 2013, 38, 200–208. [Google Scholar] [CrossRef]
- Baumert, M.; Brechtel, L.; Lock, J.; Hermsdorf, M.; Wolff, R.; Baier, V.; Voss, A. Heart rate variability, blood pressure variability, and baroreflex sensitivity in overtrained athletes. Clin. J. Sport. Med. 2006, 16, 412–417. [Google Scholar] [CrossRef]
- Bosquet, L.; Papelier, Y.; Léger, L.; Legros, P. Night heart rate variability during overtraining in male endurance athletes. J. Sports Med. Phys. Fitness 2003, 43, 506–512. [Google Scholar] [PubMed]
- Scott, J.M.; Esch, B.T.; Shave, R.; Warburton, D.E.; Gaze, D.; George, K. Cardiovascular consequences of completing a 160-km ultramarathon. Med. Sci. Sports Exerc. 2009, 41, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, Y.; Pichon, A.; Schaal, K.; Schmitt, L.; Louis, J.; Gueneron, J.; Vidal, P.P.; Hausswirth, C. Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med. Sci. Sports Exerc. 2013, 45, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Huggins, R.A.; Curtis, R.M.; Benjamin, C.L.; Adams, W.M.; Looney, D.P.; West, C.A.; Casa, D.J. Relationship Between Heart Rate Variability and Acute:Chronic Load Ratio Throughout a Season in NCAA D1 Men’s Soccer Players. J. Strength. Cond. Res. 2021, 35, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; He, Z.H.; Zhao, J.X.; Tao, D.L.; Xu, K.Y.; Earnest, C.P.; Mc Naughton, L.R. Heart rate variability threshold values for early-warning nonfunctional overreaching in elite female wrestlers. J. Strength. Cond. Res. 2013, 27, 1511–1519. [Google Scholar] [CrossRef]
- Buchheit, M.; Racinais, S.; Bilsborough, J.C.; Bourdon, P.C.; Voss, S.C.; Hocking, J.; Cordy, J.; Mendez-Villanueva, A.; Coutts, A.J. Monitoring fitness, fatigue and running performance during a pre-season training camp in elite football players. J. Sci. Med. Sport. 2013, 16, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Esco, M.R.; Allen, J.R.; Robinson, J.B.; Earley, R.L.; Fedewa, M.V.; Bragg, A.; Keith, C.M.; Wingo, J.E. Heart Rate Variability and Training Load Among National Collegiate Athletic Association Division 1 College Football Players Throughout Spring Camp. J. Strength. Cond. Res. 2018, 32, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Buchheit, M. Day-to-Day Heart-Rate Variability Recordings in World-Champion Rowers: Appreciating Unique Athlete Characteristics. Int. J. Sports Physiol. Perform. 2017, 12, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Egan-Shuttler, J.D.; Edmonds, R.; Ives, S.J. The Efficacy of Heart Rate Variability in Tracking Travel and Training Stress in Youth Female Rowers: A Preliminary Study. J. Strength. Cond. Res. 2020, 34, 3293–3300. [Google Scholar] [CrossRef]
- Liu, H.W.; Cheng, H.C.; Tsai, S.H.; Shao, Y.T. Effects of acute resistance exercise with different loads on appetite, appetite hormones and autonomic nervous system responses in healthy young men. Appetite 2023, 182, 106428. [Google Scholar] [CrossRef]
- Marasingha-Arachchige, S.U.; Rubio-Arias, J.A.; Alcaraz, P.E.; Chung, L.H. Factors that affect heart rate variability following acute resistance exercise: A systematic review and meta-analysis. J. Sport. Health Sci. 2022, 11, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Wiewelhove, T.; Raeder, C.; Flatt, A.A.; Hoos, O.; Hottenrott, L.; Schumbera, O.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; et al. Heart Rate Variability Monitoring During Strength and High-Intensity Interval Training Overload Microcycles. Front. Physiol. 2019, 10, 582. [Google Scholar] [CrossRef]
- Pareja-Blanco, F.; Rodriguez-Rosell, D.; Sanchez-Medina, L.; Ribas-Serna, J.; Lopez-Lopez, C.; Mora-Custodio, R.; Yanez-Garcia, J.M.; Gonzalez-Badillo, J.J. Acute and delayed response to resistance exercise leading or not leading to muscle failure. Clin. Physiol. Funct. Imaging 2017, 37, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Badillo, J.J.; Rodriguez-Rosell, D.; Sanchez-Medina, L.; Ribas, J.; Lopez-Lopez, C.; Mora-Custodio, R.; Yanez-Garcia, J.M.; Pareja-Blanco, F. Short-term Recovery Following Resistance Exercise Leading or not to Failure. Int. J. Sports Med. 2016, 37, 295–304. [Google Scholar] [CrossRef]
- Flatt, A.A.; Globensky, L.; Bass, E.; Sapp, B.L.; Riemann, B.L. Heart Rate Variability, Neuromuscular and Perceptual Recovery Following Resistance Training. Sports 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Thamm, A.; Freitag, N.; Figueiredo, P.; Doma, K.; Rottensteiner, C.; Bloch, W.; Schumann, M. Can Heart Rate Variability Determine Recovery Following Distinct Strength Loadings? A Randomized Cross-Over Trial. Int. J. Environ. Res. Public. Health 2019, 16, 4353. [Google Scholar] [CrossRef]
- Williams, S.; Booton, T.; Watson, M.; Rowland, D.; Altini, M. Heart Rate Variability is a Moderating Factor in the Workload-Injury Relationship of Competitive CrossFitTM Athletes. J. Sports Med. Phys. Fitness 2017, 16, 443–449. [Google Scholar]
- Myllymaki, T.; Rusko, H.; Syvaoja, H.; Juuti, T.; Kinnunen, M.L.; Kyrolainen, H. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur. J. Appl. Physiol. 2012, 112, 801–809. [Google Scholar] [CrossRef]
- DeBlauw, J.A.; Drake, N.B.; Kurtz, B.K.; Crawford, D.A.; Carper, M.J.; Wakeman, A.; Heinrich, K.M. High-Intensity Functional Training Guided by Individualized Heart Rate Variability Results in Similar Health and Fitness Improvements as Predetermined Training with Less Effort. J. Funct. Morphol. Kinesiol. 2021, 6, 102. [Google Scholar] [CrossRef]
- Vesterinen, V.; Nummela, A.; Heikura, I.; Laine, T.; Hynynen, E.; Botella, J.; Hakkinen, K. Individual Endurance Training Prescription with Heart Rate Variability. Med. Sci. Sports Exerc. 2016, 48, 1347–1354. [Google Scholar] [CrossRef]
- Gisselman, A.S.; Baxter, G.D.; Wright, A.; Hegedus, E.; Tumilty, S. Musculoskeletal overuse injuries and heart rate variability: Is there a link? Med. Hypotheses 2016, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Allen, J.R.; Keith, C.M.; Martinez, M.W.; Esco, M.R. Season-Long Heart-Rate Variability Tracking Reveals Autonomic Imbalance in American College Football Players. Int. J. Sports Physiol. Perform. 2021, 16, 1834–1843. [Google Scholar] [CrossRef]
- Nuuttila, O.P.; Nikander, A.; Polomoshnov, D.; Laukkanen, J.A.; Hakkinen, K. Effects of HRV-Guided vs. Predetermined Block Training on Performance, HRV and Serum Hormones. Int. J. Sports Med. 2017, 38, 909–920. [Google Scholar] [CrossRef]
- Javaloyes, A.; Sarabia, J.M.; Lamberts, R.P.; Plews, D.; Moya-Ramon, M. Training Prescription Guided by Heart Rate Variability Vs. Block Periodization in Well-Trained Cyclists. J. Strength. Cond. Res. 2020, 34, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Duking, P.; Zinner, C.; Reed, J.L.; Holmberg, H.C.; Sperlich, B. Predefined vs data-guided training prescription based on autonomic nervous system variation: A systematic review. Scand. J. Med. Sci. Sports 2020, 30, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Willis, S.J.; Fardel, A.; Coulmy, N.; Millet, G.P. Live high-train low guided by daily heart rate variability in elite Nordic-skiers. Eur. J. Appl. Physiol. 2018, 118, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Heart-rate variability and training-intensity distribution in elite rowers. Int. J. Sports Physiol. Perform. 2014, 9, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Paz, G.A.; Iglesias-Soler, E.; Willardson, J.M.; Maia, M.F.; Miranda, H. Postexercise Hypotension and Heart Rate Variability Responses Subsequent to Traditional, Paired Set, and Superset Resistance Training Methods. J. Strength. Cond. Res. 2019, 33, 2433–2442. [Google Scholar] [CrossRef]
- Rodriguez, D.; Nakazato, K.; Fleck, S.; Pontes, L.F., Jr.; Charro, M.A.; Alegretti, G.; Bocallini, D.S.; Figueira, A., Jr. Strength Training Methods Does Not Affect Post-Exercise Hypotension and Heart Rate Variability. J. Exerc. Physiol. Online 2017, 20, 36–51. [Google Scholar]
- De Oliveira, R.M.; Ugrinowitsch, C.; Kingsley, J.D.; Da Silva, D.G.; Bittencourt, D.; Caruso, F.R.; Borghi-Silva, A.; Libardi, C.A. Effect of individualized resistance training prescription with heart rate variability on individual muscle hypertrophy and strength responses. Eur. J. Sport. Sci. 2019, 19, 1092–1100. [Google Scholar] [CrossRef]
- da Silva, D.F.; Ferraro, Z.M.; Adamo, K.B.; Machado, F.A. Endurance Running Training Individually Guided by HRV in Untrained Women. J. Strength. Cond. Res. 2019, 33, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Juarez, M.; Gonzalez-Gomez, G.H.; Echeverria, J.C.; Lerma, C. Revisiting nonlinearity of heart rate variability in healthy aging. Sci. Rep. 2023, 13, 13185. [Google Scholar] [CrossRef] [PubMed]
- Schlagintweit, J.; Laharnar, N.; Glos, M.; Zemann, M.; Demin, A.V.; Lederer, K.; Penzel, T.; Fietze, I. Effects of sleep fragmentation and partial sleep restriction on heart rate variability during night. Sci. Rep. 2023, 13, 6202. [Google Scholar] [CrossRef] [PubMed]
- Pulopulos, M.M.; Vanderhasselt, M.A.; De Raedt, R. Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology 2018, 94, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Hall, M.; Sollers, J.J., 3rd; Fischer, J.E. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int. J. Psychophysiol. 2006, 59, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.N.; Wang, J.; Liu, H.Y.; Wu, D.; Liao, S.X. Nicotine Ingestion Reduces Heart Rate Variability in Young Healthy Adults. Biomed. Res. Int. 2022, 2022, 4286621. [Google Scholar] [CrossRef] [PubMed]
- Macartney, M.J.; Meade, R.D.; Notley, S.R.; Herry, C.L.; Seely, A.J.E.; Kenny, G.P. Fluid Loss during Exercise-Heat Stress Reduces Cardiac Vagal Autonomic Modulation. Med. Sci. Sports Exerc. 2020, 52, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Danieletto, M.; Tomalin, L.; Choi, K.H.; Zweig, M.; Golden, E.; Kaur, S.; Helmus, D.; Biello, A.; Pyzik, R.; et al. Use of Physiological Data From a Wearable Device to Identify SARS-CoV-2 Infection and Symptoms and Predict COVID-19 Diagnosis: Observational Study. J. Med. Internet Res. 2021, 23, e26107. [Google Scholar] [CrossRef] [PubMed]
- Hajduczok, A.G.; DiJoseph, K.M.; Bent, B.; Thorp, A.K.; Mullholand, J.B.; MacKay, S.A.; Barik, S.; Coleman, J.J.; Paules, C.I.; Tinsley, A. Physiologic Response to the Pfizer-BioNTech COVID-19 Vaccine Measured Using Wearable Devices: Prospective Observational Study. JMIR Form. Res. 2021, 5, e28568. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.N.; Ellis, R.J.; Hillecke, T.K.; Thayer, J.F. Heart rate variability and experimentally induced pain in healthy adults: A systematic review. Eur. J. Pain. 2014, 18, 301–314. [Google Scholar] [CrossRef]
- Koenig, J.; Loerbroks, A.; Jarczok, M.N.; Fischer, J.E.; Thayer, J.F. Chronic Pain and Heart Rate Variability in a Cross-Sectional Occupational Sample: Evidence for Impaired Vagal Control. Clin. J. Pain. 2016, 32, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Seidman, L.C.; Tsao, J.C.; Lung, K.C.; Zeltzer, L.K.; Naliboff, B.D. Heart rate variability as a biomarker for autonomic nervous system response differences between children with chronic pain and healthy control children. J. Pain. Res. 2013, 6, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.L.; Hellwinkel, J.E.; Montjoy, M.; Levi, M.; Tu, B.; Noble, J.M.; Ahmad, C.S.; Bottiglieri, T.S. Change in Heart Rate Variability after Concussion in a Collegiate Soccer Player. Neurotrauma Rep. 2020, 1, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Wilkerson, G.B.; Allen, J.R.; Keith, C.M.; Esco, M.R. Daily Heart Rate Variability before and after Concussion in an American College Football Player. Sports 2019, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Senthinathan, A.; Mainwaring, L.M.; Hutchison, M. Heart Rate Variability of Athletes Across Concussion Recovery Milestones: A Preliminary Study. Clin. J. Sport. Med. 2017, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Khandoker, A.H.; Alkhodari, M.; Hadjileontiadis, L.J.; Jelinek, H.F. Investigating the effects of beta-blockers on circadian heart rhythm using heart rate variability in ischemic heart disease with preserved ejection fraction. Sci. Rep. 2023, 13, 5828. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, O.P.; Praskurnichiĭ, E.A.; Savel’eva, S.A. Lisinopril: Antihypertensive activity, effects on heart rhythm variability and carbohydrate metabolism in patients with metabolic syndrome. Terapevticheskii Arkhiv 2008, 80, 37–41. [Google Scholar]
- Ahokas, E.K.; Hanstock, H.G.; Lofberg, I.; Nyman, M.; Wenning, P.; Kyrolainen, H.; Mikkonen, R.S.; Ihalainen, J.K. Nocturnal Heart Rate Variability in Women Discordant for Hormonal Contraceptive Use. Med. Sci. Sports Exerc. 2023, 55, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef]
- Stein, P.K.; Barzilay, J.I.; Domitrovich, P.P.; Chaves, P.M.; Gottdiener, J.S.; Heckbert, S.R.; Kronmal, R.A. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: The Cardiovascular Health Study. Diabet. Med. 2007, 24, 855–863. [Google Scholar] [CrossRef]
- Mattos, S.; Rabello da Cunha, M.; Barreto Silva, M.I.; Serfaty, F.; Tarvainen, M.P.; Klein, M.; Neves, M.F. Effects of weight loss through lifestyle changes on heart rate variability in overweight and obese patients: A systematic review. Clin. Nutr. 2022, 41, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Ooie, T.; Takahashi, N.; Taniguchi, Y.; Anan, F.; Yonemochi, H.; Saikawa, T. Influence of menstrual cycle on QT interval dynamics. Pacing Clin. Electrophysiol. 2006, 29, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Schmalenberger, K.M.; Eisenlohr-Moul, T.A.; Wurth, L.; Schneider, E.; Thayer, J.F.; Ditzen, B.; Jarczok, M.N. A Systematic Review and Meta-Analysis of Within-Person Changes in Cardiac Vagal Activity across the Menstrual Cycle: Implications for Female Health and Future Studies. J. Clin. Med. 2019, 8, 1946. [Google Scholar] [CrossRef] [PubMed]
- Colenso-Semple, L.M.; D’Souza, A.C.; Elliott-Sale, K.J.; Phillips, S.M. Current evidence shows no influence of women’s menstrual cycle phase on acute strength performance or adaptations to resistance exercise training. Front. Sports Act. Living 2023, 5, 1054542. [Google Scholar] [CrossRef] [PubMed]
- DeBlauw, J.A.; Stein, J.A.; Blackman, C.; Haas, M.; Makle, S.; Echevarria, I.; Edmonds, R.; Ives, S.J. Heart rate variability of elite female rowers in preparation for and during the national selection regattas: A pilot study on the relation to on water performance. Front. Sports Act. Living 2023, 5, 1245788. [Google Scholar] [CrossRef] [PubMed]
- Immanuel, S.; Teferra, M.N.; Baumert, M.; Bidargaddi, N. Heart Rate Variability for Evaluating Psychological Stress Changes in Healthy Adults: A Scoping Review. Neuropsychobiology 2023, 82, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Schimpchen, J.; Correia, P.F.; Meyer, T. Minimally Invasive Ways to Monitor Changes in Cardiocirculatory Fitness in Running-based Sports: A Systematic Review. Int. J. Sports Med. 2023, 44, 95–107. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Howells, D. Effects of Long-Haul Travel and the Olympic Games on Heart-Rate Variability in Rugby Sevens Medalists. Int. J. Sports Physiol. Perform. 2022, 17, 951–960. [Google Scholar] [CrossRef]
- Costa, J.A.; Figueiredo, P.; Nakamura, F.Y.; Rebelo, A.; Brito, J. Monitoring Individual Sleep and Nocturnal Heart Rate Variability Indices: The Impact of Training and Match Schedule and Load in High-Level Female Soccer Players. Front. Physiol. 2021, 12, 678462. [Google Scholar] [CrossRef]
- Manresa-Rocamora, A.; Sarabia, J.M.; Javaloyes, A.; Flatt, A.A.; Moya-Ramon, M. Heart Rate Variability-Guided Training for Enhancing Cardiac-Vagal Modulation, Aerobic Fitness, and Endurance Performance: A Methodological Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public. Health 2021, 18, 299. [Google Scholar] [CrossRef] [PubMed]
| Guidelines for Implementation in Strength and Conditioning | |
|---|---|
| Do’s | Don’ts |
| Record for a consistent duration of measurement, with best practice being at least 5 min Measure at the same time of day Measure at rest Measure with the same body positioning Use the same device settings (i.e., sampling frequency, period length, detection method, artifact removal) Know what data your app is giving you (i.e., time of measurement, arbitrary measurement vs HRV value) Track general trends with smallest worthwhile change (SWC) window using 7-day rolling averages Consider the stability of HRV within SWC rather than high or low HRV as the long-term goal of training, barring other contextual factors that affect HRV (i.e., stress) Consider the other stimuli that can affect HRV outside of training and sports Consider utilizing HRV-guided training depending on the training context | Apply population-based normalized ranges at the individual level with cut-off values Measure immediately after a “stimulating” event: exercise and high-stress life events Over-interpret small changes in HRV Change the entire training regimen over small HRV changes Rely on HRV measurements in isolation to detect overreaching and/or overtraining syndrome Act outside of the appropriate scope of practice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addleman, J.S.; Lackey, N.S.; DeBlauw, J.A.; Hajduczok, A.G. Heart Rate Variability Applications in Strength and Conditioning: A Narrative Review. J. Funct. Morphol. Kinesiol. 2024, 9, 93. https://doi.org/10.3390/jfmk9020093
Addleman JS, Lackey NS, DeBlauw JA, Hajduczok AG. Heart Rate Variability Applications in Strength and Conditioning: A Narrative Review. Journal of Functional Morphology and Kinesiology. 2024; 9(2):93. https://doi.org/10.3390/jfmk9020093
Chicago/Turabian StyleAddleman, Jennifer S., Nicholas S. Lackey, Justin A. DeBlauw, and Alexander G. Hajduczok. 2024. "Heart Rate Variability Applications in Strength and Conditioning: A Narrative Review" Journal of Functional Morphology and Kinesiology 9, no. 2: 93. https://doi.org/10.3390/jfmk9020093
APA StyleAddleman, J. S., Lackey, N. S., DeBlauw, J. A., & Hajduczok, A. G. (2024). Heart Rate Variability Applications in Strength and Conditioning: A Narrative Review. Journal of Functional Morphology and Kinesiology, 9(2), 93. https://doi.org/10.3390/jfmk9020093






