Analysis of Muscle Oxygenation after a Normobaric Hypoxia Tolerance Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Procedure
2.4. Variables
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calbet, J.A.L.; Lundby, C. Air to muscle O2 delivery during exercise at altitude. High. Alt. Med. Biol. 2009, 10, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Girard, O.; Willis, S.J.; Purnelle, M.; Scott, B.R.; Millet, G.P. Separate and combined effects of local and systemic hypoxia in resistance exercise. Eur. J. Appl. Physiol. 2019, 119, 2313–2325. [Google Scholar] [CrossRef] [PubMed]
- Albertus-Cámara, I.; Ferrer-López, V.; Martínez-González-Moro, I. The Effect of Normobaric Hypoxia in Middle-and/or Long-Distance Runners. Biology 2022, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ayán, M.P.; Crespo González-Calero, M.; Izquierdo García, J.; González Alcázar, C.; de Juan-Bagudá, J.; Arranz Escudero, A.; Avellanas-Chavala, M.L.; Esteva, S.; Castillo Martín, J.I. Intermittent hypoxic-hyperoxic conditioning for heart failure rehabilitation. REC Cardioclinics 2023, 58, 79–85. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Xi, L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Exp. Biol. Med. 2016, 241, 1708–1723. [Google Scholar] [CrossRef] [PubMed]
- Bassovitch, O.; Serebrovskaya, T.V. Equipment and Regimes for Intermittent Hypoxia Therapy. In Intermittent Hypoxia: From Molecular Mechanisms to Clinical Applications; Xi, L., Serebrovskaya, T.V., Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 589–601. [Google Scholar]
- Albertus-Cámara, I.; Rochel-Vera, C.; Lomas-Albaladejo, J.L.; Ferrer-López, V.; Martínez-González-Moro, I. Ventilatory pattern influences tolerance to normobaric hypoxia in healthy adults. Tolerance to Normobaric Hypoxia in Healthy Adults. IJRPH 2023, 20, 4935. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.; Girard, O.; Ihsan, M.; Fairchild, T. The Use of the SpO2 to FiO2 Ratio to Individualize the Hypoxic Dose in Sport Science, Exercise, and Health Settings. Front. Physiol. 2020, 11, 570472. [Google Scholar] [CrossRef] [PubMed]
- Serebrovska, T.V.; Serebrovska, Z.O.; Egorov, E. Fitness and therapeutic potential of intermittent hypoxia training: A matter of dose. Fiziol. Zhurnal 2016, 62, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Stöggl, T.; Born, D.P. Near infrared spectroscopy for muscle specific analysis of intensity and fatigue during cross-country skiing competition a case report. Sensors 2021, 21, 2535. [Google Scholar] [CrossRef]
- Farzam, P.; Starkweather, Z.; Franceschini, M.A. Validation of a novel wearable, wireless technology to estimate oxygen levels and lactate threshold power in the exercising muscle. Physiol. Reports. 2018, 6, 13. [Google Scholar] [CrossRef]
- Klusiewicz, A.; Rębiś, K.; Ozimek, M.; Czaplicki, A. The use of muscle near-infrared spectroscopy (NIRS) to assess the aerobic training loads of world-class rowers. Biol. Sport. 2021, 38, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, K.; Hamanaka, Y.; Niwayama, M. Investigation of oxygen saturation in regions of skin by near infrared spectroscopy. Skin. Res. Technol. 2020, 1, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Humon, I.O. The Humon Training Zones. 2020. Available online: https://humon.io/use-case/ (accessed on 2 April 2023).
- Hamaoka, T.; McCully, K.K. Review of early development of near infrared spectroscopy and recent advancement of studies on muscle oxygenation and oxidative metabolism. J. Physiol. Sci. 2019, 69, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Turnes, T.; Penteado Dos Santos, R.; Alves de Aguiar, R.; Loch, T.; Trevisol Possamani, L.; Caputo, F. Association between deoxygenated hemoglobin breaking-point, anaerobic threshold and rowing performance. Int. J. Sports. Physiol. Perform. 2019, 14, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Ruiz, M.J.; Jódar-Reverte, M.; Ferrer-López, V.; Martínez-González-Moro, I. Quadriceps muscle oxygenation during a maximum stress test in middle-aged athletes. Monten. J. Sports Sci. Med. 2020, 9, 43–49. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Sumi, D.; Hayashi, N.; Ota, N.; Ienaga, K.; Goto, K. Effects of combined hot and hypoxic conditions on muscle blood flow and muscle oxygenation during repeated cycling sprints. Eur. J. Appl. Physiol. 2021, 121, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.; Feriche, B.; Olcina, G.; Schoenfeld, B.J.; Camacho-Cardenosa, A.; Almeida, F.; Martínez-Guardado, I.; Timon, R.; Padial, P. Inter-set rest configuration effect on acute physiological and performance-related responses to a resistance training session in terrestrial vs simulated hypoxia. Peer J. 2022, 10, e13469. [Google Scholar] [CrossRef] [PubMed]
- Nell, H.J.; Castelli, L.M.; Bertani, D.; Jipson, A.A.; Meagher, S.F.; Melo, L.T.; Zabjek, K.; Reid, W.D. The effects of hypoxia on muscle deoxygenation and recruitment deoxygenation and recruitment in the flexor digitorum superficialis during submaximal intermittent handgrip exercise. BMC Sports. Sci. Med. Rehabil. 2020, 12, 16. [Google Scholar] [CrossRef]
- Calbet, J.A.; Radegran, G.; Boushel, R.; Saltin, B. On the mechanisms that limit oxygen uptake uptake during exercise in acute and chronic hypoxia: Role of muscle mass. J. Physiol. 2009, 587, 477–490. [Google Scholar] [CrossRef]
- Deldicque, L. Does normobaric hypoxic resistance training confer benefit over normoxic training in athletes? A Narrative Review. J. Sci. Sport. Exerci. 2022, 4, 306–314. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Tomas-Carus, P.; Timón, R.; Olcina, G.; Burtscher, M. Acute physiological response to a normobaric hypoxic exposure: Sex differences. Int. J. Biometeorol. 2022, 66, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Meng, L.; Zhu, H.; Wan, L.; Chen, F. Effect of normobaric oxygen inhalation intervention on microcirculatory blood flow and fatigue elimination of college students after exercise. Front. Genet. 2022, 13, 901862. [Google Scholar] [CrossRef] [PubMed]
- Suhr, F.; Knuth, S.; Achtzehn, S.; Mester, J.; de Marees, M. Acute exhaustive exercise under normoxic and normobaric hypoxic conditions differentially regulates angiogenic biomarkers in humans. Medicina 2021, 57, 727. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Ruiz, M.J.; Jódar-Reverte, M.; Martínez-González-Moro, I.; Ferrer-López, V. Effects of gender on oxygen saturation of thigh muscles during maximal treadmill Exercise Testing. Sport Mont 2021, 19, 7–11. [Google Scholar] [CrossRef]
- Hobbins, L.; Hunter, S.; Gaoua, N.; Girar, O. Short-Term perceptually regulated interval-walk training in hypoxia and normoxia in overweight-to-obese adults. J. Sports Sci. Med. 2021, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Inglis, E.C.; Iannetta, D.; Murias, J.M. Evaluating the NIRS-derived microvascular O2 extraction “reserve” in groups varying in sex and training status using leg blood flow occlusions. PLoS ONE 2019, 14, e0220192. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Carranza, V.; González-Mohíno, F.; Turner, A.P.; Rodriguez-Barbero, S.; González-Ravé, J.M. Using a portable near-infrared spectroscopy device to estimate the second ventilatory threshold. Int. J. Sports Med. 2021, 42, 905–910. [Google Scholar] [CrossRef]
- Iannetta, D.; Qahtani, A.; Millet, G.Y.; Murias, J.M. Quadriceps muscles O2 extraction and EMG breakpoints during a ramp incremental test. Front. Physiol. 2017, 8, 686. [Google Scholar] [CrossRef]
- Timon, R.; González-Custodio, A.; Vasquez-Bonilla, A.; Olcina, G.; Leal, A. Intermittent hypoxia as a therapeutic tool to improve health parameters in older adults. Int. J. Environ. Res. Public Health. 2022, 19, 5339. [Google Scholar] [CrossRef]



| Gender | Mean | SD | ANOVA (F) | p Value | |
|---|---|---|---|---|---|
| Aged (years) | Men | 30.9 | 10.5 | 1.756 | 0.191 |
| Women | 27.1 | 8.4 | |||
| Weekly Physical Exercise (Hours) | Men | 7.24 | 4.60 | 4.535 | 0.038 |
| Women | 4.65 | 2.94 | |||
| Height (cm) | Men | 177.14 | 6.74 | 35.877 | <0.000 |
| Women | 165.18 | 6.97 | |||
| Body Weight (Kg) | Men | 77.64 | 9.57 | 29.239 | <0.001 |
| Women | 62.58 | 9.34 | |||
| BMI (Kg/m2) | Men | 24.72 | 2.24 | 7.231 | 0.010 |
| Women | 22.89 | 2.50 | |||
| Fat percentage (%) | Men | 19.06 | 5.21 | 30.702 | <0.001 |
| Women | 28.22 | 6.52 | |||
| Muscle Percentage (%) | Men | 42.50 | 5.39 | 8.501 | 0.005 |
| Women | 37.91 | 5.33 | |||
| Waist circumference (cm) | Men | 80.77 | 6.53 | 22.317 | <0.001 |
| Women | 71.49 | 7.07 | |||
| Hip circumference (cm) | Men | 95.74 | 6.78 | 0.079 | 0.780 |
| Women | 95.21 | 5.59 | |||
| SBP (mmHg) | Men | 129.08 | 13.71 | 16.047 | <0.001 |
| Women | 113.53 | 12.15 | |||
| DBP (mmHg) | Men | 80.73 | 9.04 | 1.242 | 0.270 |
| Women | 77.65 | 10.30 |
| Mean | SD | Mean Dif | SD Dif | t-Paired | p-Value | d Cohen | ||
|---|---|---|---|---|---|---|---|---|
| SpO2 (%) | Pre | 99.17 | 1.22 | 14.46 | 4.61 | 23.043 | <0.000 | 3.103 |
| Post | 84.70 | 4.68 | ||||||
| SmO2 (%) | Pre | 59.85 | 12.99 | 3.11 | 3.52 | 6.502 | <0.000 | 0.803 |
| Post | 56.74 | 13.00 | ||||||
| HR (bpm) | Pre | 73.83 | 13.99 | −6.65 | 9.48 | −5.156 | <0.000 | −0.685 |
| Post | 80.48 | 12.79 |
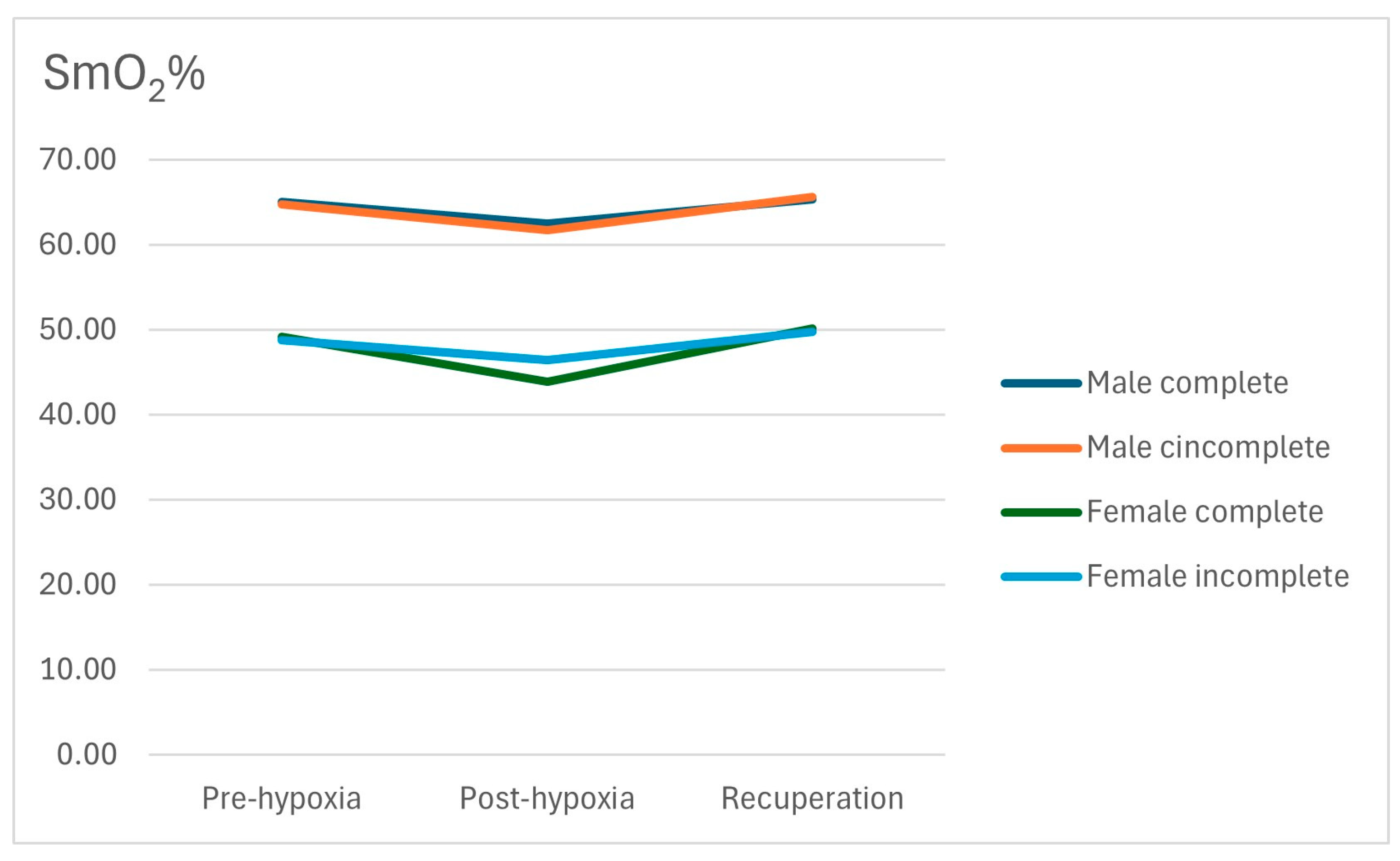
| SmO2 (%) | Group | n | Mean | SD | |
|---|---|---|---|---|---|
| Men (n = 37) | Pre | Incomplete | 24 | 64.75 | 10.22 |
| Complete | 13 | 65.08 | 5.74 | ||
| Post | Incomplete | 24 | 61.71 | 10.23 | |
| Complete | 13 | 62.46 | 5.92 | ||
| 10 min | Incomplete | 24 | 65.67 | 8.83 | |
| Complete | 13 | 65.31 | 6.22 | ||
| Women (n = 17) | Pre | Incomplete | 10 | 48.80 | 14.09 |
| Complete | 7 | 49.14 | 15.31 | ||
| Post | Incomplete | 10 | 46.40 | 10.50 | |
| Complete | 7 | 43.86 | 17.74 | ||
| 10 min | Incomplete | 10 | 49.80 | 9.59 | |
| Complete | 7 | 50.14 | 17.85 | ||
| All subjects (n = 54) | Pre | Incomplete | 34 | 60.06 | 13.46 |
| Complete | 20 | 59.50 | 12.48 | ||
| Post | Incomplete | 34 | 57.21 | 12.38 | |
| Complete | 20 | 55.95 | 14.30 | ||
| 10 min | Incomplete | 34 | 61.00 | 11.54 | |
| Complete | 20 | 60.00 | 13.42 |
| SmO2 | Gender (p Value) | Group (p Value) |
|---|---|---|
| Pre NHTT | <0.000 | 0.914 |
| Post NHTT | <0.000 | 0.913 |
| 10 min NHTT | <0.000 | 0.963 |
| Post—Pre NHTT | 0.532 | 0.513 |
| 10 min—PreNHTT | 0.772 | 0.696 |
| Men | Women | |||
|---|---|---|---|---|
| r Pearson | p Value | r Pearson | p Value | |
| Height (cm) | 0.193 | 0.252 | 0.331 | 0.194 |
| Body Weight (Kg) | 0.095 | 0.578 | 0.252 | 0.330 |
| Muscle mass (Kg) | −0.199 | 0.237 | 0.290 | 0.259 |
| Fat mass (Kg) | 0.096 | 0.572 | 0.213 | 0.413 |
| BMI (Kg/m2) | −0.037 | 0.826 | 0.104 | 0.691 |
| Fat percentage (%) | 0.060 | 0.726 | 0.076 | 0.772 |
| Muscle Percentage (%) | −0.318 | 0.055 | 0.142 | 0.586 |
| Waist circumference (cm) | 0.112 | 0.509 | 0.242 | 0.349 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albertus-Cámara, I.; Paredes-Ruiz, M.-J.; Martínez-González-Moro, I. Analysis of Muscle Oxygenation after a Normobaric Hypoxia Tolerance Test. J. Funct. Morphol. Kinesiol. 2024, 9, 86. https://doi.org/10.3390/jfmk9020086
Albertus-Cámara I, Paredes-Ruiz M-J, Martínez-González-Moro I. Analysis of Muscle Oxygenation after a Normobaric Hypoxia Tolerance Test. Journal of Functional Morphology and Kinesiology. 2024; 9(2):86. https://doi.org/10.3390/jfmk9020086
Chicago/Turabian StyleAlbertus-Cámara, Inés, María-José Paredes-Ruiz, and Ignacio Martínez-González-Moro. 2024. "Analysis of Muscle Oxygenation after a Normobaric Hypoxia Tolerance Test" Journal of Functional Morphology and Kinesiology 9, no. 2: 86. https://doi.org/10.3390/jfmk9020086
APA StyleAlbertus-Cámara, I., Paredes-Ruiz, M.-J., & Martínez-González-Moro, I. (2024). Analysis of Muscle Oxygenation after a Normobaric Hypoxia Tolerance Test. Journal of Functional Morphology and Kinesiology, 9(2), 86. https://doi.org/10.3390/jfmk9020086







