Abstract
Multiple sclerosis is a disease that concerns a growing number of people, especially females. There are different interventions proposed for this population, and physical activity is one of them. A proper and well-structured physical activity program can be a cheap, feasible, and practical instrument to help this population improve their quality of life. Consequently, the present study aimed to analyze, through an umbrella review, published articles to evaluate the protocols and the effect of intervention on different types of multiple sclerosis and eventually to propose a standardized intervention for this population. Systematic reviews and meta-analyses of randomized controlled trials on multiple sclerosis and physical activity effects were searched for on the electronic databases PubMed, Web of Science, and Scopus up to 22 December 2022. The quality of the studies included was determined and the results were narratively analyzed. The included studies present heterogeneity in the population, in the study design and protocols, and in the outcomes evaluated. Most of the studies detected positive outcomes on the physical function of people with multiple sclerosis. This study highlights the necessity of future studies on a population with similar characteristics, adopting similar protocols to evaluate their feasibility and validity to make physical intervention prescribed as a medicine.
1. Introduction
Multiple sclerosis (MS) is an immune-mediated disease that affects the central nervous system and is one of the most prevalent neurological conditions and leading causes of impairment in young adults [1]. It was stated that there would be about 2.8 million people with MS worldwide by 2020, and females are twice as likely to have the disease as males [2]. This could be due to the higher survival rate among the female population [3]. MS is characterized by inflammatory demyelination with disruption to terminal axonal structures [4]. It leads to irreversible neurological damage [4]. MS is considered a two-stage disease that starts with early inflammation, which is the cause of the relapsing-remitting form, and delayed neurodegeneration, which is the cause of the progression of the non-relapsing form [5]. The most frequent form of MS is relapsing-remitting, characterized by the onset of recurrent clinical symptoms followed by total or partial recovery; moreover, after 10 to 15 years, a stage of the disease defined as secondary progressive MS causes progressive degeneration over time, worsening the clinical symptoms [6]. On the other hand, in the form of primary progressive MS, there is a gradual deterioration from the onset and the disease progression is unstoppable [7].
The pharmacological treatment of MS targets acute attacks, reduction in symptoms, and biological activity [8]. Among the most common symptoms are depression, pain, and walking difficulties [9]. There is also fatigue, usually perceived fatigue, which limits the activities of daily living and impacts anxiety, depression, cognition, and sleep quality, reducing the quality of life [10,11]. Pain, gait dysfunction, and fatigue, in that order, influence the perceived health of people with MS [12]. Most of the common symptoms can lead to a lower quality of life and activity restrictions, and they include cognitive deficiencies and muscular stiffness [13]. Related to muscle problems, it has been detected that people with MS generally present leg weakness, limiting their performance [14], especially their walking performance. The walking ability of this population is reduced in terms of velocity [15] but also in terms of cadence and stride length [16]. Postural balance control capacity is also reduced [16], limiting the daily activities and further worsening quality of life. This creates a circle that deteriorates the person’s state of health. Drugs that target the immunological signaling proteins or the immune cell populations are often adopted to treat MS; however, these treatments do not cure all of the symptoms of the disease but rather mainly decrease inflammation in these patients [17].
Exercise training has emerged as a useful rehabilitation strategy to control symptoms, regain function, improve quality of life, promote well-being, and increase involvement in activities of daily living [18]. Physical training has an important role in reducing perceived fatigue [19,20], and this indirectly improves quality of life. However, different training methods seem to have different effects. For example, the effects of aerobic exercise can be cited as leading to an improvement in the satisfaction of MS patients with their physical, mental, and social functioning [21]. On the other hand, resistance training seems to positively influence the production of neurotrophies and thus indirectly limit the progression of the disease [22]. Also, mindfulness training such as yoga seems to improve postural balance, speed, and endurance for walking, reducing fatigue, stress, anxiety, and depression and improving quality of life [23,24]. In summary, in people with MS, a supervised and customized exercise program can improve physical fitness, functional capacity, quality of life, cognitive impairments [25], aerobic capacity, and muscular strength, and it may improve mobility, fatigue, and health-related quality of life [26].
Neurologists, advanced practice clinicians, and other medical professionals can recommend physical activity and exercise, highlighting the advantages of treating the symptoms, increasing general health and quality of life, and motivating their patients throughout the treatment [27]. The common goal should be to delay or avoid irreversible neurological damage and maximize self-sufficiency, especially in activities of daily living. People with MS are generally less physically active, highlighting the necessity of proper and adapted intervention to improve patients’ adherence and compliance [28].
A standard operating procedure is a step-by-step description of the intervention to improving its quality and allow for repetition [29]. Thousands of articles are published each year on the topic of MS and physical activity or exercise; most of them are heterogeneous in terms of population and intervention. Therefore, this umbrella review evaluated previously published systematic reviews and meta-analyses of randomized controlled trials on the same topic [30] to evaluate the protocols adopted and their effects on each different type of MS and, eventually, to propose a detailed intervention for this population. The investigation of physical activity’s impacts on MS and the extrapolation of information about exercise training were also taken into consideration.
2. Materials and Methods
This umbrella review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [31].
2.1. Search Strategy
The search for relevant articles was conducted on the electronic databases PubMed, Web of Science, and Scopus. The inclusion criteria for the articles were systematic reviews and meta-analyses published up to 22 December 2022. The search used various keywords, including “multiple sclerosis”, “MS”, “exercise”, “exercise training”, “physical activity”, “review”, and “meta-analysis”. The keywords were combined using the Boolean operators AND or OR. To search the three databases, a string was used: (“multiple sclerosis” OR “MS”) AND (exercise OR “physical activity” OR “exercise training”) AND (“systematic review” OR “meta-analysis”).
2.2. Eligibility Criteria
The inclusion and exclusion criteria for the population, intervention, comparison, outcomes, and study design (PICO-S) were carefully considered. The population under investigation was individuals with MS, regardless of age and MS typology. Reviews were excluded if the sample investigated included other concomitant pathologies. Studies were excluded if the physical exercise interventions were not structured and presented. The intervention had to include physical exercise. The comparison and the outcomes were not necessary because our focus was on the intervention’s structures. Other studies designed differently than systematic reviews and meta-analyses of randomized controlled trials were excluded. Only articles written in the English language were included regardless of the country of publication.
2.3. Data Sources, Study Sections, and Data Extraction
In the first step, manuscripts were stored in EndNote X8 (EndNote version X8; Thompson Reuters, New York, NY, USA), and duplicate selection was performed. In the second phase, two independent investigators screened the reviews against the eligibility criteria based on the title, abstract, and full text. Any disagreements between the two investigators were resolved by the principal investigator.
Information related to the first author and year of publication, review methodology, databases screened, number of reviews included, objective of the study, risk of bias assessment and score, conclusion of the study, population screened, training characteristics, and main results were stored in tables. A descriptive and narrative synthesis was adopted to describe the results. A meta-analysis was not performed due to the possibility of including studies considered in more than one systematic review, which increases the risk of bias [32].
2.4. Quality Assessment
The quality of the included systematic reviews and meta-analyses of randomized controlled trials was assessed using the rating scale “Assessment of Multiple Systematic Reviews” (AMSTAR) [33]. This scale comprises 11 items and has demonstrated reliability and validity [34]. Studies with a final score between 0 and 4 were considered of poor quality, those with a score between 5 and 7 were considered of moderate quality, and those with a score above 8 were considered of high quality. A score of 0 was assigned if no sufficient information was available, and a score of 1 was assigned if enough information was collected. All included reviews were independently scored by two investigators, and any disagreements were resolved by the principal investigator.
3. Results
A total of 1561 studies (PubMed 626; Web of Science 413; Scopus 522) were found after the search of the electronic database. After the removal of duplicate articles, 1099 remained. After title and abstract screening, a total of 65 studies were collected for full-text analysis. A final total of 16 systematic reviews and meta-analyses of randomized controlled trials were included in this umbrella review. The screening process is summarized in Figure 1.
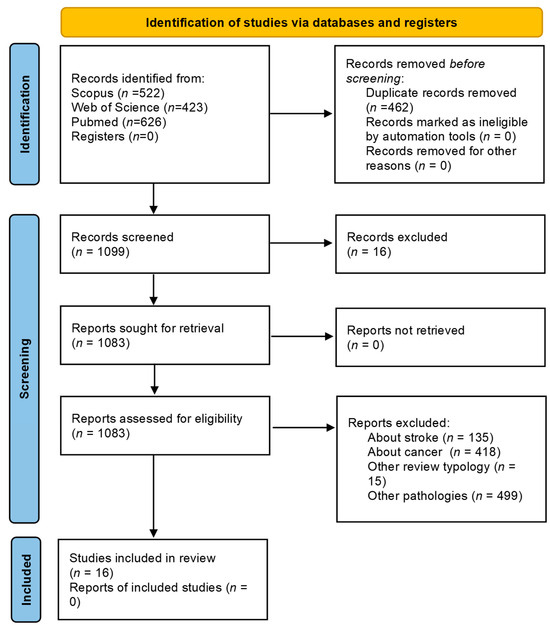
Figure 1.
Flow diagram of the study identification.
3.1. Characteristics of the Included Studies
Fourteen (of 16) studies adopted PRISMA guidelines, while in two studies information was not provided. The minimum number of databases searched was three, and all studies performed a search on MEDLINE (PubMed). The second most searched database was Cochrane (n = 10), followed by Embase and SPORTDiscus (n = 9), Scopus (n = 8), PEDro (n = 7), and Web of Science (n = 6). Other databases were searched, but the number was minimal.
To assess the risk of bias, the Physiotherapy Evidence Database (PEDro) scale was adopted in six studies, while the Cochrane tools were adopted in two studies, such as the Tool for the Assessment of Study Quality and Reporting in Exercise (TESTEX). Other studies adopted different methods, and two studies did not provide this information.
Eleven studies assessed disability with the Expanded Disability Status Scale (EDSS). Five studies did not use the Expanded Disability Status Scale (EDSS). The range of the EDSS varied widely among the studies. The type of MS was very heterogeneous. The different types of MS, including relapsing-remitting, primary progressive, and secondary progressive, were considered as a whole without particular differences within the same systematic review. There were no systematic reviews that included studies with only one type of MS.
Most of the studies evaluated the effects of an intervention on fatigue and postural balance (n = 4). Muscle function was investigated three times. Quality of life and walking ability was investigated twice (n = 2). Only one time were cardiorespiratory fitness, depressive symptoms, and cognitive performance investigated.
From a physical point of view, exercise training had positive effects on postural balance (n = 4), muscle function (n = 3), aerobic capacity, walking ability, physical function, functional mobility, strength, general physical performance, flexibility, and core stability (n = 1). One study detected mixed results for dynamic balance [35]. One study [36] found that, in the functional reach test, Pilates exercises were effective, but there was no difference with the control group. One study detected no differences in functional mobility and cardiorespiratory fitness [37].
Three studies detected positive effects of training on fatigue. It seems that yoga had only short-term effects on fatigue and mood and temporary benefits for depression. One study detected a lack of effects on depression [37]. Physical training also had positive effects on quality of life (n = 4). Training also had positive outcomes on pain. It seems that training did not work for cognitive performance [38]. More details about the study characteristics are provided in Table 1.

Table 1.
Characteristics of the included studies.
3.2. Characteristics of the Interventions
The frequency ranged from one to six times a week, with most of the studies having a mean of three times a week. The intensity ranged from low to vigorous, with some studies highlighting that it progressively increased. The time (duration of the session) ranged from 15 to 135 min, with a mean of about 50 min. The duration of the intervention ranged from 3 to 26 weeks. One study highlighted that it was not clear whether exercise frequency and duration/volume modalities positively influenced depressive symptoms [41].
Aerobic and resistance training was proposed in nine studies: In eight of them, the authors investigated both training modalities individually and combined. Pilates and yoga as standalone interventions were proposed in two studies and aquatic therapy and equine-assisted therapy in one study. Studies on aerobic and resistance training were contradictory, with some studies associating the two training modalities and demonstrating a reduction in perceived fatigue [49] and other studies presenting a negative association between aerobic training and fatigue, whereas muscle strength training presented heterogeneity in the results [51]. Yoga seems to have had positive effects on fatigue [48]. A significant improvement in self-perceived fatigue was detected after Pilates interventions [37], hippotherapy [35], and aquatic therapy [47]. Yoga and aerobic training were more effective in improving dynamic and static balance; aquatic and aerobic training were more effective in improving functional walking ability [43]. Resistance training demonstrated the strongest improvement in the 6 min walking test, while combined training showed the greatest improvement in walking endurance [46]. Another study detected positive effects of Pilates training on postural balance, but it was comparable to aerobic and traditional exercises [36].
Nine studies did not report information on whether the intervention was supervised or not, while the remaining study detected both supervised and home-based methodologies, making it difficult to extrapolate a clear result from the intervention mode.
Related to dropout rate, four studies reported percentages that ranged from 0 to maxima of 18.4% [44], 22% [41], 32% [39], and 47% [42]. More details about the study characteristics are provided in Table 2.

Table 2.
Characteristics of the interventions.
3.3. Risk of Bias Assessment
The quality of the included studies ranged from 4 to 10, with a mean of 8/11. Within the included studies, the overall quality was mainly moderate and the risk of bias was medium. Four studies had no scores. The results of the two reviews were unclear. A summary is provided in Table 1 and Table 3.

Table 3.
Quality assessment through the Assessment of Multiple Systematic Reviews” (AMSTAR) of the included systematic reviews.
4. Discussion
The main finding of the study was the heterogeneity that still exists in the participants’ characteristics and the protocols adopted in the studies that wanted to associate physical activity intervention and MS. Our findings are in line with another review of reviews [52], which highlighted the heterogeneity in the results with different types/modes of exercise interventions, comparison groups, and/or study populations. Even though several years had passed between the two works, as the other review of reviews was written in 2017 [52], it was also for us difficult to provide information about which specific combination of exercise duration, frequency, and intensities can be suggested. Despite the heterogeneity of the studies, one kind of activity adopted in most of the interventions was mindfulness activities such as yoga and Pilates, which seem safe and feasible, making them ideal as basic interventions. Below is a possible training intervention that should be personalized according to the characteristics of the participants.
A comparison of people with MS with different ages or different disease courses was hard if not impossible to execute. Most of the studies correctly evaluated their samples with the EDSS, which rates the central nervous system’s functioning and defines the development of the disease [53]. Unfortunately, not all studies adopted this scale, and due to the differences within and between the studies, it was hard to create groups. This limitation was also noted in other studies [54,55]. Based on these findings, it is advisable for future studies to be more consistent about this aspect. Only this way will it be possible to create standardized protocols that physicians and kinesiologists can adopt as an intervention. The second limitation is in the protocols adopted. The differences were too broad to make the protocols comparable and generalize the findings. The differences between the studies and the necessity of well-designed interventions were also noted in other studies within the reviews [56,57], making it difficult to synthesize the results [49]. Despite these significant limitations, most of the studies agreed that physical activity is a feasible, cheap, and easy-to-adopt intervention to improve the physical sphere of people with MS (see Table 1). In summary, exercise training has positive effects on postural balance [58], muscle function, aerobic capacity [45], walking ability, physical function, functional mobility, strength [44], general physical performance, flexibility core stability [59], and fatigue [60], cognition [61], depression, pain, and on quality of life [62]. It seems to have effects on symptoms of fatigue, poor functionality, postural balance, and quality of life [63] as well as on chronic levels of BDNF [56,64].
The following is the rationale for the training protocol proposal in terms of frequency, intensity, time, and type. It is fundamental to highlight that the proposal has to be personalized according to the indication of the family doctor or the expert who follows the patient from a medical point of view. The frequency in the studies ranged from one to six times a week, but most of the studies based their weekly frequency on three times a week, making this a reference point. The intensity should be adapted to the patient, but, as suggested in some of the included studies, it should be gradually increased to moments of vigorous intensity. Also, the time ranged significantly, from a few minutes to hours, but the mean was about 50 min, making it an appropriate indication of good training. Lastly, the type of activity: Aerobic and resistance training were proposed in most of the studies (see Table 2), including combined and in the water, and mindfulness activities were also included. According to the included studies, it seems that more physically oriented training (aerobic and resistance training) affects the body characteristics, whereas mindfulness activities have positive effects on fatigue and depression. Considering the importance of muscle strength in older adults and considering the age that current PwMS have reached, it is fundamental to include in the intervention strength-based resistance training such as high-intensity interval exercise (HIIT) to improve strength, gait speed, and quality of life [65] and to prevent sarcopenia [66].
An important aspect to consider in the interventions is the wish to change behavior [67]. Regarding the location, home-based or supervised were both proposed without distinction. Because not all people with MS can constantly go to intervention centers, technology-based distance physical rehabilitation interventions could be a good solution with positive outcomes [68]. Furthermore, home-based exercises are potentially able to reduce fall outcomes in ambulatory PwMS [69], so this training modality can be proposed. This standard operating procedure is a proposal, but future studies on exercise training should deeply standardize their procedures; indeed, this type of intervention has positive outcomes without prescription as a pharmacotherapy requirement [70]. Despite the training typology or structure, physical activity should be suggested because of its benefits and also considering the low dropout rate detected in the included studies (Table 2).
This study is a review of reviews, and its main limitation is that it reports findings that were extrapolated and interpreted from other studies. Despite the effort to minimize the risk of bias and include only high-quality research, some of the findings could have presented errors due to this double indirect topic evaluation. Another significant limitation is related to the impossibility of analyzing the pharmaceutical medication of the patients and the associated physical activity dose. Despite that, this work provides an overview of the topic, providing some feedback for future studies. In the next few years, scientists should try to propose standardized and validated interventions based on a person’s macro-characteristics to prescribe physical activity like medicine.
5. Conclusions
The study highlights the necessity of well-planned and structured interventions with standardized protocols proposed in a similar population with multiple sclerosis. These protocols can be adapted and integrated with the medical doctor’s indications to make them personalized to the person’s characteristics and necessities. The protocols should be validated and standardized in order to prescribe them as medicine. Physical activity is a feasible, cheap, and easy-to-adopt intervention to improve the physical, mental, and social health of people with MS.
Author Contributions
M.S.: study design and conceptualization, data interpretation, and writing—original draft. L.P.: methodology, study design, writing—original draft, data extraction, and revision. B.T.: study design and methodology, analysis, and revision. A.A.: data interpretation, critical review, and revision. A.C.: critical review and revision V.D.: investigation, critical review, and revision. G.M. (Grazia Maugeri): data interpretation, critical review, and data extraction. G.M. (Giuseppe Musumeci): investigation, critical review, revision, study design, and conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University Research Project Grant PIACERI Found—NATURE-OA—2020–2022, Department of Biomedical and Biotechnological Sciences (BIOMETEC), University of Catania, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the tables.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16 (Suppl. S6), s53–s59. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A.C. Treatment of multiple sclerosis: A review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Zhang, Y.; Taylor, B.V.; Simpson, S.; Blizzard, L.; Campbell, J.A.; Palmer, A.J.; van der Mei, I. Feelings of depression, pain and walking difficulties have the largest impact on the quality of life of people with multiple sclerosis, irrespective of clinical phenotype. Mult. Scler. J. 2021, 27, 1262–1275. [Google Scholar] [CrossRef]
- Rooney, S.; Wood, L.; Moffat, F.; Paul, L. Prevalence of fatigue and its association with clinical features in progressive and non-progressive forms of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 276–282. [Google Scholar] [CrossRef]
- Bakshi, R. Fatigue associated with multiple sclerosis: Diagnosis, impact and management. Mult. Scler. J. 2003, 9, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Cutter, G.; Friendly, M.; Kister, I. Which symptoms contribute the most to patients’ perception of health in multiple sclerosis? Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317728301. [Google Scholar] [CrossRef]
- 13. Weld-Blundell, I.V.; Grech, L.; Learmonth, Y.C.; Marck, C.H. Lifestyle and complementary therapies in multiple sclerosis guidelines: Systematic review. Acta Neurol. Scand. 2022, 145, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Bowser, B.; O’Rourke, S.; Brown, C.N.; White, L.; Simpson, K.J. Sit-to-stand biomechanics of individuals with multiple sclerosis. Clin. Biomech. 2015, 30, 788–794. [Google Scholar] [CrossRef]
- Thoumie, P.; Lamotte, D.; Cantalloube, S.; Faucher, M.; Amarenco, G. Motor determinants of gait in 100 ambulatory patients with multiple sclerosis. Mult. Scler. J. 2005, 11, 485–491. [Google Scholar] [CrossRef]
- Yahia, A.; Ghroubi, S.; Mhiri, C.; Elleuch, M. Relationship between muscular strength, gait and postural parameters in multiple sclerosis. Ann. Phys. Rehabil. Med. 2011, 54, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Gholamzad, M.; Ebtekar, M.; Ardestani, M.S.; Azimi, M.; Mahmodi, Z.; Mousavi, M.J.; Aslani, S. A comprehensive review on the treatment approaches of multiple sclerosis: Currently and in the future. Inflamm. Res. 2019, 68, 25–38. [Google Scholar] [CrossRef]
- Motl, R.W.; Sandroff, B.M.; Kwakkel, G.; Dalgas, U.; Feinstein, A.; Heesen, C.; Feys, P.; Thompson, A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017, 16, 848–856. [Google Scholar] [CrossRef]
- Heine, M.; Van De Port, I.; Rietberg, M.B.; van Wegen, E.E.H.; Kwakkel, G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2015, 9, CD009956. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Greenlee, T.A.; Motl, R.W.; Nickrent, M.S.; Petruzzello, S.J. Effects of exercise training on fatigue in multiple sclerosis: A meta-analysis. Psychosom. Med. 2013, 75, 575–580. [Google Scholar] [CrossRef]
- Alphonsus, K.B.; Su, Y.; D’arcy, C. The effect of exercise, yoga and physiotherapy on the quality of life of people with multiple sclerosis: Systematic review and meta-analysis. Complement. Ther. Med. 2019, 43, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Ragonese, P.; Ingoglia, S.; Schiera, G.; Schirò, G.; Di Liegro, C.M.; Salemi, G.; Di Liegro, I.; Proia, P. Lactate Threshold Training Program on Patients with Multiple Sclerosis: A Multidisciplinary Approach. Nutrients 2021, 13, 4284. [Google Scholar] [CrossRef]
- Ahmadi, A.; Nikbakh, M.; Arastoo, A.; Habibi, A.-H. The effects of a yoga intervention on balance, speed and endurance of walking, fatigue and quality of life in people with multiple sclerosis. J. Hum. Kinet. 2010, 23, 71–78. [Google Scholar] [CrossRef]
- Shohani, M.; Badfar, G.; Nasirkandy, M.P.; Kaikhavani, S.; Rahmati, S.; Modmeli, Y.; Soleymani, A.; Azami, M. The effect of yoga on stress, anxiety, and depression in women. Int. J. Prev. Med. 2018, 9, 21. [Google Scholar]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Martin-Ginis, K.A.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of Exercise Training on Fitness, Mobility, Fatigue, and Health-Related Quality of Life Among Adults with Multiple Sclerosis: A Systematic Review to Inform Guideline Development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828.e3. [Google Scholar] [CrossRef]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult. Scler. J. 2020, 26, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; McAuley, E.; Snook, E.M. Physical activity and multiple sclerosis: A meta-analysis. Mult. Scler. J. 2005, 11, 459–463. [Google Scholar] [CrossRef]
- Petrigna, L.; Pajaujiene, S.; Delextrat, A.; Gómez-López, M.; Paoli, A.; Palma, A.; Bianco, A. The importance of standard operating procedures in physical fitness assessment: A brief review. Sport Sci. Health 2022, 18, 21–26. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar]
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 2011, 11, 15. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef]
- Shea, B.J.; Hamel, C.; Wells, G.A.; Bouter, L.; Kristjansson, E.; Grimshaw, J.; Henry, D.; Boers, M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009, 62, 1013–1020. [Google Scholar] [CrossRef]
- Suárez-Iglesias, D.; Bidaurrazaga-Letona, I.; Sanchez-Lastra, M.A.; Gil, S.M.; Ayán, C. Effectiveness of equine-assisted therapies for improving health outcomes in people with multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2021, 55, 103161. [Google Scholar] [CrossRef]
- Arik, M.I.; Kiloatar, H.; Saracoglu, I. Do Pilates exercises improve balance in patients with multiple sclerosis? A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022, 57, 103410. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lastra, M.A.; Martínez-Aldao, D.; Molina, A.J.; Ayán, C. Pilates for people with multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2019, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Gharakhanlou, R.; Wesselmann, L.; Rademacher, A.; Lampit, A.; Negaresh, R.; Kaviani, M.; Oberste, M.; Motl, R.W.; Sandroff, B.M.; Bansi, J.; et al. Exercise training and cognitive performance in persons with multiple sclerosis: A systematic review and multilevel meta-analysis of clinical trials. Mult. Scler. J. 2021, 27, 1977–1993. [Google Scholar] [CrossRef]
- Afkar, A.; Ashouri, A.; Rahmani, M.; Sigaroudi, A.E. Effect of exercise therapy on quality of life of patients with multiple sclerosis in Iran: A systematic review and meta-analysis. Neurol. Sci. 2017, 38, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Lauche, R.; Azizi, H.; Dobos, G.; Langhorst, J. Yoga for multiple sclerosis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e112414. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E.; Sloth, M. The effect of exercise on depressive symptoms in multiple sclerosis based on a meta-analysis and critical review of the literature. Eur. J. Neurol. 2015, 22, 443-e34. [Google Scholar] [CrossRef]
- Dennett, R.; Madsen, L.T.; Connolly, L.; Hosking, J.; Dalgas, U.; Freeman, J. Adherence and drop-out in randomized controlled trials of exercise interventions in people with multiple sclerosis: A systematic review and meta-analyses. Mult. Scler. Relat. Disord. 2020, 43, 102169. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, X.; Chen, P. Effects of Different Exercise Therapies on Balance Function and Functional Walking Ability in Multiple Sclerosis Disease Patients—A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 7175. [Google Scholar] [CrossRef]
- Jørgensen, M.; Dalgas, U.; Wens, I.; Hvid, L. Muscle strength and power in persons with multiple sclerosis—A systematic review and meta-analysis. J. Neurol. Sci. 2017, 376, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Langeskov-Christensen, M.; Heine, M.; Kwakkel, G.; Dalgas, U. Aerobic capacity in persons with multiple sclerosis: A systematic review and meta-analysis. Sports Med. 2015, 45, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; Dieberg, G.; Smart, N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: A meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1339–1348.e7. [Google Scholar] [CrossRef] [PubMed]
- Shariat, A.; Najafabadi, M.G.; Fard, Z.S.; Nakhostin-Ansari, A.; Shaw, B.S. A systematic review with meta-analysis on balance, fatigue, and motor function following aquatic therapy in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 68, 104107. [Google Scholar] [CrossRef]
- Shohani, M.; Kazemi, F.; Rahmati, S.; Azami, M. The effect of yoga on the quality of life and fatigue in patients with multiple sclerosis: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Clin. Pract. 2020, 39, 101087. [Google Scholar] [CrossRef]
- Taul-Madsen, L.; Connolly, L.; Dennett, R.; Freeman, J.; Dalgas, U.; Hvid, L.G. Is Aerobic or Resistance Training the Most Effective Exercise Modality for Improving Lower Extremity Physical Function and Perceived Fatigue in People With Multiple Sclerosis? A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 102, 2032–2048. [Google Scholar] [CrossRef]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Reina-Gutiérrez, S.; Álvarez-Bueno, C.; Guzmán-Pavón, M.J.; Pozuelo-Carrascosa, D.P.; Fernández-Rodríguez, R.; Sanchez-López, M.; Cavero-Redondo, I. Effect of Exercise on Fatigue in Multiple Sclerosis: A Network Meta-analysis Comparing Different Types of Exercise. Arch. Phys. Med. Rehabil. 2022, 103, 970–987.e18. [Google Scholar] [CrossRef]
- Rooney, S.; Wood, L.; Moffat, F.; Paul, L. Is Fatigue Associated with Aerobic Capacity and Muscle Strength in People With Multiple Sclerosis: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 2193–2204. [Google Scholar] [CrossRef]
- Safari, R.; van der Linden, M.L.; Mercer, T.H. Effect of exercise interventions on perceived fatigue in people with multiple sclerosis: Synthesis of meta-analytic reviews. Neurodegener. Dis. Manag. 2017, 7, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Edwards, T.; Michelsen, A.S.; Fakolade, A.O.; Dalgas, U.; Pilutti, L.A. Exercise training improves participation in persons with multiple sclerosis: A systematic review and meta-analysis. J. Sport Health Sci. 2022, 11, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Amatya, B.; Khan, F.; Ng, L.; Galea, M. Rehabilitation for people with multiple sclerosis: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2019, 1, Cd012732. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, P.; Karimi, A.; Momtazmanesh, S.; Teixeira, A.L.; Teunissen, C.E.; van Wegen, E.E.H.; Hirsch, M.A.; Yekaninejad, M.S.; Rezaei, N. Exercise-induced increase in blood-based brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis: A systematic review and meta-analysis of exercise intervention trials. PLoS ONE 2022, 17, e0264557. [Google Scholar] [CrossRef]
- Torres-Pareja, M.; Sánchez-Lastra, M.A.; Iglesias, L.; Suárez-Iglesias, D.; Mendoza, N.; Ayán, C. Exercise Interventions for Improving Flexibility in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Medicina 2019, 55, 726. [Google Scholar] [CrossRef]
- Cameron, M.H.; Lord, S. Postural control in multiple sclerosis: Implications for fall prevention. Curr. Neurol. Neurosci. Rep. 2010, 10, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Bulguroglu, I.; Guclu-Gunduz, A.; Yazici, G.; Ozkul, C.; Irkec, C.; Nazliel, B.; Batur-Caglayan, H. The effects of Mat Pilates and Reformer Pilates in patients with Multiple Sclerosis: A randomized controlled study. NeuroRehabilitation 2017, 41, 413–422. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.; Shen, J.; Yang, H.; Xu, W.; Shao, M.; Pan, F. Effect of Exercise on Fatigue in Multiple Sclerosis Patients: A Network Meta-analysis. Int. J. Sports Med. 2021, 42, 1250–1259. [Google Scholar] [CrossRef]
- Abasıyanık, Z.; Ertekin, Ö.; Kahraman, T.; Yigit, P.; Özakbaş, S. The effects of Clinical Pilates training on walking, balance, fall risk, respiratory, and cognitive functions in persons with multiple sclerosis: A randomized controlled trial. Explore 2020, 16, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tollár, J.; Nagy, F.; Tóth, B.E.; Török, K.; Szita, K.; Csutorás, B.; Moizs, M.; Hortobágyi, T. Exercise Effects on Multiple Sclerosis Quality of Life and Clinical–Motor Symptoms. Med. Sci. Sports Exerc. 2020, 52, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.L.; Whillier, S. Effects of Nonpharmaceutical Treatments on Symptom Management in Adults with Mild or Moderate Multiple Sclerosis: A Meta-analysis. J. Manip. Physiol. Ther. 2019, 42, 514–531. [Google Scholar] [CrossRef] [PubMed]
- Diechmann, M.D.; Campbell, E.; Coulter, E.; Paul, L.; Dalgas, U.; Hvid, L.G. Effects of Exercise Training on Neurotrophic Factors and Subsequent Neuroprotection in Persons with Multiple Sclerosis—A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 1499. [Google Scholar] [CrossRef]
- García, J.D.J.; Martínez-Amat, A.; De La Torre-Cruz, M.J.; Fábrega-Cuadros, R.; Díaz, D.C.; Aibar-Almazán, A.; Achalandabaso-Ochoa, A.; Hita-Contreras, F. Suspension training HIIT improves gait speed, strength and quality of life in older adults. Int. J. Sports Med. 2019, 40, 116–124. [Google Scholar] [CrossRef]
- Cannataro, R.; Cione, E.; Bonilla, D.A.; Cerullo, G.; Angelini, F.; D’Antona, G. Strength training in elderly: An useful tool against sarcopenia. Front. Sports Act. Living 2022, 4, 950949. [Google Scholar] [CrossRef]
- Kim, Y.; Mehta, T.; Lai, B.; Motl, R.W. Immediate and Sustained Effects of Interventions for Changing Physical Activity in People with Multiple Sclerosis: Meta-analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2020, 101, 1414–1436. [Google Scholar] [CrossRef]
- Rintala, A.; Hakala, S.; Paltamaa, J.; Heinonen, A.; Karvanen, J.; Sjögren, T. Effectiveness of technology-based distance physical rehabilitation interventions on physical activity and walking in multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. Disabil. Rehabil. 2018, 40, 373–387. [Google Scholar] [CrossRef]
- Abou, L.; Qin, K.; Alluri, A.; Du, Y.; Rice, L.A. The effectiveness of physical therapy interventions in reducing falls among people with multiple sclerosis: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2022, 29, 74–85. [Google Scholar] [CrossRef]
- Ensari, I.; Motl, R.W.; Pilutti, L.A. Exercise training improves depressive symptoms in people with multiple sclerosis: Results of a meta-analysis. J. Psychosom. Res. 2014, 76, 465–471. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).