Sitting Less, Recovering Faster: Investigating the Relationship between Daily Sitting Time and Muscle Recovery following Intense Exercise: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
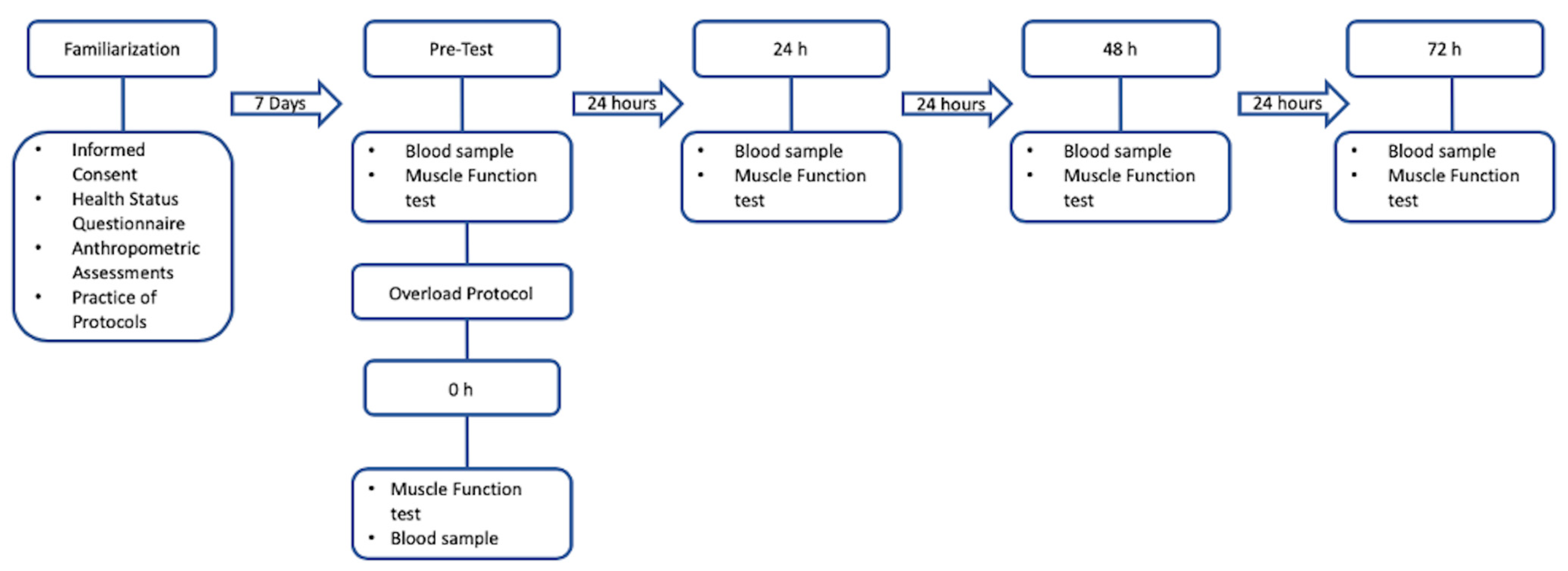
2.2. Research Design
2.3. Procedure
2.4. Muscle Function Test Protocol
2.5. Overload Protocol
2.6. Lifestyle Logging Protocol
2.7. Blood Sample Protocol
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Descriptive Statistics
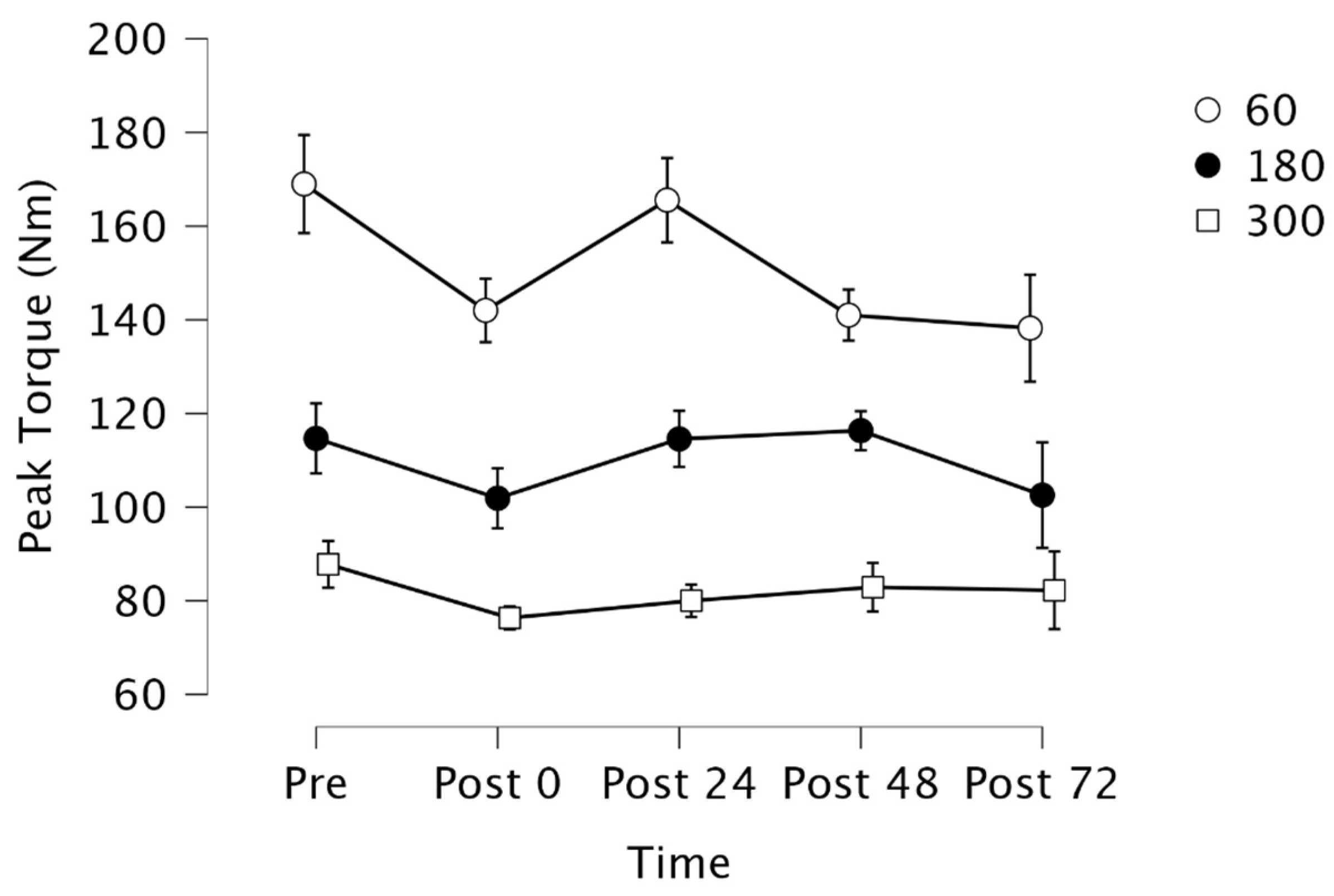
3.3. Peak Torque
3.4. Muscle Soreness and Inflammation Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sesso, H.D.; Paffenbarger, R.S.; Lee, I.M. Physical activity and coronary heart disease in men. Circulation 2000, 102, 975–980. [Google Scholar] [CrossRef]
- Paffenbarger, R.S.; Hyde, R.T.; Wing, A.L.; Lee, I.M.; Jung, D.L.; Kampert, J.B. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N. Engl. J. Med. 1993, 328, 538–545. [Google Scholar] [CrossRef]
- Lee, I.M.; Hsieh, C.C.; Paffenbarger, R.S. Exercise intensity and longevity in men. The Harvard Alumni Health Study. JAMA 1995, 273, 1179–1184. [Google Scholar] [CrossRef]
- Paffenbarger, R.S.; Kampert, J.B.; Lee, I.M.; Hyde, R.T.; Leung, R.W.; Wing, A.L. Changes in physical activity and other lifeway patterns influencing longevity. Med. Sci. Sports Exerc. 1994, 26, 857–865. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G. Physical activity in the prevention of cardiovascular disease. Sports Med. 2001, 31, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The Evolving Definition of “Sedentary”. Exerc. Sport Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef]
- Healy, G.N.; Eakin, E.G.; LaMontagne, A.D.; Owen, N.; Winkler, E.A.H.; Wiesner, G.; Gunning, L.; Neuhaus, M.; Lawler, S.; Fjeldsoe, B.S.; et al. Reducing sitting time in office workers: Short-term efficacy of a multicomponent intervention. Prev. Med. 2013, 57, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.C.; Owen, N.; Yates, T.E.; Kingwell, B.A.; Dunstan, D.W. Sitting Less and Moving More: Improved Glycaemic Control for Type 2 Diabetes Prevention and Management. Curr. Diabetes Rep. 2016, 16, 114. [Google Scholar] [CrossRef]
- Peddie, M.C.; Bone, J.L.; Rehrer, N.J.; Skeaff, C.M.; Gray, A.R.; Perry, T.L. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: A randomized crossover trial. Am. J. Clin. Nutr. 2013, 98, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C.A. Interrupting Prolonged Sitting in Overweight, and Obese Adults and Glycaemic Responses: A Randomized Crossover Study in Free-Living Conditions. Master’s Thesis, University of Bedfordshire, Bedfordshire, UK, 2018. Available online: https://uobrep.openrepository.com/handle/10547/623335 (accessed on 23 July 2020).
- Bailey, D.P.; Locke, C.D. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J. Sci. Med. Sport 2015, 18, 294–298. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Verin, E.; Hamilton, M.T.; Shaw, J.E.; Bertovic, D.A.; Zimmet, P.Z.; Salmon, J.; et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchel, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults. Ann. Intern. Med. 2015, 162, 123. [Google Scholar] [CrossRef]
- Weller, I.; Corey, P. The impact of excluding non-leisure energy expenditure on the relation between physical activity and mortality in women. Epidemiology 1998, 9, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Greenland, P.; LaCroix, A.Z.; Stefanish, M.L.; Mouton, C.P.; Oberman, A.; Perri, M.G.; Sheps, D.S.; Pettinger, M.B.; Siscovick, D.S. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N. Engl. J. Med. 2002, 347, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Everhart, J.E.; Patel, K.V.; Schoeller, D.A.; Colbert, L.H.; Visser, M.; Tylavsky, F.; Bauer, D.C.; Goodpaster, B.; Harris, T.B. Daily activity energy expenditure and mortality among older adults. JAMA 2006, 296, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.H.; Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur. Heart J. 2011, 32, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Howard, B.; Healy, G.N.; Owen, N. Too much sitting–A health hazard. Diabetes Res. Clin. Pract. 2012, 97, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Thomas, D.M.; Tudor-Locke, C.; Katzmarzyk, P.T.; Earnest, C.P.; Rodarte, R.Q.; Martin, C.K.; Blair, S.N.; Bouchard, C. Trends over 5 decades in u.s. occupation-related physical activity and their associations with obesity. PLoS ONE 2011, 6, 19657. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Shook, R.P.; Thomas, D.M.; Church, T.S.; Katzmarzyk, P.T.; Hebert, J.R.; McIver, K.L.; Hand, G.A.; Lavie, C.J.; Blair, S.N. 45-year trends in women’s use of time and household management energy expenditure. PLoS ONE 2013, 8, 56620. [Google Scholar] [CrossRef]
- Patel, A.V.; Bernstein, L.; Deka, A.; Feigelson, H.S.; Campbell, P.T.; Gapstur, G.A.C.; Thun, M.J. Leisure time spent sitting in relation to total mortality in a prospective cohort of us adults. Am. J. Epidemiol. 2010, 172, 419–429. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health. Available online: https://www.who.int/publications/i/item/9789241599979 (accessed on 22 July 2020).
- Centers for Disease Control and Prevention (CDC). Prevalence of Adults Meeting Who Physical Activity Recommendations. Available online: https://www.cdc.gov/nchs/products/databriefs/db443.htm (accessed on 2 June 2020).
- Henson, J.; Yates, T.; Edwardson, C.L.; Khunti, K.; Talbot, D.; Gray, L.J.; Leigh, T.M.; Carter, P.; Davies, M.J. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS ONE 2013, 8, 78350. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Barr, E.L.M.; Healy, G.N.; Salmon, J.; Shaw, J.E.; Balkau, B.; Magliano, D.J.; Cameron, A.J.; Zimmet, P.Z.; Owen, N. Television viewing time and mortality. Circulation 2010, 121, 384–391. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Dunstan, D.W.; Salmon, J.; Cerin, E.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Breaks in sedentary time: Beneficial associations with metabolic risk. Diabetes Care 2008, 31, 661–666. [Google Scholar] [CrossRef] [PubMed]
- León-Muñoz, L.M.; Martínez-Gómez, D.; Balboa-Castillo, T.; López-García, E.; Guallar-Castillón, P.; Rodríguez-Artalejo, F. Continued sedentariness, change in sitting time, and mortality in older adults. Med. Sci. Sports Exerc. 2013, 45, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Bielko, S.L.; Mather, K.J.; Johnston, J.D.; Wallace, J.P. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med. Sci. Sports Exerc. 2015, 47, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Bellettiere, J.; Winkler, E.A.H.; Chastin, S.F.M.; Kerr, J.; Owen, N.; Dunstan, D.W.; Healy, G.N. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS ONE 2017, 12, 0180119. [Google Scholar] [CrossRef] [PubMed]
- Bey, L.; Hamilton, M.T. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: A molecular reason to maintain daily low-intensity activity. J. Physiol. 2003, 551, 673–682. [Google Scholar] [CrossRef]
- Jeppesen, J.; Hollenbeck, C.B.; Zhou, M.Y.; Coulston, A.M.; Jones, C.; Chen, Y.D.I.; Reaven, G.M. Relation between insulin resistance, hyperinsulinemia, postheparin plasma lipoprotein lipase activity, and postprandial lipemia. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 320–324. [Google Scholar] [CrossRef]
- Kim, I.Y.; Park, S.; Chou, T.H.; Trombold, J.R.; Coyle, E.F. Prolonged sitting negatively affects the postprandial plasma triglyceride-lowering effect of acute exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E891–E898. [Google Scholar] [CrossRef]
- Worthman, C.M.; Costello, E.J. Tracking biocultural pathways in population health: The value of biomarkers. Ann. Hum. Biol. 2009, 36, 281–297. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Eltoft, A.; Arntzen, K.A.; Hansen, J.B.; Wilsgaard, T.; Mathiesen, E.B.; Johnsen, S.H. C-reactive protein in atherosclerosis–A risk marker but not a causal factor? A 13-year population-based longitudinal study: The Tromsø study. Atherosclerosis 2017, 263, 293–300. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wolf, P.A.; Castelli, W.P.; D’Agostino, R.B. Fibrinogen and risk of cardiovascular disease: The Framingham Study. JAMA 1987, 258, 1183–1186. [Google Scholar] [CrossRef]
- Yarnell, J.W.; Baker, I.A.; Sweetnam, P.M.; Bainton, D.; O’Brien, J.R.; Whitehead, P.J.; Elwood, P.C. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease. The Caerphilly and Speedwell collaborative heart disease studies. Circulation 1991, 83, 836–844. [Google Scholar] [CrossRef]
- Meade, T.W.; Brozovic, M.; Chakrabarti, R.R.; North, W.R.; Haines, A.P.; Stirling, Y.; Imeson, J.D.; Thompson, S.G. Haemostatic function and ischaemic heart disease: Principal results of the northwick park heart study. Lancet 1986, 328, 533–537. [Google Scholar] [CrossRef]
- Hamer, M.; Smith, L.; Stamatakis, E. Prospective association of TV viewing with acute phase reactants and coagulation markers: English Longitudinal Study of Ageing. Atherosclerosis 2015, 239, 322–327. [Google Scholar] [CrossRef]
- Gordon, J.A.; Hoffman, J.R.; Arroyo, E.; Varanoske, A.N.; Coker, N.A.; Gepner, Y.; Wells, A.J.; Stout, J.R.; Fukuda, D.H. Comparisons in the Recovery Response from Resistance Exercise Between Young and Middle-Aged Men. J. Strength Cond. Res. 2017, 31, 3454–3462. [Google Scholar] [CrossRef]
- Cockburn, E.; Hayes, P.R.; French, D.N.; Stevenson, E.; St Clair, G.A. Acute milk-based protein–CHO supplementation attenuates exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2008, 33, 775–783. [Google Scholar] [CrossRef]
- Farias-Junior, L.F.; Browne, R.A.V.; Freire, Y.A.; Oliveira-Dantas, F.F.; Lemos, T.M.A.M.; Galvao-Coelho, N.L.; Hardcastke, S.J.; Okano, A.H.; Aoki, M.S.; Costa, E.C. Psychological responses, muscle damage, inflammation, and delayed onset muscle soreness to high-intensity interval and moderate-intensity continuous exercise in overweight men. Physiol. Behav. 2019, 199, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Croisier, J.L.; Camus, G.; Deby-Dupont, G.; Bertrand, F.; Lhermerout, C.; Crielaard, J.M.; Juchmes-Ferir, A.; Deby, C.; Albert, A.; Lamy, M. myocellular enzyme leakage, polymorphonuclear neutrophil activation and delayed onset muscle soreness induced by isokinetic eccentric exercise. Arch. Physiol. Biochem. 1996, 104, 322–329. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ho, C.S.; Chu, L.M.; Donnan, S. Relation between plasma creatinine and body size. Clin. Chem. 1986, 32, 371–373. [Google Scholar] [CrossRef]
- Nikolaidis, M.; Protosygellou, M.; Petridou, A.; Tsalis, G.; Tsigilis, N.; Mougios, V. Hematologic and biochemical profile of juvenile and adult athletes of both sexes: Implications for clinical evaluation. Int. J. Sports Med. 2003, 24, 506–511. [Google Scholar] [CrossRef]
- Newton, M.J.; Morgan, G.T.; Sacco, P.; Chapman, D.W.; Nosaka, K. comparison of responses to strenuous eccentric exercise of the elbow flexors between resistance-trained and untrained men. J. Strength Cond. Res. 2008, 22, 597–607. [Google Scholar] [CrossRef]
- Vincent, H.K.; Vincent, K.R. The effect of training status on the serum creatine kinase response, soreness and muscle function following resistance exercise. Int. J. Sports Med. 1997, 28, 431–437. [Google Scholar] [CrossRef]
- Karamizrak, S.O.; Ergen, E.; Töre, I.R.; Akgün, N. Changes in serum creatine kinase, lactate dehydrogenase and aldolase activities following supramaximal exercise in athletes. J. Sports Med. Phys. Fit. 1994, 34, 141–146. [Google Scholar]
- Garry, J.P.; McShane, J.M. Postcompetition elevation of muscle enzyme levels in professional football players. MedGenMed Medscape Gen. Med. 2000, 2, E4. [Google Scholar]
- Fehrenbach, E.; Niess, A.M.; Schlotz, E.; Passek, F.; Dickhuth, H.H.; Northoff, H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J. Appl. Physiol. 1985, 89, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in sports and exercise: Tracking health, performance, and recovery in athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Meredith, C.N.; Cannon, J.G.; Dinarello, C.A.; Frontera, W.R.; Hughes, V.A.; Jones, B.H.; Knuttgen, H.G. Metabolic changes following eccentric exercise in trained and untrained men. J. Appl. Physiol. 1986, 61, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Akins, J.D.; Crawford, C.K.; Burton, H.M.; Wolfe, A.S.; Vardarli, E.; Coyle, E.F. Inactivity induces resistance to the metabolic benefits following acute exercise. J. Appl. Physiol. 2019, 126, 1088–1094. [Google Scholar] [CrossRef]
- Burton, H.M.; Coyle, E.F. Daily step count and postprandial fat metabolism. Med. Sci. Sports Exerc. 2021, 53, 333–340. [Google Scholar] [CrossRef]
- Altenburg, T.M.; Rotteveel, J.; Dunstan, D.W.; Salmon, J.; Chinapaw, M.J.M. The effect of interrupting prolonged sitting time with short, hourly, moderate-intensity cycling bouts on cardiometabolic risk factors in healthy, young adults. J. Appl. Physiol. 2013, 115, 1751–1756. [Google Scholar] [CrossRef]
- Wolfe, A.S.; Burton, H.M.; Vardarli, E.; Coyle, E.F. Hourly 4-s sprints prevent impairment of postprandial fat metabolism from inactivity. Med. Sci. Sports Exerc. 2020, 52, 2262–2269. [Google Scholar] [CrossRef]
- Miyashita, M.; Burns, S.F.; Stensel, D.J. Exercise and postprandial lipemia: Effect of continuous compared with intermittent activity patterns. Am. J. Clin. Nutr. 2006, 83, 24–29. [Google Scholar] [CrossRef]
- Hamer, M.; Stamatakis, E. The accumulative effects of modifiable risk factors on inflammation and haemostasis. Brain Behav. Immun. 2008, 22, 1041–1043. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Sandhu, M.S.; Day, N.E.; Luben, R.; Bingham, S.A.; Peters, R.J.G.; Wareham, N.J.; Khaw, K.T. Physical activity, C-reactive protein levels and the risk of future coronary artery disease in apparently healthy men and women: The EPIC–Norfolk prospective population study. Eur. J. Cardiovasc Prev. Rehabil. 2006, 13, 970–976. [Google Scholar] [CrossRef]
- Teixeira, V.; Valente, H.; Casal, S.; Marques, F.; Moreira, P. Antioxidant status, oxidative stress, and damage in elite trained kayakers and canoeists and sedentary Controls. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.J.; Pereira, R.; Machado, M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Interact. 2014, 14, 68–77. [Google Scholar] [PubMed]
- Howard, B.J.; Fraser, S.F.; Sethi, P.; Cerin, E.; Hamilton, M.C.; Owen, N.; Dunstan, D.W.; Kingwell, B.A. Impact on Hemostatic Parameters of Interrupting Sitting with Intermittent Activity. Med. Sci. Sports Exerc. 2013, 45, 1285–1291. [Google Scholar] [CrossRef]
- Hamer, M.; Sabia, S.; Batty, G.D.; Shipley, M.J.; Tabak, A.G.; Singh-Manoux, A.; Kivimaki, M. Physical Activity and Inflammatory Markers Over 10 Years. Circulation 2012, 126, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.J.; Hu, L.; Valentine, R.J.; McAuley, E.; Evans, A.M.; Baynard, T.; Woods, J.A. Reduction in trunk fat predicts cardiovascular exercise training-related reductions in C-reactive protein. Brain Behav. Immun. 2009, 23, 485–491. [Google Scholar] [CrossRef]
- Orri, J.C.; Carter, S.R.; Howington, E.B. Gender comparison of C-reactive protein and cardiovascular disease risk in college students and intercollegiate athletes. J. Sports Med. Phys. Fit. 2010, 50, 72–78. [Google Scholar]
- Wang, S.; Reed, D.B.; Goli, S.; Goswami, D. Blood leptin and C-reactive protein provide more sensitive assessment than blood lipids and other inflammatory biomarkers in overweight university students. Nutr. Res. 2011, 31, 586–593. [Google Scholar] [CrossRef]
- Vainshelboim, B.; Brennan, G.M.; LoRusso, S.; Fitzgerald, P.; Wisniewski, K.S. Sedentary behavior and physiological health determinants in male and female college students. Physiol. Behav. 2019, 204, 277–282. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Pereira, M.A.; Curran, K.M. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med. Sci. Sports Exerc. 2000, 32, 1601–1609. [Google Scholar] [CrossRef]
- Buckworth, J.; Nigg, C. Physical Activity, Exercise, and Sedentary Behavior in College Students. J. Am. Coll. Health 2004, 53, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.S.; Truelove, S.; Burke, S.M.; Irwin, J.D. Sedentary time among undergraduate students: A systematic review. J. Am. Coll. Health 2019, 10, 237–244. [Google Scholar] [CrossRef]
- Yang, L.; Cao, C.; Kantor, E.D.; Nguyen, L.H.; Zheng, X.; Park, Y.; Giovannucci, E.L.; Matthews, C.E.; Colditz, G.A.; Cao, Y. Trends in Sedentary Behavior among the US Population, 2001–2016. JAMA 2019, 321, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Hume, P.A.; Maxwell, L. Delayed onset muscle soreness. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Miilunpalo, S.; Vuori, I.; Oja, P.; Pasanen, M.; Urponen, H. Self-rated health status as a health measure: The predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J. Clin. Epidemiol. 1997, 50, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Madoni, S.N.; Costa, P.B.; Coburn, J.W.; Galpin, A.J. Effects of foam rolling on range of motion, peak torque, muscle activation, and the hamstrings-to-quadriceps strength ratios. J. Strength Cond. Res. 2018, 32, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.D.; Housh, T.J.; Cochrane, K.C.; Bergstorm, H.C.; Traylor, D.A.; Lewis, R.W.; Buckner, S.L.; Schmidt, R.J.; Johnson, G.O.; Cramer, J.T. Effects of anatabine and unilateral maximal eccentric isokinetic muscle actions on serum markers of muscle damage and inflammation. Eur. J. Pharmacol. 2014, 728, 161–166. [Google Scholar] [CrossRef]
- Cornish, S.M.; Johnson, S.T. Systemic cytokine response to three bouts of eccentric exercise. Results Immunol. 2014, 4, 23–29. [Google Scholar] [CrossRef][Green Version]
- Costa, P.B.; Herda, T.J.; Herda, A.A.; Cramer, J.T. Effects of dynamic stretching on strength, muscle imbalance, and muscle activation. Med. Sci. Sports Exerc. 2014, 46, 586–593. [Google Scholar] [CrossRef]
- Ruggieri, R.M.; Coburn, J.W.; Galpin, A.J.; Costa, P.B. Effects of a vibrating foam roller on ipsilateral and contralateral neuromuscular function and the hamstrings-to-quadriceps ratios. Int. J. Exerc. Sci. 2021, 14, 304–323. [Google Scholar] [PubMed]
- Depner, C.M.; Kirwan, R.D.; Frederickson, S.J.; Miles, M.P. Enhanced inflammation with high carbohydrate intake during recovery from eccentric exercise. Eur. J. Appl. Physiol. 2010, 109, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Weight, L.M.; Alexander, D.; Jacobs, P. Strenuous exercise: Analogous to the acute-phase response? Clin. Sci. 1991, 81, 677–683. [Google Scholar] [CrossRef]
- Stupka, N.; Lowther, S.; Chorneyko, K.; Bourgeois, J.M.; Hogben, C.; Tarnopolsky, M.A. Gender differences in muscle inflammation after eccentric exercise. J. Appl. Physiol. 2000, 89, 2325–2332. [Google Scholar] [CrossRef]
- Toft, A.D.; Jensen, L.B.; Bruunsgaard, H.; Ibfelt, T.; Halkjaer-Kristensen, J.; Febbraio, M.; Pedersen, B.K. Cytokine response to eccentric exercise in young and elderly humans. Am. J. Physiol. Cell Physiol. 2002, 283, C289–C295. [Google Scholar] [CrossRef]
- Kasapis, C.; Thompson, P.D. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef]
- Taylor, C.G.; Rogers, G.J.; Goodman, C.; Baynes, R.D.; Bothwell, T.H.; Bezwoda, W.R.; Kramer, F.; Hattingh, J. Hematologic, iron-related, and acute-phase protein responses to sustained strenuous exercise. J. Appl. Physiol. 1987, 62, 464–469. [Google Scholar] [CrossRef]
- Geffken, D.F.; Cushman, M.; Burke, G.L.; Polak, J.F.; Sakkinen, P.A.; Tracy, R.P. Association between physical activity and markers of inflammation in a healthy Elderly Population. Am. J. Epidemiol. 2001, 153, 242–250. [Google Scholar] [CrossRef]
- Strachan, A.F.; Noakes, T.D.; Kotzenberg, G.; Nel, A.E.; de Beer, F.C. C reactive protein concentrations during long distance running. BMJ 1984, 289, 1249–1251. [Google Scholar] [CrossRef]
- Malm, C.; Sjödin, B.; Sjöberg, B.; Lenkei, R.; Renstrom, P.; Lundberg, I.E.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar] [CrossRef]
- Nosaka, K.; Clarkson, P.M. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med. Sci. Sports Exerc. 1996, 28, 953–961. [Google Scholar] [CrossRef]
- Milias, G.A.; Nomikos, T.; Fragopoulou, E.; Athanasopoulos, S.; Antonopoulou, S. Effects of eccentric exercise-induced muscle injury on blood levels of platelet activating factor (PAF) and other inflammatory markers. Eur. J. Appl. Physiol. 2005, 95, 504–513. [Google Scholar] [CrossRef]
- Ghanbari, A.; Tayebi, S.M. The Effect of a Single Session of Eccentric Resistance Exercise on Some Parameters of White Blood Cells. Ann. Appl. Sport Sci. 2013, 1, 17–26. [Google Scholar]
- MacIntyre, D.L.; Reid, W.D.; Lyster, D.M.; Szasz, I.J.; McKenzie, D.C. Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J. Appl. Physiol. 1996, 80, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L. Effects of dietary carbohydrate on delayed onset muscle soreness and reactive oxygen species after contraction induced muscle damage. Br. J. Sports Med. 2005, 39, 948–953. [Google Scholar] [CrossRef]
- Miles, M.P.; Pearson, S.D.; Andring, J.M.; Kidd, J.R.; Volpe, S.L. Effect of carbohydrate intake during recovery from eccentric exercise on interleukin-6 and muscle-damage markers. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.; Lee, J. Effect of timing of whey protein supplement on muscle damage markers after eccentric exercise. J. Exerc. Rehabil. 2017, 13, 436–440. [Google Scholar] [CrossRef]
- Chen, J.; Berkman, W.; Bardouh, M.; Ng, C.Y.K.; Allman-Farinelli, M. The use of a food logging app in the naturalistic setting fails to provide accurate measurements of nutrients and poses usability challenges. Nutrition 2019, 57, 208–216. [Google Scholar] [CrossRef]
- Haskell, W.L. Physical activity by self-report: A brief history and future issues. J. Phys. Act. Health 2012, 9, S5–S10. [Google Scholar] [CrossRef]
- Clark, B.K.; Thorp, A.A.; Winkler, E.A.; Gardiner, P.A.; Healy, G.N.; Owen, N.; Dunstan, D.W. Validity of self-reported measures of workplace sitting time and breaks in sitting time. Med. Sci. Sports Exerc. 2011, 43, 1907–1912. [Google Scholar] [CrossRef]
- Tu, Y.K. Testing the relation between percentage change and baseline value. Sci. Rep. 2016, 6, 23247. [Google Scholar] [CrossRef]
| Variable | Pre | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|---|
| WBC (K/μL) | 6.41 ± 2.97 | 7.31 ± 2.49 | 6.73 ± 1.58 | 6.28 ± 1.71 | 6.21 ± 1.29 |
| CRP (mg/L) | 2.08 ± 3.09 | 2.08 ± 3.18 | 2.2 ± 2.89 | 2.1 ± 2.62 | 1.79 ± 2.58 |
| CK (U/L) | 366.00 ± 464.48 | 365.00 ± 416.44 | 414.56 ± 286.98 | 622.33 ± 349.77 | 2112.33 ± 2347.3 |
| Mb (mcg/L) | 45.11 ± 22.52 | 57.22 ± 23.81 | 47.89 ± 21.99 | 247.22 ± 286.04 | 422.89 ± 670.45 |
| Variable | 0–24 h | 0–48 h | 0–72 h | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Sitting Time | ||||||
| WBC (K/μL) | 0.558 | 0.118 | 0.551 | 0.124 | 0.465 | 0.207 |
| CRP (mg/L) | 0.010 | 0.979 | 0.001 | 0.997 | −0.024 | 0.951 |
| CK (U/L) | 0.232 | 0.548 | 0.496 | 0.175 | 0.738 * | 0.023 * |
| Mb (mcg/L) | 0.280 | 0.466 | 0.71 * | 0.033 * | 0.889 ** | 0.001 ** |
| Physical Activity | ||||||
| WBC (K/μL) | 0.517 | 0.154 | 0.399 | 0.287 | 0.310 | 0.417 |
| CRP (mg/L) | 0.176 | 0.650 | 0.157 | 0.687 | 0.105 | 0.789 |
| CK (U/L) | −0.057 | 0.885 | 0.081 | 0.835 | 0.469 | 0.203 |
| Mb (mcg/L) | −0.157 | 0.688 | 0.388 | 0.303 | 0.729 * | 0.026 * |
| Protein Intake | ||||||
| WBC (K/μL) | 0.218 | 0.573 | 0.211 | 0.585 | 0.562 | 0.116 |
| CRP (mg/L) | 0.299 | 0.435 | 0.179 | 0.645 | 0.217 | 0.575 |
| CK (U/L) | −0.173 | 0.656 | 0.251 | 0.514 | 0.042 | 0.915 |
| Mb (mcg/L) | 0.278 | 0.468 | 0.112 | 0.775 | −0.096 | 0.807 |
| Carbohydrate Intake | ||||||
| WBC (K/μL) | 0.18 | 0.642 | 0.642 | 0.063 | 0.177 | 0.649 |
| CRP (mg/L) | 0.127 | 0.745 | 0.138 | 0.724 | 0.120 | 0.759 |
| CK (U/L) | 0.170 | 0.661 | 0.513 | 0.157 | 0.551 | 0.124 |
| Mb (mcg/L) | −0.078 | 0.842 | 0.479 | 0.192 | 0.208 | 0.592 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodden, J.; Ortega, D.G.; Costa, P.B. Sitting Less, Recovering Faster: Investigating the Relationship between Daily Sitting Time and Muscle Recovery following Intense Exercise: A Pilot Study. J. Funct. Morphol. Kinesiol. 2024, 9, 24. https://doi.org/10.3390/jfmk9010024
Rodden J, Ortega DG, Costa PB. Sitting Less, Recovering Faster: Investigating the Relationship between Daily Sitting Time and Muscle Recovery following Intense Exercise: A Pilot Study. Journal of Functional Morphology and Kinesiology. 2024; 9(1):24. https://doi.org/10.3390/jfmk9010024
Chicago/Turabian StyleRodden, Jaime, Dolores G. Ortega, and Pablo B. Costa. 2024. "Sitting Less, Recovering Faster: Investigating the Relationship between Daily Sitting Time and Muscle Recovery following Intense Exercise: A Pilot Study" Journal of Functional Morphology and Kinesiology 9, no. 1: 24. https://doi.org/10.3390/jfmk9010024
APA StyleRodden, J., Ortega, D. G., & Costa, P. B. (2024). Sitting Less, Recovering Faster: Investigating the Relationship between Daily Sitting Time and Muscle Recovery following Intense Exercise: A Pilot Study. Journal of Functional Morphology and Kinesiology, 9(1), 24. https://doi.org/10.3390/jfmk9010024







